Abstract
Adenomyosis is a common benign gynecological disorder presenting with dysmenorrhea, menorrhagia, and pressure symptoms. Magnetic resonance imaging–guided focused ultrasound surgery (MRgFUS) utilizes precisely focused USG waves to generate and maintain high temperatures within the targeted tissue to achieve protein denaturation and coagulative necrosis. The heat generated is monitored using MRI images acquired in real-time in three planes. We present two cases of focal adenomyosis treated with MRgFUS showing good symptomatic relief at 3 and 6 months follow-up.
Keywords: Magnetic resonance imaging–guided focused ultrasound surgery, MRgFUS, focal adenomyosis, fibroid
Introduction
Adenomyosis is a common benign gynecological disorder affecting premenopausal women and having a significant impact on a woman's health and quality of life.[1] During the past several years, MRI guided focused ultrasound (MRgFUS) has been established as a safe and effective technology for noninvasive treatment of uterine fibroids and focal adenomyosis.[2–4] Our case reports briefly discuss the successful use of MRgFUS to treat symptomatic focal adenomyosis in two patients.
Case Reports
Case 1
The patient was a 39-year-old nulliparous Indian woman with a body mass index (BMI) of 25.11. She presented to her gynecologist with symptoms of dysmenorrhea, heavy bleeding, passing clots during the menstrual cycle, and fatigue for the past six months. Her symptom severity score (SSS) was 47.5 points on the 0 to 100 scale of The Uterine Fibroid Symptom and Quality of Life (UFS-QoL) questionnaire.[5] In addition, as this questionnaire is fibroid specific and does not address menstrual pain symptoms that are typical of adenomyosis, the patient was also asked to grade her menstrual pain on a scale of 0 to 10, and she graded her pain as 10, the highest on the scale.
Screening USG done elsewhere was suggestive of adenomyosis and the patient was referred to our center for MRI to evaluate her suitability for the MRgFUS treatment. MRI of the pelvis showed marked thickening of the junctional zone, with 5.4 × 4.8 × 5.9 cm (volume: 91 cc) focal adenomyosis involving the posterior myometrium [Figure 1A]. To evaluate the viability, and as part of our screening routine prior to MRgFUS, SPGR (spoiled gradient-recalled acquisition in the steady state) images with contrast were acquired. Post-gadolinium contrast images revealed homogenous enhancement [Figure 1B].
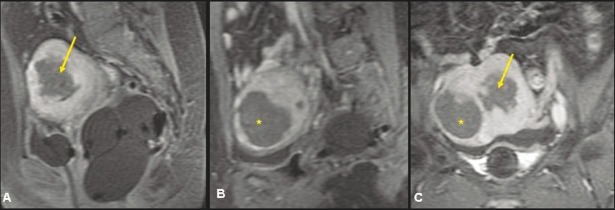
Figure 1 (A, B).
A 39-year-old nulliparous woman with focal adenomyosis. Sagittal T2W screening MRI of the pelvis (A) shows focal adenomyosis (yellow arrow) involving the posterior myometrium. Contrast-enhanced spoiled gradient-recalled acquisition in the steady state (SPGR) (B) shows homogenous enhancement in the focal adenomyosis (asterisk)
After consulting with the patient about the potential benefits and risks involved, she opted for treatment with MRgFUS. Treatment was performed using the ExAblate® 2000 system (InSightec, Haifa, Israel) and Signa® HDxt 1.5T MRI (GE Healthcare, Milwaukee, US).
The treatment was done under conscious sedation (one ampoule of fentanyl) to alleviate pain and reduce motion. A urinary catheter was inserted to prevent uterine shift during the treatment due to bladder filling. Bowels obstructing the beam path were mitigated with a rectal balloon inflated with 250cc of water and the uterus was pushed towards the anterior abdominal wall.
T2W images were acquired for treatment planning. Treatment duration was 1h 50 min (from first to last sonication), utilizing 31 focal spots or sonications [Figure 2] with a mean energy of 3516 Joules and a frequency of 1.15 MHz. A nominal HD treatment protocol was used with nominal large and elongated spots. No overlap of focal spots was used and the subendometrial zone was also targeted. The average temperature achieved was 73.4°C (min: 43°C; max: 102°C). Contrast-enhanced SPGR images were acquired post treatment and showed a nonperfused volume (NPV) of 40 cc, achieving an NPV ratio (ratio of the NPV to the volume of the focal adenomyosis or myoma) of 44% [Figure 3].
Figure 2 (A-C).
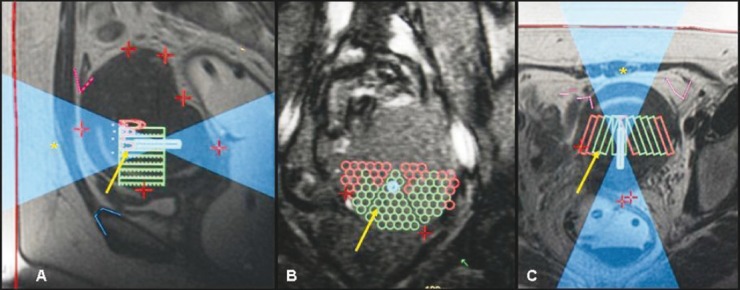
A 39-year-old nulliparous woman with focal adenomyosis. Sagittal (A), coronal (B), and axial (C) T2W images obtained during treatment show the sonication spots (yellow arrow) and the ultrasound beam path (asterisk). (Red line: skin surface; blue line: pubic bone; pink line: bowel; red plus symbol: fiducials to detect movement.)
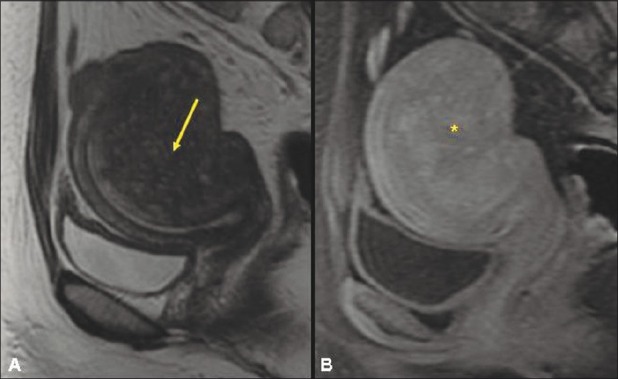
Figure 3 (A, B).
A 39-year-old nulliparous woman with focal adenomyosis. Contrast-enhanced SPGR sagittal (A) and axial (B) MRIs of the pelvis after MRgFUS treatment show a nonenhancing area (arrow) corresponding to a nonperfused volume of 44%
Treatment was completed without complications and the patient was discharged 30 min later after her recovery from conscious sedation.
During her follow-up phone call the next day, she reported returning to work and feeling well. There had been no adverse events. The patient provided a similar report in a phone call seven days post treatment. At three months follow-up, the patient had a SSS score of 32.5 and menstrual pain score of four. At six months follow-up, her SSS score had reduced to 25 points and she was totally free of the menstrual pain, with a score of 0 points.
Case 2
The patient was a 43-year-old multiparous Indian woman with a BMI of 24.2 and had the same complaints as the first case. Her SSS and menstrual pain score were 50 and 8, respectively. Screening USG done elsewhere was suggestive of adenomyosis. MRI showed marked thickening of the junctional zone, with a 4.4 × 3.4 × 5.0 cm (volume: 47 cc) focal adenomyosis involving the posterior myometrium [Figure 4A]. In addition, a 4.6 × 3.6 × 4.2 cm (volume: 35 cc) intramural fibroid, homogenously hypointense on T2W images, was noted involving the right side of the fundus of the uterus [Figure 4B]. Post-gadolinium contrast images revealed homogenous enhancement in the focal adenomyosis and heterogenous enhancement in the fibroid [Figure 5].
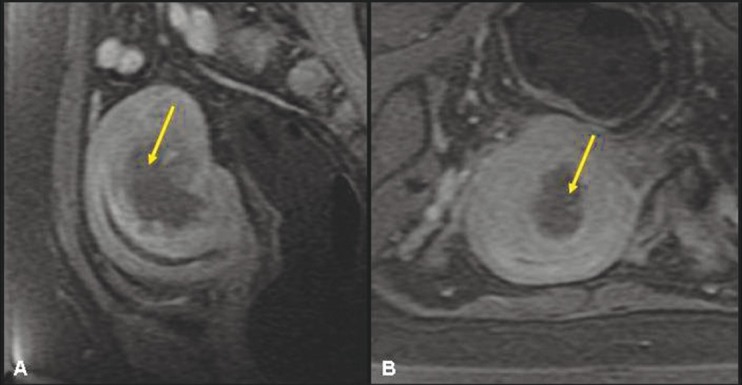
Figure 4 (A, B).
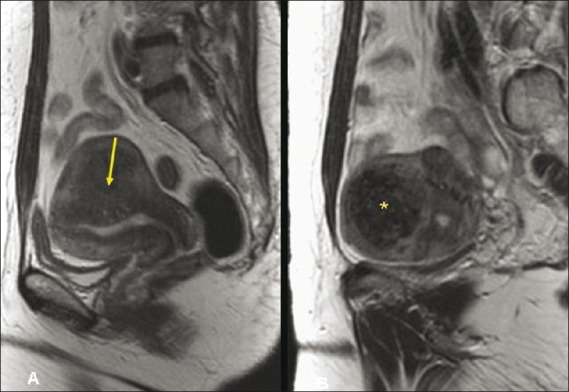
A 43-year-old multiparous woman. Sagittal T2W screening MR of the pelvis (A, B) shows focal adenomyosis (yellow arrow) involving the posterior myometrium and homogenously hypointense intramural fibroid (asterisk) involving the fundus
Figure 5 (A, B).
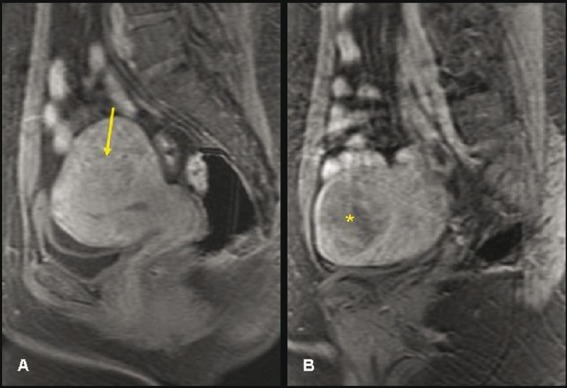
A 43-year-old multiparous woman. Contrast-enhanced SPGR sagittal screening MRI of the pelvis (A, B) shows homogenous enhancement in the focal adenomyosis (arrow) and heterogenous enhancement in the fundal fibroid (asterisk)
Treatment was performed under conscious sedation. A urinary catheter was inserted and the bowels were mitigated with a cut gel pad and rectal balloon [Figure 6]. Both the fibroid and the adenomyosis were treated in 2h 40 min, using 64 sonications with a mean energy of 1250.78 Joules and a frequency of 1.15 MHz. Nominal HD treatment protocol was used with nominal small and large spots. The average temperature achieved was 85.5°C (min: 53°C; max: 148°C) [Figure 7]. Post treatment, a non-perfused volume of 30 cc and NPV ratio of 63.8% was achieved in the focal adenomyosis. Nonperfused volume of 30 cc and NPV ratio of 85.7% was achieved in the fibroid [Figure 8].
Figure 6.
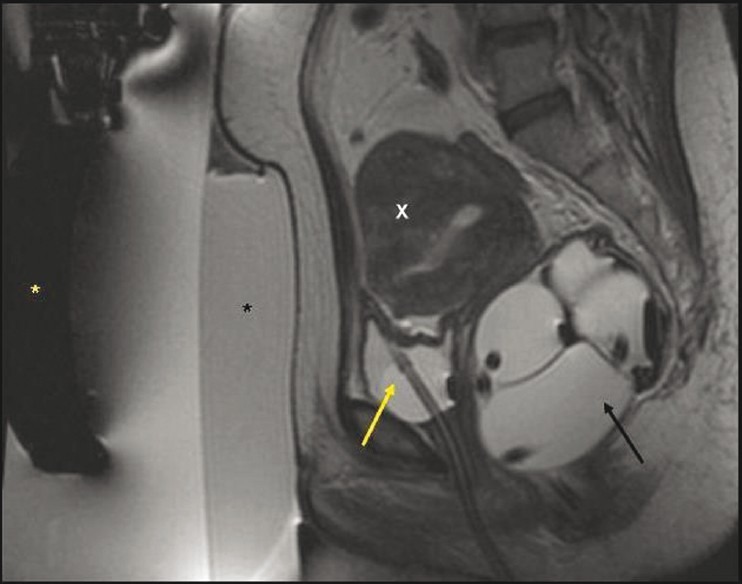
A 43-year-old multiparous woman. T2W sagittal MR of the pelvis during treatment planning shows an inflated rectal balloon (black arrow) and an empty urinary bladder with Foley catheter in situ (yellow arrow), which have together brought the focal adenomyosis (X) close to the anterior abdominal wall. (Yellow asterisk: transducer; black asterisk: gel pad.)
Figure 7 (A-C).
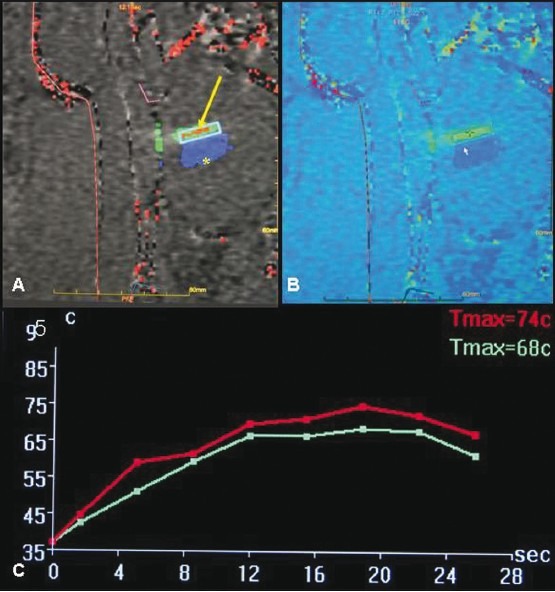
Gray-scale (A) and colour-coded (B) thermal maps showing the temperatures attained in a sonicated spot (arrow). Previously ablated tissue is shown in blue (asterisk). Thermal graph (C) shows temperatures attained in the sonicated spot
Figure 8 (A-C).
A 43-year-old multiparous woman. Contrast-enhanced SPGR sagittal (A, B) and coronal (C) MRIs of the pelvis after MRgFUS treatment show a nonenhancing area corresponding to a nonperfused volume of 63.8% in the focal adenomyosis (arrow) and 85.7% in the fibroid (asterisk)
No complications were encountered and the patient was discharged 30 min later. She returned to work the next day and reported no adverse effects. At three months follow-up, the patient had a SSS score of 40 and menstrual pain score of five. At six months follow-up, her SSS score had reduced to 24 points and she was totally free of the menstrual pain, with a score of 0 points.
Discussion
Adenomyosis is a common benign gynecological disorder affecting premenopausal women. It is characterized by the presence of ectopic endometrial glands and stroma in the myometrium and hyperplasia of adjacent smooth muscles.[6] The symptoms of adenomyosis include dysmenorrhea, menorrhagia, abnormal uterine bleeding, and diffuse uterine enlargement, sometimes leading to pelvic pressure and frequent urination. Hysterectomy, which eliminates the underlying source of symptoms, is the most common treatment for adenomyosis.[7] For women who wish to preserve their uterus, the alternative options include hormonal therapy,[8–11] endometrial ablation therapy,[12,13] and uterine artery embolization (UAE).[14–16] Hormonal treatment offer only temporary alleviation of symptoms and is also associated with side effects.[17] Endometrial ablation is an alternative when the depth of myometrial penetration is limited.[12,13] UAE has elicited mixed responses.[14–16]
During the past several years, the application of MRgFUS for the treatment of symptomatic uterine fibroids has been established as safe and effective, achieving fibroid shrinkage and symptom alleviation in diverse ethnic populations.[2,18,19] Recently, there have been reports that this technology has also been successfully applied to patients suffering from adenomyosis.[3,4,20] MRgFUS is noninvasive, thus avoiding scar formation in the uterus.
In our center, MRgFUS is currently used for the treatment of symptomatic uterine myomas and focal adenomyosis. The treatment procedure incorporates a focused ultrasound system (ExAblate® 2000, Insightec, Haifa, Israel) fully integrated with a 1.5T MRI scanner (Signa® HD, GE Healthcare, Milwaukee, WI, USA). The patient table docks to the MRI scanner. The patient lies prone and a gel pad couples the patient's abdomen to the phased array transducer housed in a sealed water bath in the patient table. Coronal, sagittal, and axial T2W MRI images are obtained to localize the lesion and to define the region of treatment. The focused ultrasound system generates a high-intensity acoustic beam (sonication) that is focused onto the target. Each sonication creates an elongated elliptical focus of high temperature (60–80°C), resulting in tissue necrosis.[21] The heat generated during the course of these sonications is monitored using MRI images acquired in real-time in three planes. At the end of treatment, the results are evaluated by assessing the nonperfused regions on contrast-enhanced SPGR images. These areas are summed to create the NPV. The ratio of the NPV to the volume of the focal adenomyosis or myoma-the NPV ratio–has been correlated with treatment effectiveness, reduction of symptom severity score and shrinkage of the ablated tissue.[3,19,22,23]
In both our patients, there was a steady decrease in symptoms over the course of the follow-up period, as demonstrated by the decline in the SSS. Both patients had high menstrual pain score before treatment (10 and 8, respectively, in patients 1 and 2). This gradually decreased and they were free of menstrual pain by the end of 6 months. They reported a significant improvement in their quality of life. In both patients no adverse events or complications were recorded during the treatment or follow-up period.
In case 2, there was a fibroid associated with the focal adenomyosis, and both were treated in the same sitting.
Our case reports suggest that clinical improvement in symptomatic adenomyosis is achievable within a short period of time after treatment with MRgFUS. MRgFUS is a noninvasive, day-care procedure, requiring no admission and having a low complication rate. MRgFUS may be a promising alternative to hysterectomy for the patient with adenomyosis who wishes to preserve her uterus. A number of reports of successful treatment have been published[20,24–26] that also show the feasibility of pregnancy following MRgFUS.
Acknowledgement
We would like to thank management and staff of Godavari Imaging Sciences and Research Center, GSL medical college and GSL trust for their support.
Footnotes
Source of Support: Godavari Imaging Sciences and Research Center, GSL medical college and GSL trust
Conflict of Interest: None declared.
References
- 1.Ota H, Igarashi S, Hatazawa J, Tanaka T. Is adenomyosis an immune disease? Hum Reprod Update. 1998;4:360–7. doi: 10.1093/humupd/4.4.360. [DOI] [PubMed] [Google Scholar]
- 2.Stewart EA, Gostout B, Rabinovici J, Kim HS, Regan L, Tempany CMC. Sustained relief of leiomyoma symptoms by using focused ultrasound surgery. Obstet Gynecol. 2007;110:279–87. doi: 10.1097/01.AOG.0000275283.39475.f6. [DOI] [PubMed] [Google Scholar]
- 3.Fukunishi H, Funaki K, Sawada K, Yamaguchi K, Maeda T, Kaji Y. Early results of magnetic resonance-guided focused ultrasound surgery of adenomyosis : Analysis of 20 cases. J Minim Invasive Gynecol. 2008;15:571–9. doi: 10.1016/j.jmig.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Yoon SW, Kim KA, Cha SH, Kim YM, Lee C, Na YJ, et al. Successful use of magnetic resonance-guided focused ultrasound surgery to relieve symptoms in a patient with symptomatic focal adenomyosis. Fertil Steril. 2008;90:2018. e13–5. doi: 10.1016/j.fertnstert.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 5.Spies JB, Coyne K, Guaou Guaou N, Boyle D, Skyrnarz-Murphy K, Gonzalves SM. The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomyomata. Obstet Gynecol. 2002;99:290–300. doi: 10.1016/s0029-7844(01)01702-1. [DOI] [PubMed] [Google Scholar]
- 6.Tamai K, Togashi K, Ito T, Morisawa N, Fujiwara T, Koyama T. MR imaging findings of adenomyosis: Correlation with histopathologic features and diagnostic pitfalls. Radiographics. 2005;25:21–40. doi: 10.1148/rg.251045060. [DOI] [PubMed] [Google Scholar]
- 7.Ascher SM, Jha RC, Reinhold C. Benign myometrial conditions: Leiomyomas and adenomyosis. Top Magn Reson Imaging. 2003;14:281–304. doi: 10.1097/00002142-200308000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Bragheto AM, Caserta N, Bahamondes L, Petta CA. Effectiveness of the levonorgestrel-releasing intrauterine system in the treatment of adenomyosis diagnosed and monitored by magnetic resonance imaging. Contraception. 2007;76:195–9. doi: 10.1016/j.contraception.2007.05.091. [DOI] [PubMed] [Google Scholar]
- 9.Freundl G, Gödtke K, Gnoth C, Godehardt E, Kienle E. Steroidal ‘add-back’ therapy in patients treated with GnRH agonists. Gynecol Obstet Invest. 1998;45(Suppl 1):22–30. doi: 10.1159/000052848. discussion 35. [DOI] [PubMed] [Google Scholar]
- 10.Imaoka I, Ascher SM, Sugimura K, Takahashi K, Li H, Cuomo F, et al. MR imaging of diffuse adenomyosis changes after GnRH analog therapy. J Magn Reson Imaging. 2002;15:285–90. doi: 10.1002/jmri.10060. [DOI] [PubMed] [Google Scholar]
- 11.Igarashi M. A new therapy for pelvic endometriosis and uterine adenomyosis: Local effect of vaginal and intrauterine danazol application. Asia Oceania J Obstet Gynaecol. 1990;16:1–12. doi: 10.1111/j.1447-0756.1990.tb00207.x. [DOI] [PubMed] [Google Scholar]
- 12.McCausland AM, McCausland VM. Depth of endometrial penetration in adenomyosis helps determine outcome of rollerball ablation. Am J Obstet Gynecol. 1996;174:1786–93. doi: 10.1016/s0002-9378(96)70211-9. [DOI] [PubMed] [Google Scholar]
- 13.Kanaoka Y, Hirai K, Ishiko O. Successful microwave endometrial ablation in a uterus enlarged by adenomyosis. Osaka City Med J. 2004;50:47–51. [PubMed] [Google Scholar]
- 14.Smith SJ, Sewall LE, Handelsman A. A clinical failure of uterine fibroid embolization due to adenomyosis. J Vasc Interv Radiol. 1999;10:1171–4. doi: 10.1016/s1051-0443(99)70216-2. [DOI] [PubMed] [Google Scholar]
- 15.Pelage JP, Jacob D, Fazel A, Namur J, Laurent A, Rymer R, et al. Midterm results of uterine artery embolization for symptomatic adenomyosis: Initial experience. Radiology. 2005;234:948–53. doi: 10.1148/radiol.2343031697. [DOI] [PubMed] [Google Scholar]
- 16.Lohle PN, De Vries J, Klazen CA, Boekkooi PF, Vervest HA, Smeets AJ, et al. Uterine artery embolization for symptomatic adenomyosis with or without uterine leiomyomas with the use of calibrated tris-acryl gelatin microspheres: Midterm clinical and MR imaging follow-up. J Vasc Interv Radiol. 2007;18:835–41. doi: 10.1016/j.jvir.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 17.Levgur M. Therapeutic options for adenomyosis : A review. Arch Gynecol Obstet. 2007;276:1–15. doi: 10.1007/s00404-006-0299-8. [DOI] [PubMed] [Google Scholar]
- 18.Hindley J, Gedroyc WM, Regan L, Stewart E, Tempany C, Hynyen K, et al. MRI guidance of focused ultrasound therapy of uterine fibroids : e0 arly results. AJR Am J Roentgenol. 2004;183:1713–9. doi: 10.2214/ajr.183.6.01831713. [DOI] [PubMed] [Google Scholar]
- 19.Morita Y, Ito N, Hikida H, Takeuchi S, Nakamura K, Ohashi H. Non-invasive magnetic resonance imaging-guided focused ultrasound treatment for uterine fibroids - early experience. Eur J Obstet Gynecol Reprod Biol. 2008;139:199–203. doi: 10.1016/j.ejogrb.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 20.Rabinovici J, Inbar Y, Eylon SC, Schiff E, Hananel A, Freundlich D. Pregnancy and live birth after focused ultrasound surgery for symptomatic focal adenomyosis : A case report. Hum Reprod. 2006;21:1255–9. doi: 10.1093/humrep/dei458. [DOI] [PubMed] [Google Scholar]
- 21.Fennessy FM, Tempany CM. A review of magnetic resonance imaging-guided focused ultrasound surgery of uterine fibroids. Top Magn Reson Imaging. 2006;17:173–9. doi: 10.1097/RMR.0b013e3180337e1f. [DOI] [PubMed] [Google Scholar]
- 22.Funaki K, Fukunishi H, Funaki T, Sawada K, Kaji Y, Maruo T. Magnetic resonance-guided focused ultrasound surgery for uterine fibroids: Relationship between the therapeutic effects and signal intensity of preexisting T2-weighted magnetic resonance images. Am J Obstet Gynecol. 2007;196:184.e1–6. doi: 10.1016/j.ajog.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 23.Kim KA, Yoon SW, Lee C, Seong SJ, Yoon BS, Park H. Short-term results of magnetic resonance imaging-guided focused ultrasound surgery for patients with adenomyosis: Symptomatic relief and pain reduction. Fertil Steril. 2011;95:1152–5. doi: 10.1016/j.fertnstert.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 24.Gavrilova-Jordan LP, Rose CH, Traynor KD, Brost BC, Gostout BS. Successful term pregnancy following MR-guided focused ultrasound treatment of uterine leiomyoma. J Perinatol. 2007;27:59–61. doi: 10.1038/sj.jp.7211624. [DOI] [PubMed] [Google Scholar]
- 25.Hanstede MM, Tempany CM, Stewart EA. Focused ultrasound surgery of intramural leiomyomas may facilitate fertility : A case report. Fertil Steril. 2007;88:497.e5–7. doi: 10.1016/j.fertnstert.2006.11.103. [DOI] [PubMed] [Google Scholar]
- 26.Morita Y, Ito N, Ohashi H. Pregnancy following MR-guided focused ultrasound surgery for a uterine fibroid. Int J Gynaecol Obstet. 2007;99:56–7. doi: 10.1016/j.ijgo.2007.03.053. [DOI] [PubMed] [Google Scholar]