Abstract
Background:
Effect on hemodynamic changes and experience of robot-assisted laparoscopic radical prostatectomy (RALRP) in steep Trendelenburg position (45°) with high-pressure CO2 pneumoperitoneum is very limited. Therefore, we planned this prospective clinical trial to study the effect of steep Tredelenburg position with high-pressure CO2 pneumoperitoneum on hemodynamic parameters in a patient undergoing RALRP using FloTrac/Vigileo™1.10.
Methods:
After ethical approval and informed consent, 15 patients scheduled for RALRP were included in the study. In the operation room, after attaching standard monitors, the radial artery was cannulated. Anesthesia was induced with fentanyl (2 μg/kg) and thiopentone (4–7 mg/kg), and tracheal intubation was facilitated by vecuronium bromide (0.1 mg/kg). The patient's right internal jugular vein was cannulated and the Pre Sep™ central venous oximetry catheter was connected to it. Anesthesia was maintained with isoflurane in oxygen and nitrous oxide and intermittent boluses of vecuronium. Intermittent positive-pressure ventilation was provided to maintain normocapnea. After CO2 pneumoperitoneum, position of the patient was gradually changed to 45° Trendelenburg over 5 min. The robot was then docked and the robot-assisted surgery started. Intraoperative monitoring included central venous pressure (CVP), stroke volume (SV), stroke volume variation (SVV), cardiac output (CO), cardiac index (CI) and central venous oxygen saturation (ScvO2).
Results:
After induction of anesthesia, heart rate (HR), SV, CO and CI were decreased significantly from the baseline value (P>0.05). SV, CO and CI further decreased significantly after creating pneumoperitoneum (P>0.05). At the 45° Trendelenburg position, HR, SV, CO and CI were significantly decreased compared with baseline. Thereafter, CO and CI were persistently low throughout the 45° Trendelenburg position (P=0.001). HR at 20 min and 1 h, SV and mean arterial blood pressure after 2 h decreased significantly from the baseline value (P>0.05) during the 45° Trendelenburg position. CVP increased significantly after creating pneumoperitoneum and at the 45° Trendelenburg position (after 5 and 20 min) compared with the baseline postinduction value (P>0.05). All these parameters returned to baseline after deflation of CO2 pneumoperitoneum in the supine position. There were no significant changes in SVV and ScvO2 throughout the study period.
Conclusions:
The steep Trendelenburg position and CO2 pneumoperitoneum, during RALRP, leads to significant decrease in stroke volume and cardiac output.
Keywords: Flotrac/vigileo™, hemodynamic changes, pneumoperitoneum, steep trendelenburg position
INTRODUCTION
Carcinoma prostate is one of the most common malignancies in men. Technical advances in the surgical field have led to an evolution in radical prostatectomy from open to minimally invasive methods. Although laparoscopic surgery is well tolerated by most of the patients, cardiovascular changes may have adverse consequences in those patients with limited cardiac reserve.[1] Recently, introduction of an advanced technology, da Vinci Robotic system (Intuitive Surgical, Sunnyvale, CA, USA), circumvented all the disadvantages of conventional laparoscopy. The first reported robot-assisted laparoscopic prostatectomy (RALRP) using the da Vinci system was described by Abbou et al. in 2001.[2]
Numerous studies have documented the impact of carbon dioxide (CO2) pneumoperitoneum on hemodynamic variables. In most of the studies, a rise in mean blood pressure and either a decreased or no changes in heart rate (HR) and cardiac output (CO) have been reported.[3–14] These changes are proportional to changes in intraabdominal pressure (IAP) attained during pneumoperitoneum, and are observed more when the patient is in the 15° Trendelenburg position.[15] RALRP requires much steeper Trendelenburg position (40–45°) and higher pressure (16–18 mmHg) of CO2 pneumoperitoneum to allow optimal visualization of the surgical field. Also, retroperitoneal dissection increases the CO2 absorption. This may lead to even more significant hemodynamic and respiratory consequences, creating major problems for the patient and challenges to the anesthesiologist. The pulmonary artery thermodilution technique has been used in most of the previous studies in laparoscopy for measuring hemodynamic changes. Although this invasive method is the gold standard, it is associated with known complications such as cardiac arrhythmias, and requires high technical skills for its insertion. Recently, FloTrac/Vigileo™ 1.10, a semiinvasive pulse contour analysis device, is being used to measure the changes in hemodynamic parameters as it is less invasive, easy to operate and require less technical skills. Also, the Flotrec can be used with an already existing arterial line often indicated in RALRP. Moreover, previous studies have also shown the FloTrac/Vigileo™ to be comparable to the thermodilution technique to measure hemodynamics.[16–18]
Effect on hemodynamic changes and experience of RALRP in steep Trendelenburg position (45°) with high-pressure CO2 pneumoperitoneum is very limited, and only few case reports have been published till now. Therefore, we planned this prospective clinical trial to study the effect of steep Tredelenburg position with high-pressure CO2 pneumoperitoneum on hemodynamic parameters in a patient undergoing RALRP using FloTrac/Vigileo™ 1.10 (Edwards Lifesciences LLC, Irvine, CA, USA).
METHODS
After institutional ethical committee approval and informed written consent, 15 ASA physical status I–II male patients scheduled for RALRP were included in the study. Patients with significant coronary artery disease, valvular heart disease, severe chronic obstructive pulmonary disease or other respiratory diseases and morbid obesity (body mass index >35 kg/m2) were excluded from the study. All patients were operated by the same surgical team.
In the operation room, after attaching standard monitors [electrocardiogram (ECG), noninvasive blood pressure (NIBP), pulse oximeter (SpO2)], a peripheral venous access was secured. Under local anesthesia, the patient's radial artery was cannulated with a 20 G arterial catheter. Anesthesia was induced intravenously with fentanyl (2 μg/kg) and thiopentone (4–7 mg/kg) and tracheal intubation was facilitated by vecuronium bromide (0.1 mg/kg). The patient's right internal jugular vein was cannulated under full aseptic precaution and a Pre Sep™ central venous oximetry catheter (Edwards Lifesciences™ LLC) was connected to it. The sensor/transducer (FloTrac) was connected to the radial artery catheter to measure the waveform. It was then attached to the processing/display unit (Vigileo), which applies a proprietary algorithm to the digitalized wave, and reports patient's CO, cardiac index (CI), stroke volume (SV) and stroke volume variation (SVV). The Pre-sep central venous oximetry catheter (Edwards Lifesciences™) was also connected to a Vigileo monitor to get central venous oxygen saturation (ScvO2) and to a Datex Ohmeda monitor (Datex Ohmeda S5 Avance GE Healthcare, GE Healthcare Finland Oy, FIN-00031 GE, Finland.) to measure the central venous pressure (CVP) of the patient.
Anesthesia was maintained with isoflurane (minimum alveolar concentration 1–1.2) in oxygen and nitrous oxide (50:50) and intermittent boluses of vecuronium. Intermittent positive-pressure ventilation was provided with an initial tidal volume of 8–10 mL/kg and respiratory rate of 10/min. Ventilatory parameters were adjusted after creating CO2 pneumoperitoneum to maintain normocapnea (EtCO2 35–40 mmHg). Intraoperative analgesia was provided by administration of intravenous boluses of fentanyl (0.5–1 μg /kg) when HR or blood pressure raised by 20% of the baseline. To maintain body temperature, a warming blanket (Geratherm Patient Warming System OP, Gaschwenda, Germany) was used and balanced salt solution was administered through a Hotline fluid warmer (Smiths Medical ASD Inc., Rockland, MA, USA). Fasting fluid requirement of the patient was replaced as per standard guidelines, but the intraoperative maintenance fluid was restricted to 2 mL/kg/h till the completion of the urethro-vesical anastomosis. After that, intravenous fluid administration was done as per standard fluid management guidelines based on replacement of fluid deficit, third-space fluid loss and blood loss.
After induction of anesthesia, a nasogastric tube and Foley's catheter were also inserted. Initially, the patient was in a supine position. Cushioned stirrups were used to place the patient in the modified lithotomy position and both arms and hands were generously padded and placed at the patient's sides. Cross-shoulder braces were also used to prevent falling down of the patient from the operating table. All the monitors were rechecked after positioning of the patient. A total of six ports were made on the patient's abdomen in an inverted V-shaped configuration. The Veress needle was used to establish the CO2 pneumoperitoneum. After placing these ports, the position of the patient was gradually changed to the 45° Trendelenburg position over 5 min. The robot was then docked and the robot-assisted surgery was started.
At the end of the surgery, when the patient was made supine, approximately 1–2 L of balanced salt solution was administered to all the patients due to restriction of fluid during the dissection of prostate and repair of urethra. Patient's residual neuromuscular blockade was reversed with neostigmine (50 mg/kg) and glycopyrrolate (20 mg/kg) and the trachea was extubated after achieving adequate ventilatory parameters. All patients were then shifted to the post anesthesia care unit (PACU), where postoperative nausea vomiting and any other complications were noted. Postoperative analgesia was maintained by intravenous paracetamol (10–15 mg/kg) 8-hourly and boluses of fentanyl (0.5 mg/kg) if required.
Intraoperative monitoring included continuous ECG, HR, mean arterial blood pressure (MAP), SpO2, inspired oxygen fraction (FiO2), peak airway pressure (PIP), end tidal carbon dioxide (EtCO2), temperature, minimum alveolar concentration (MAC) and CVP. The variables noted intraoperatively include SV, SVV, CO, CI and ScvO2 from the Vigileo monitor. Readings were taken at the following intervals: preinduction (baseline value), after 5 min of induction of anesthesia, after 5 min of creating CO2 pneumoperitoneum, after 5 min of 45° Trendelenburg position with CO2 pneumoperitoneum, after 20 min of 45° Trendelenburg position with CO2 pneumoperitoneum and then hourly till the end of surgery and at the end of surgery in the supine position after deflation of CO2 pneumoperitoneum.
Statistical analysis
Because this was an observational prospective pilot study, we did not determine the sample size. Statistical analysis was performed using SPSS software version 15 (SPSS Inc., Chicago, IL, USA). All data were expressed as mean±standard deviation. Changes in hemodynamic variables with time were analyzed by analysis of variance for repeated measurements and post hoc was done by Bonnferoni test. The preinduction value was considered as baseline, to which subsequent readings were compared. For CVP and ScvO2 postinduction values were considered as baseline. A P>0.05 was considered as statistically significant.
RESULTS
On our study, of the 19 patients, only 15 patients were recruited for the study as four patients did not met the inclusion criteria. All the patients recruited were included for statistical analysis. The patient characteristics are described in Table 1. Among these 15 patients, three patients were diabetic, five were hypertensive and one had both. Only two patients required transfusion of two units of packed red blood cells each during the surgical procedure.
Table 1.
Demographic parameters

After induction of anesthesia, HR, SV, CO and CI were decreased significantly from the baseline value (P>0.05) [Figures 1–7]. SV, CO and CI further decreased significantly after creating pneumoperitoneum (P>0.05) [Figures 4 and 5]. At the 45° Trendelenburg position, HR, SV, CO and CI were significantly decreased compared with baseline. Thereafter, CO and CI were persistently low throughout the 45° Trendelenburg position (P=0.001). HR at 20 min and 1 h, SV and MAP after 2 h decreased significantly from the baseline value (P>0.05) during the 45° Trendelenburg position. CVP increased significantly after creating pneumoperitoneum and at the 45° Trendelenburg position (after 5 and 20 min) compared with the baseline postinduction value (P>0.05). All these parameters returned to baseline after deflation of CO2 pneumoperitoneum in the supine position. There were no significant changes in SVV and ScvO2 throughout the study period [Figures 3 and 7].
Figure 1.
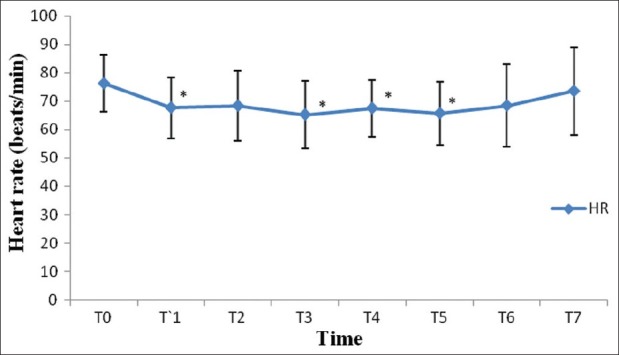
Changes in heart rate (beats/min) with time. T0: baseline (before induction); T1: postinduction; T2: after CO2 pneumoperitoneum; T3: after 5 min of 45° Trendelenburg position; T4: after 20 min of 45° Trendelenburg position; T5: after 1 h; T6: after 2 T7: after deflation of CO2 pneumoperitoneum in supine position. *Statistically significant compared with the baseline value (P>0.05)
Figure 7.
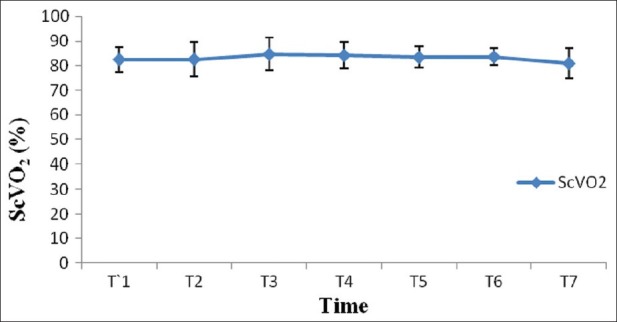
Changes in central venous oxygen saturation (ScvO2) with time. T1: postinduction (baseline); T2: after CO2 pneumoperitoneum; T3: after 5 min of 45° Trendelenburg position; T4: after 20 min of 45° Trendelenburg position; T5: after 1 h; T6: after 2 h; T7: after deflation of CO2 pneumoperitoneum in the supine position
Figure 4.
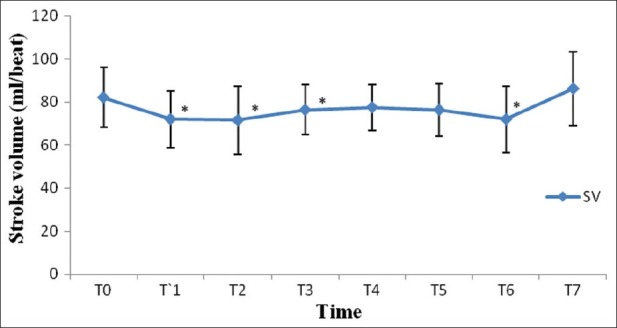
Changes in stroke volume (mL/beat) with time. T0: baseline (before induction); T1: postinduction; T2: after CO2 pneumoperitoneum; T3: after 5 min of 45° Trendelenburg position; T4: after 20 min of 45° Trendelenburg position; T5: after 1 h; T6: after 2 h; T7: after deflation of CO2 pneumoperitoneum in the supine position. *Statistically significant compared with the baseline value (P>0.05)
Figure 5.
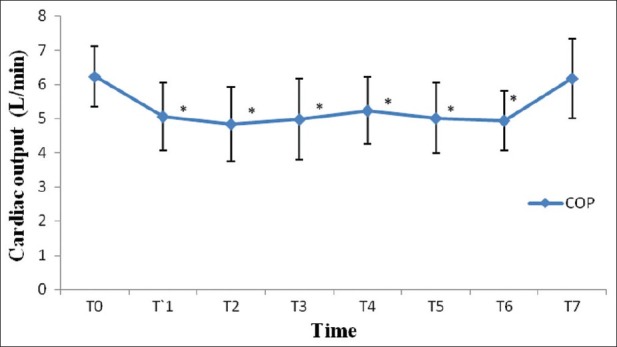
Changes in cardiac output (L/min) with time. T0: baseline (before induction); T1: postinduction; T2: after CO2 pneumoperitoneum; T3: after 5 min of 45° Trendelenburg position; T4: after 20 min of 45° Trendelenburg position; T5: after 1 h; T6: after 2 h; T7: after deflation of CO2 pneumoperitoneum in the supine position. *Statistically significant compared with the baseline value (P>0.05)
Figure 3.
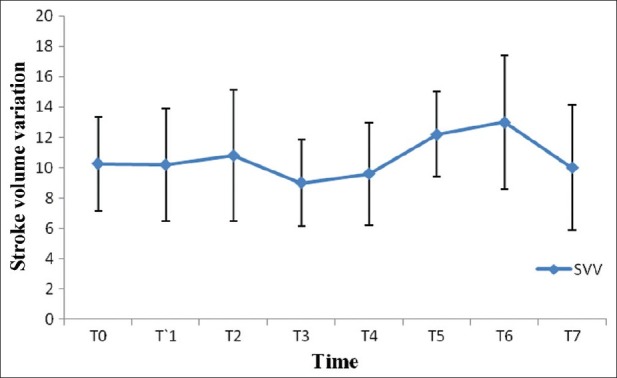
Changes in stroke volume variation with time. T0: baseline (before induction); T1: postinduction; T2: after CO2 pneumoperitoneum; T3: after 5 min of 45° Trendelenburg position; T4: after 20 min of 45° Trendelenburg position; T5: after 1 h; T6: after 2 h; T7: after deflation of CO2 pneumoperitoneum in the supine position
Figure 2.
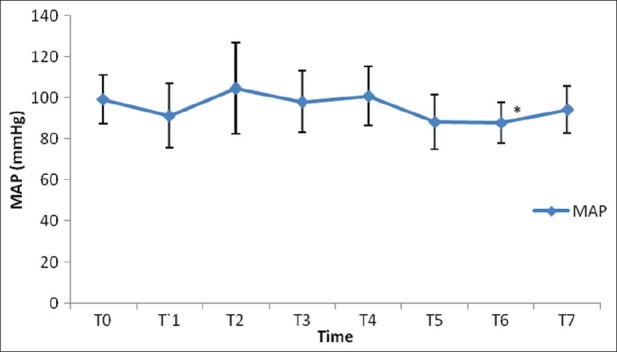
Changes in mean arterial blood pressure (mmHg) with time. T0: baseline (before induction); T1: postinduction; T2: after CO2 pneumoperitoneum; T3: after 5 min of 45° Trendelenburg position; T4: after 20 min of 45° Trendelenburg position; T5: after 1 h; T6: after 2 h; T7: after deflation of CO2 pneumoperitoneum in supine position. *Statistically significant compared with the baseline value (P>0.05)
Figure 6.
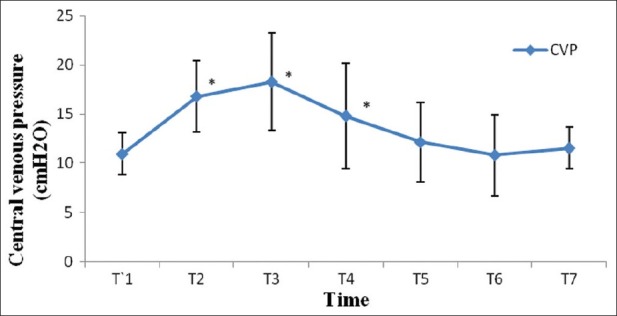
Changes in central venous pressure (cm H2O) with time. T1: postinduction (baseline); T2: after CO2 pneumoperitoneum; T3: after 5 min of 45° Trendelenburg position; T4: after 20 min of 45° Trendelenburg position; T5: after 1 h; T6: after 2 h; T7: after deflation of CO2 pneumoperitoneum in the supine position. *Statistically significant compared with the baseline value (P>0.05)
DISCUSSION
In our study, we found significant hemodynamic changes like decrease in HR after induction of general anesthesia at the 45° Trendelenburg position at 20 min and 1 h after positioning, but no significant change after initiation of CO2 pneumoperitoneum. There was significant decrease in MAP after 2 h of CO2 pneumoperitoneum. SV, CO and CI were also decreased significantly after induction, insufflations of CO2 pnemoperitoneum and immediately after the 45° Trendelenburg position. CO and CI remained significantly low throughout the surgery. Although these hemodynamic variables were decreased compared with baseline, they were within the physiological normal limits. All these parameters returned to baseline after deflation of pnemoperotoneum in the supine position.
In our study, general anesthesia was induced with fentanyl, thiopentone sodium and vecuronium bromide. The combination of fentanyl and vecuronium causes bradycardia, which explains the reduced HR after induction.[19] Our study included elderly patients who may have depressed b-receptor response secondary to decreased receptor affinity and alterations in signal transduction. They may also have depressed baroreceptor mechanism, which itself explain reduced HR in such groups of patients.[20] In our study, there was no change in HR during insufflations of CO2 pneumoperitoneum, as reported by earlier studies.[21–24]
In our study, we noticed a significant decrease in MAP after 2 h of CO2 pneumoperitoneum compared with baseline, even though it was within the normal range. This could be due to the combined effects of restrictive intravenous fluid administration in such operations to improve visibility during dissection of urethra and CO2 pneumoperitoneum in steep Trendelenburg position. Our observations are similar to that as reported by Meininger et al. in their study of hemodynamic changes during RALRP.[21] However, studies by Falabella et al. and Meininger et al. showed a significant increase in MAP after insufflation of CO2 pneumoperitoneum.[22,23] This difference is probably due to the different methodology used in our study when compared with these two previous studies. Both these studies compared MAP with postinduction MAP values; they did not measure baseline MAP before induction. However, our study is unique in the sense that preinduction MAP was considered as the baseline value to compare the subsequent readings.
We observed a significant decrease in SV, CO and CI after induction, after pneumoperitoneum and immediately after the 45° Tredelenburg position. Some fall in hemodynamic parameters is expected after induction with thiopentone and isoflurane–fentanyl anesthesia. This fall in hemodynamics might be further compounded with CO2 pneumoperitoneum, as it is known to cause increase in systemic vascular resistance and afterload, thereby reducing the stroke volume.[25] However, the studies by Falabella and Meininger et al. showed results that are in contrast to our study.[22,23] This difference may be due to the following reasons. First, both these studies did not measure hemodynamic variables before induction; therefore, the impact of anesthesia on these variables was unknown. In our study, we measured baseline hemodynamic variables before induction to which subsequent hemodynamic variables were compared. Second, our study is also different from their studies as they excluded patients with cardiopulmonary disease, including hypertension. However, we have included patients with hypertension and controlled cardiac diseases. In our study, seven of 15 patients were hypertensive patients and one had coronary artery disease coronary artery disease, who were receiving beta adrenergic and or calcium channel blocker drugs. This also may have contributed to an impaired inotropic response. Third, the IAP after CO2 insufflations were different. In the first study, it was 15 mmHg and in the second study, it was 12 mmHg. But, in our study, the IAP was 14–16 mmHg. This higher IAP might be one of the reasons for a decreased CI in our study.
Hirvonen et al. reported a decrease in SV and CO after induction and after CO2 pneumoperitoneum with Trendelenburg position during laparoscopic hysterectomy in 20 patients, which is similar to our findings.[26] They also demonstrated an increase in CVP after CO2 pneumoperitoneum and 45° Trendelenburg position, similar to our study. There are multiple reasons for this rise in CVP. The CO2 pneumoperitoneum may have caused an autotransfusion of pooled venous blood from the splanchnic region to the central compartment and also transmission of increased IAP to the thorax.[27] In our study, toward the end of laparoscopy, the CVP returned to baseline. Perhaps some shifts of blood from total blood volume take place during prolonged CO2 pneumoperitoneum. Such shifts may occur by transcapillary fluid filtration into the interstitial space in dependent areas of the body.[26] This mechanism also explains the upper body edema in many of these patients after surgery despite a controlled intravenous fluid regimen. Therefore, CVP may neither be a reliable indicator as reflecting the right atrial pressure nor guiding intravascular volume status in RALRP surgery.
We found no significant change in SVV in any part of our study period. In view of this, we are of the opinion that SVV may be of use in guiding the intravascular volume status in RALRP surgery with steep Trendelenburg, where CVP may not be reliable. Previous studies by Cannesson et al. and Kobayashi et al. demonstrated that Flotrac (version) 1.10 SVV provided reliable data in determining fluid responsiveness with acceptable sensitivity and specificity.[28,29] However, as per our knowledge and the literature available, no previous study included SVV changes during RALRP. Because there was no change in SVV with CO2 pneumoperitoneum and at the 45° Trendelenburg position in contrast to CVP, we suggest that SVV may be a useful predictor of intravascular volume status in patients undergoing RALRP.
In conclusion, steep Trendelenburg position and CO2 pneumoperitoneum during RALRP leads to a significant decrease in SV and CO.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Cunningham AJ, Brull SJ. Laparoscopic cholecystectomy: Anaesthetic implications. Anesth Analg. 1993;76:1120–33. doi: 10.1213/00000539-199305000-00035. [DOI] [PubMed] [Google Scholar]
- 2.Abbou CC, Hoznek A, Salomon L, Olsson LE, Lobontiu A, Saint F, et al. Laparoscopic radical prostatectomy with a remote controlled robot. J Urol. 2001;165:1964–6. doi: 10.1097/00005392-200106000-00027. [DOI] [PubMed] [Google Scholar]
- 3.Koliopanos A, Zografos G, Skiathitis S, Stithos D, Voukena V, Karampinis A, et al. Esophageal Doppler (ODM II) improves intraoperative haemodynamic monitoring during laparoscopic surgery. Surg Laparosc Endosc Percutan Tech. 2005;15:332–8. doi: 10.1097/01.sle.0000191631.66505.4a. [DOI] [PubMed] [Google Scholar]
- 4.Myre K, Buanes T, Smith G, Stokland O. Simultaneous haemodynamic and echocardiographic changes during abdominal gas insufflation. Surg Laparosc Endosc. 1997;7:415–9. [PubMed] [Google Scholar]
- 5.O’Leary E, Hubbard K, Tormey W, Cunningham AJ. Laparoscopic cholecystectomy: Haemodynamic and neuroendocrine responses after CO2 pneumoperitoneum and changes in position. Br J Anaesth. 1996;76:640–4. doi: 10.1093/bja/76.5.640. [DOI] [PubMed] [Google Scholar]
- 6.Harris SN, Ballantyne GH, Luther MA, Perrino AC., Jr Alterations of cardiovascular performance during laparoscopic colectomy: A combined haemodynamic and echocardiographic analysis. Anesth Analg. 1996;83:482–7. doi: 10.1097/00000539-199609000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Haxby EJ, Gray MR, Rodriguez C, Nott D, Springall M, Mythen M. Assessment of cardiovascular changes during laparoscopic hernia repair using oesophageal Doppler. Br J Anaesth. 1997;78:515–9. doi: 10.1093/bja/78.5.515. [DOI] [PubMed] [Google Scholar]
- 8.Casati A, Comotti L, Tommasino C, Leggieri C, Bignami E, Tarantino F, et al. Effects of pneumoperitoneum and reverse Trendelenburg position on cardiopulmonary function in morbidly obese patients receiving laparoscopic gastric banding. Eur J Anaesthesiol. 2000;17:300–5. doi: 10.1046/j.1365-2346.2000.00662.x. [DOI] [PubMed] [Google Scholar]
- 9.Alfonsi P, Vieillard-Baron A, Coggia M, Guignard B, Goeau-Brissonniere O, Jardin F, et al. Cardiac function during intraperitoneal CO2 insufflation for aortic surgery: A transesophageal echocardiographic study. Anesth Analg. 2006;102:1304–10. doi: 10.1213/01.ane.0000202473.17453.79. [DOI] [PubMed] [Google Scholar]
- 10.Larsen JF, Svendsen FM, Pedersen V. Randomized clinical trial of the effect of pneumoperitoneum on cardiac function and haemodynamics during laparoscopic cholecystectomy. Br J Surg. 2004;91:848–54. doi: 10.1002/bjs.4573. [DOI] [PubMed] [Google Scholar]
- 11.Girardis M, Broi UD, Antonutto G, Pasetto A. The effect of laparoscopic cholecystectomy on cardiovascular function and pulmonary gas exchange. Anesth Analg. 1996;83:134–40. doi: 10.1097/00000539-199607000-00024. [DOI] [PubMed] [Google Scholar]
- 12.Greim CA, Broscheit J, Kortländer J, Roewer N, Schulte am Esch J. Effects of intra-abdominal CO2-insufflation on normal and impaired myocardial function: An experimental study. Acta Anaesthesiol Scand. 2003;47:751–60. doi: 10.1034/j.1399-6576.2003.00135.x. [DOI] [PubMed] [Google Scholar]
- 13.Andersson L, Lindberg G, Bringman S, Ramel S, Anderberg B, Odeberg-Wernerman S. Pneumoperitoneum versus abdominal wall lift: Effects on central haemodynamics and intrathoracic pressure during laparoscopic cholecystectomy. Acta Anaesthesiol Scand. 2003;47:838–46. doi: 10.1034/j.1399-6576.2003.00117.x. [DOI] [PubMed] [Google Scholar]
- 14.Hachenberg T, Ebel C, Czorny M, Thomas H, Wendt M. Intrathoracic and pulmonary blood volume during CO2 pneumoperitoneum in humans. Acta Anaesthesiol Scand. 1998;42:794–8. doi: 10.1111/j.1399-6576.1998.tb05324.x. [DOI] [PubMed] [Google Scholar]
- 15.Zollinger A, Krayer S, Singer T, Seifert B, Heinzelmann M, Schlumpf R, et al. Haemodynamic effects of CO2 pneumoperitoneum in elderly patients with an increased cardiac risk. Eur J Anaesthesiol. 1997;14:266–75. doi: 10.1046/j.1365-2346.1997.00078.x. [DOI] [PubMed] [Google Scholar]
- 16.Monnet X, Anguel A, Naudin B, Jabot J, Richard C, Teboul JL. Arterial Pressure-based cardiac output in septic patients: different accuracy of pulse contour and uncalibrated pressure waveform devices. Crit Care. 2012;14:R109. doi: 10.1186/cc9058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayer J, Boldt J, Wolf MW, Lang J, Suttner S. Cardiac output derived from arterial pressure waveform analysis in patients undergoing cardiac surgery: Validity of a second generation device. Anesth Analg. 2008;106:867–72. doi: 10.1213/ane.0b013e318161964d. [DOI] [PubMed] [Google Scholar]
- 18.Button D, Weibel L, Reuthebuch O, Genomi M, Zollinger A, Hofer CK. Clinical evaluation of the Flotrac/Vigileo system and two established continuous cardiac output monitoring devices in patients undergoing cardiac surgery. Br J Anaesthesia. 2007;99:329–36. doi: 10.1093/bja/aem188. [DOI] [PubMed] [Google Scholar]
- 19.Inoue K, Banayosi A, Stolarski L, Reichelt W. Vecuronium induced bradycardia following induction of anaesthesia with etomidate or thiopentone, with or without fentanyl. Br J Anaesth. 1988;1:10–7. doi: 10.1093/bja/60.1.10. [DOI] [PubMed] [Google Scholar]
- 20.Rook GA. Autonomic and cardiovascular function in the geriatric patient. Anesthesiol Clin North America. 2000;18:31–4. doi: 10.1016/s0889-8537(05)70147-4. [DOI] [PubMed] [Google Scholar]
- 21.Meininger D, Byhahn C, Bueck M, Binder J, Kramer W, Kessler P, et al. Effects of prolonged pneumoperitoneum on haemodynamics and acid-base balance during totally endoscopic robot-assisted radical prostatectomies. World J Surg. 2002;26:1423–7. doi: 10.1007/s00268-002-6404-7. [DOI] [PubMed] [Google Scholar]
- 22.Falabella A, Moore-Jeffries E, Sullivan MJ, Nelson R, Lew M. Cardiac function during steep Trendelenburg position and CO 2 pneumoperitoneum for robotic-assisted prostatectomy: A trans-oesophageal Doppler probe study. Int J Med Robot. 2007;3:312–5. doi: 10.1002/rcs.165. [DOI] [PubMed] [Google Scholar]
- 23.Meininger D, Westphal K, Bremerich DH, Runkel H, Probst M, Zwissler B, et al. Effects of posture and prolonged pneumoperitoneum on haemodynamic parameters during laparoscopy. World J Surg. 2008;32:1400–5. doi: 10.1007/s00268-007-9424-5. [DOI] [PubMed] [Google Scholar]
- 24.Park EY, Koo BN, Min KT, Nam SH. The effect of pneumoperitoneum in the steep Trendelenburg position on cerebral oxygenation. Acta Anaesthesiol Scand. 2009;53:895–9. doi: 10.1111/j.1399-6576.2009.01991.x. [DOI] [PubMed] [Google Scholar]
- 25.Hirvonen EA, Nuutinen LS, Kauko M. Haemodynamic changes due to Trendelenburg positioning and pneumoperitoneum during laparoscopic hysterectomy. Acta Anaesthesiol Scand. 1995;39:949–55. doi: 10.1111/j.1399-6576.1995.tb04203.x. [DOI] [PubMed] [Google Scholar]
- 26.Odeberg S, Ljungqvist O, Svenberg T, Gannedahl P, Bäckdahl M, von Rosen A, et al. Haemodynamic effects of pneumoperitoneum and the influence of posture during anaesthesia for laparoscopic surgery. Acta Anaesthesiol Scand. 1994;38:276–83. doi: 10.1111/j.1399-6576.1994.tb03889.x. [DOI] [PubMed] [Google Scholar]
- 27.Asopa A, Karthik S, Subramaniam B. Current status of dynamic parameters of fluid loading. Int Anesthesiol Clin. 2010;48:23–36. doi: 10.1097/AIA.0b013e3181bff887. [DOI] [PubMed] [Google Scholar]
- 28.Cannesson M, Musrd H, Desebbe O, Boucau C, Simon R, Hénaine R, et al. The ability of stroke volume variations obtained with Vigileo/Flo Trac system to monitor fluid responmsiveness in mechanically ventilated patients. Anesth Analg. 2009;108:513–7. doi: 10.1213/ane.0b013e318192a36b. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi M, Ko M, Kimura T, Meguro E, Hayakawa Y, Irinoda T, et al. Perioperative monitoring of fluid responsiveness after esophageal surgery using stroke volume variation. Expert Rev Med Devices. 2008;5:311–6. doi: 10.1586/17434440.5.3.311. [DOI] [PubMed] [Google Scholar]


