Abstract
Background:
Intrathecal magnesium has been found to prolong the duration of analgesia in various surgical procedures like lower limb surgeries and as adjuncts to general anesthesia for pain management. The present study was designed to examine whether addition of intrathecal magnesium sulfate would enhance the analgesic efficacy of intrathecal bupivacaine and fentanyl in patients undergoing total abdominal hysterectomy.
Methods:
After taking informed consent, 60 patients were randomised into two groups with 30 patients. Group “S” received 2.5 mL (12.5 mg) of hyperbaric bupivacaine + 0.5 mL (25 mcg) of fentanyl + 0.5 mL of normal saline and Group “M” received 2.5 mL (12.5 mg) of hyperbaric bupivacaine + 0.5 mL (25 mcg) of fentanyl + 0.5 mL (100 mg) of magnesium sulfate. Onset of sensory, motor block and duration of analgesia was noted.
Results:
Demographic profile and duration of surgery were comparable (P>0.5). Time of onset of sensory and motor blockade was delayed in Group M compared with Group S, and this was statistically significant. A statistically significant longer duration of analgesia was observed in Group M compared with the control Group S. However, the recovery of motor blockade was found to be statistically insignificant in both the groups. The hemodynamic parameters were comparable in the perioperative period (P>0.05). The incidence of side-effects in both the groups were also comparable (P>0.05).
Conclusion:
The addition of 100 mg intrathecal magnesium led to prolonged duration of analgesia significantly without increasing the incidence of side-effects. Also, there was a significant delay in the onset of both sensory and motor blockade.
Keywords: Abdominal hysterectomy, analgesia, magnesium sulfate, subarachnoid block
INTRODUCTION
Techniques involving the smaller doses of opioid in combination with nonopioid adjuvant drugs are becoming increasingly popular approaches for perioperative pain management. Noxious stimulation leads to the release of glutamate and aspartate neurotransmitters, which bind to various subclasses of excitatory amino acid receptors, including the N-methyl D-aspartate (NMDA) receptor. Activation of NMDA receptors leads to calcium and sodium influx into the cell, with an efflux of potassium and initiation of central sensitization and wind-up.[1,2] NMDA receptor signalling may be important in determining the duration and intensity of postoperative pain.[1] Magnesium blocks NMDA channels in a voltage-dependent way, and the addition of magnesium produces a reduction of NMDA-induced currents.[3] Magnesium sulfate has been used systemically, and has shown antinociceptive effects, but results are not consistent.[4–6] A limitation to the parenteral application of magnesium for modulation of antinociception via NMDA channel antagonism is insufficient blood–brain barrier penetration to achieve effective cerebrospinal fluid concentrations.[4,7] Intrathecal magnesium has been found to prolong the duration of analgesia in various surgical procedures like lower limb surgeries and as adjuncts to general anesthesia for pain management.[8–17] However, the role of intrathecal magnesium has not been studied in patients undergoing total abdominal hysterectomy.
We hypothesize that intrathecal magnesium could potentiate opioid spinal analgesia and avoid the potential side-effect of the larger doses of intravenous magnesium that may be required to observe antinociception modulation in humans. Therefore, the present study was designed to examine whether addition of intrathecal magnesium sulfate would enhance the analgesic efficacy of intrathecal bupivacaine and fentanyl in patients undergoing total abdominal hysterectomy.
METHODS
This prospective randomized study was carried after approval by the institutional ethical committee. After taking written informed consent, 60 patients of ASA physical status I and II, aged between 18 and 60 years, scheduled for total abdominal hysterectomy, were selected. Patients who had a past history of reaction to study drugs, on analgesic therapy and calcium channel blockers, major hepatic, renal or cardiovascular dysfunction and any contraindication to central neuraxial blockade were excluded from the study. Patients were explained in detail about the procedure of the study during the preanesthetic visit. Patients were familiarized with the visual analogue scale (VAS) (0 - no pain, 10 - worst pain) 1 day before surgery. They were advised overnight fasting and were premedicated with oral pantoprazole 40 mg in the morning of the surgery.
Patients were randomised by a computer-generated random number table into two groups of 30 patients each:
Group S: Intrathecal administration of 2.5 mL (12.5 mg) of hyperbaric bupivacaine + 0.5 mL (25 mcg) of fentanyl + 0.5 mL of normal saline.
Group M: Intrathecal administration of 2.5 mL (12.5 mg) of hyperbaric bupivacaine + 0.5 mL (25 mcg) of fentanyl + 0.5 mL (100 mg) of 20% magnesium sulfate.
In the operation room, after attaching routine monitors (electrocardiogram, noninvasive blood pressure, pulse oximeter), intravenous access was secured. All patients were preloaded with 15 mL/kg of Ringer's lactate solution. The subarachnoid block was administered in the left lateral position at the L3-L4 interspace with a 25 G Whitacre tip spinal needle and 3.5 mL of the drug solution was injected intrathecally over a 30-s period as per the group allocation. Patients were then placed in the supine position. Oxygenation was given via a Hudson mask at the rate of 4 L/min. All local anesthetic solutions and adjuvant drugs were prepared by an anesthesiologist not involved in the performance of spinal anesthesia, patient care or data collection.
Sensory block was assessed by a pinprick test. The onset of sensory blockade (defined as the time from the injection of intrathecal drugs to the absence of pain at the T8 dermatome) was recorded every minute till the T8 level was achieved. Onset of motor blockade was assessed at 5-min intervals till 15 min (i.e., B5, B10 and B15) according to the modified Bromage scale[18] [0 - no motor block, 1 - inability to flex the hip [hip blocked], 2 - inability to flex the knee [hip and knee blocked], 3 - inability to flex the ankle [hip, knee and ankle blocked]). Grades of sedation during surgery were assessed by the Ramsay's sedation scale[19] (1 - anxious and agitated or restless, or both, 2 - cooperative, oriented, tranquil, 3 - responding to commands only, 4 - brisk response to light glabellar tap, 5 - sluggish response to light glabellar tap).
Blood pressure (systolic, diastolic and mean), heart rate, respiratory rate and peripheral oxygen saturation (SpO2) were recorded 5 min before the intrathecal injection (0) and at 5, 10, 15, 20, 25 and 30 min after the injection, and subsequently every 15 min. Hypotension (defined as systolic blood pressure of less than 90 mmHg or less than 20% of baseline blood pressure) was treated with intravenous fluid initially (250 mL boluses repeated twice) and intravenous mephentermine 6 mg, if required. Bradycardia (defined as heart rate of less than 50) was treated with intravenous 0.6 mg atropine sulfate. Postoperatively, in patients with a VAS score of >4, intramuscular diclofenac (75 mg) was administered as rescue analgesic and the duration of analgesia (time from the administration of intrathecal drugs and administration of first rescue analgesic) was noted. Patients were also assessed for side-effects like nausea, vomiting, hypotension and bradycardia.
Statistical analysis
Sample size analysis determined that n=30 per group was required to detect a 25 min difference in the duration of analgesia (primary outcome variable) between groups, with a power of 90% and a significance level of 5%. Statistical analysis was performed with SPSS for windows version 15.0. Statistical comparison was carried out using the Chi-square or Fisher's exact tests and Independent Student's t-test where appropriate. A value of P<0.05 was considered statistically significant. The results are expressed as mean (SD).
RESULTS
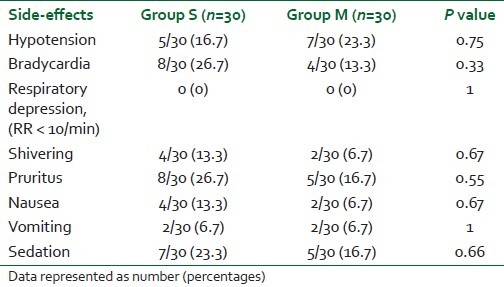
Sixty-eight patients were considered for the study, but 60 patients were randomized for the study as the other eight patients did not meet our inclusion criteria. All 60 patients recruited and randomized completed the study and were included in the analysis. There was no statistically significant (P>0.5) difference among the two groups in terms of demographic data and duration of surgery [Table 1]. Time of onset of sensory and motor blockade was delayed in Group M compared with Group S, and was statistically significant [Table 2]. Motor blockade (Bromage scale) in the two groups at 5 min was statistically insignificant and, however, the motor blockade at 10 min was found to be statistically significant, i.e. onset of motor complete motor block was delayed in Group M compared with Group S [Table 2]. A statistically significant longer duration of analgesia was observed in Group M compared with the control Group S (P value 0.001) [Table 2]. However, the recovery of motor blockade was found to be statistically insignificant in both the groups [Table 3]. The hemodynamic parameters (blood pressure, heart rate) were comparable in the perioperative period (P>0.05). The incidence of side-effects in both the groups was also comparable (P>0.05) [Table 3].
Table 1.
Demographic data of Groups S and M
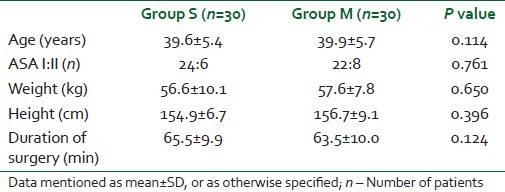
Table 2.
Study parameters in the Groups S and M
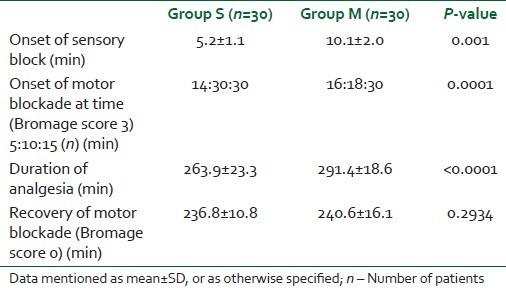
Table 3.
Side-effects in the Groups S and M
DISCUSSION
The results of our study showed a significant increase in duration of analgesia when magnesium was added to intrathecal bupivacaine and fentanyl in patients undergoing total abdominal hysterectomy. Also, addition of magnesium intrathecally significantly delays the onset of sensory and motor blockade.
The safety of intrathecal magnesium administration has been evaluated in animal and human studies.[5] The safety and utility of intrathecal magnesium in patients undergoing caesarean section is also described. At doses used in this study, intrathecally administered magnesium has been reported to prolong the duration of spinal opioid analgesia without increasing adverse events in parturients.[10] A recent human study found no deleterious effects of intrathecal magnesium on spinal opioid analgesia in labouring parturients.[10] Thus, intrathecal magnesium seems to have a good safety profile. This is comparable to our study, where there were no side-effects related to the drug used. Our study demonstrates no clinically significant difference in the hemodynamic parameters and adverse effects among the two groups. The incidence of hypotension was 16.66% in our study, which is less than that in other studies.[15,16]
Magnesium-induced augmentation of analgesia when supplemented to bupivacaine and fentanyl has been shown in other studies.[10–12] But, the effect of intrathecal magnesium for total abdominal hysterectomy has not been studied. Use of intrathecal magnesium reduces postoperative epidural analgesic requirement.[13] In studies in which 50 mg magnesium sulfate was administered intrathecally in patients undergoing total knee replacement, knee artroscopy and thoracic surgery observed that the VRS scores at 120 min, first analgesic requirement and 36-h morphine requirement were significantly lower after intrathecal injection of magnesium sulfate, respectively, as compared with the control group (P<0.05).[10,13] In patients undergoing caesarean section under spinal anesthesia, the addition of intrathecal magnesium sulfate (100 mg) to morphine 100 mg improved the quality and the duration of postoperative analgesia without increasing the incidence of adverse effects.[20] In parturients with mild preeclampsia undergoing caesarean delivery, the addition of magnesium sulfate 50 mg to the intrathecal combination of bupivacaine and fentanyl prolongs the duration of analgesia and reduces the postoperative analgesic requirements without additional side-effects.[17] Intrathecal magnesium has been used for labor analgesia in the dose of 50 mg in addition to fentanyl, and the authors observed that there was significant prolongation in the median duration of analgesia (75 min) in the magnesium plus fentanyl group compared with the fentanyl alone group (60 min).[8] Our results are in contrast to the study by Unlugenc and colleagues, who reported a decrease in the duration of analgesia with the addition of intrathecal magnesium.[16] This may be due to the lesser dose of intrathecal bupivacaine (10 mg), magnesium (50 mg) and different surgical procedure (caesarean section) as compared with our study, where we used 100 mg of magnesium for total abdominal hysterectomy.
In our study, we have found that the onset of sensory and motor blockade was delayed with the addition of intrathecal magnesium, which suggests that intrathecal magnesium causes delay in surgical anesthesia, which is similar to other studies.[9,16] The time to complete motor recovery was 240±16.149 min in our study, which is higher than other studies possibly due to the higher doses of bupivacaine and magnesium in our study.[9,15] The delayed onset of both sensory and motor blockade in patients receiving intrathecal magnesium sulfate along with bupivacaine, fentanyl combination has been explained.[9] The delayed onset could be due to the solution of magnesium sulfate having a different pH, which might explain our findings. However, they cannot offer a satisfactory explanation for this delay and further studies are needed. Also, increase in metabolism of bupivacaine due to the activation of cytochrome P450 (CYP) by magnesium may be responsible for the delayed onset.[21,22] But, it still could not explain the similar motor recovery.
The addition of intrathecal magnesium (50 mg) in patients undergoing knee arthroscopy prolonged the time for regression of two segments in the maximum block height and time to L2 regression, but did not affect the time to complete recovery of motor function and also did not affect maximum sensory level or the time to reach the highest level of sensory block.[15] In our study, we have used 100 mg of magnesium sulfate, but it did not affect the time to complete motor recovery.
The optimal dose of intrathecal magnesium has not been reported. Earlier studies with 50 and 100 mg gave satisfactory analgesia without significant added adverse effects. With 100 mg of intrathecal magnesium, we observed increased duration of analgesia as compared with previous studies without increasing any adverse effects.[16] The increased dose may carry the risk of respiratory depression, but none of our patients had any episodes of respiratory depression.[23] Use of intrathecal magnesium did not find any signs of systemic toxicity, such as arterial hypotension, cardiac arrhythmias, somnolence, double vision, slurred speech or weakness, either intraoperatively or during the postoperative course in patients treated with magnesium sulfate who underwent major orthopedic surgery.[11] We too did not find any of the above-mentioned complications during the intraoperative or in the postoperative periods.
CONCLUSION
To conclude, our results show that addition of 100 mg intrathecal magnesium led to prolonged duration of analgesia significantly without increasing the incidence of side-effects. Also, there was a significant delay in the onset of both sensory and motor blockade.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation: Implications for the treatment of post-injury pain and hypersensitivity states. Pain. 1991;44:293–9. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- 2.Woolf CJ, Chong MS. Preemptive analgesia: Treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg. 1993;77:362–79. doi: 10.1213/00000539-199377020-00026. [DOI] [PubMed] [Google Scholar]
- 3.Ascher P, Nowak L. Electrophysiological studies of NMDA receptors. Trends Neurosci. 1987;10:284–8. [Google Scholar]
- 4.Ko SH, Lim HR, Kim DC, Han YJ, Choe H, Song HS. Magnesium sulphate does not reduce postoperative analgesic requirements. Anesthesiology. 2001;95:640–6. doi: 10.1097/00000542-200109000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Mebazaa MS, Ouerghi S, Frikha N, Moncer N, Mestiri K, James MF, et al. Is magnesium sulfate by the intrathecal route efficient and safe? Ann Fr Anesth Reanim. 2011;30:47–50. doi: 10.1016/j.annfar.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Wilder-Smith CH, Knopfli R, Wilder-Smith OH. Perioperative magnesium infusion and postoperative pain. Acta Anaesthesiol Scand. 1997;41:1023–7. doi: 10.1111/j.1399-6576.1997.tb04830.x. [DOI] [PubMed] [Google Scholar]
- 7.Thuranu GR, Kemp DB, Jarvis A. Cerebrospinal fluid levels of magnesium in patients with preeclampsia after treatment with intravenous magnesium sulfate: A preliminary report. Am J Obstet Gynecol. 1987;157:1435–8. doi: 10.1016/s0002-9378(87)80239-9. [DOI] [PubMed] [Google Scholar]
- 8.Buvanendran A, McCarthy RJ, Kroin JS, Leong W, Perry P, Tuman KJ. Intrathecal magnesium prolongs fentanyl analgesia: A prospective, randomized, controlled trial. Anesth Analg. 2002;95:661–6. doi: 10.1097/00000539-200209000-00031. [DOI] [PubMed] [Google Scholar]
- 9.Ozalevli M, Cetin TO, Unlugence H, Guler T, Isik G. The effect of adding intrathecal magnesium sulphate to bupivacaine fentanyl spinal anaesthesia. Acta Anaesthesiol Scand. 2005;49:1514–9. doi: 10.1111/j.1399-6576.2005.00793.x. [DOI] [PubMed] [Google Scholar]
- 10.Lee JW, Kim MK, Shin YS, Koo BN. The analgesic effect of single dose of intrathecal magnesium sulfate. Korean J Anesthesiol. 2007;52:72–6. [Google Scholar]
- 11.Arcioni R, Palmisani S, Tigano S, Santorsola C, Sauli V, Romanò S, et al. Combined intrathecal and epidural magnesium sulfate supplementation of spinal anesthesia to reduce postoperative analgesic requirements. Acta Anaesthesiol Scand. 2007;51:482–9. doi: 10.1111/j.1399-6576.2007.01263.x. [DOI] [PubMed] [Google Scholar]
- 12.Bohannon TW, Estes MD. Evaluation of subarachnoid fentanyl for postoperative analgesia (Abstract) Anesthesiology. 1987;67:237. [Google Scholar]
- 13.Biswas BN, Rudra A, Bose BK, Nath S, Chakrabarty S, Bhattacharjee S. Intrathecal fentanyl with hyperbaric bupivacaine improves analgesia during caesarean delivery and in early postoperative period. Indian J Anaesth. 2002;66:469–72. [Google Scholar]
- 14.Singh V, Gupta LK, Singh GP. Comparison among intrathecal fentanyl and butorphanol in combination with bupivacaine for lower limb surgeries. J Anaesth Clin Pharmacol. 2006;22:371–5. [Google Scholar]
- 15.Dayioglu H, Baykara ZN, Salbes A, Solak M, Toker K. Effects of adding magnesium to bupivacaine and fentanyl for spinal anesthesia in knee arthroscopy. J Anesth. 2009;23:19–25. doi: 10.1007/s00540-008-0677-4. [DOI] [PubMed] [Google Scholar]
- 16.Unlugenc H, Ozalevli M, Gunduz M, Gunasti S, Urunsak IF, Guler T, et al. Comparison of intrathecal magnesium, fentanyl, or placebo combined with bupivacaine 0.5% for parturients undergoing elective cesarean delivery. Acta Anaesthesiol Scand. 2009;53:346–53. doi: 10.1111/j.1399-6576.2008.01864.x. [DOI] [PubMed] [Google Scholar]
- 17.Malleeswaran S, Panda N, Mathew P, Bagga R. A randomised study of magnesium sulphate as an adjuvant to intrathecal bupivacaine in patients with mild preeclampsia undergoing caesarean section. Int J Obstet Anesth. 2010;19:161–6. doi: 10.1016/j.ijoa.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Breen TW, Shapiro T, Glass B, Foster-Payne D, Oriol NE. Epidural anesthesia for labor in the ambulatory patient. Anesth Analg. 1993;77:919–24. doi: 10.1213/00000539-199311000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Schulte-Tamburen AM, Scheier J, Briegel J, Schwender D, Peter K. Comparison of five sedation scoring systems by means of auditory evoked potentials. Intensive Care Med. 1999;25:377–82. doi: 10.1007/s001340050861. [DOI] [PubMed] [Google Scholar]
- 20.Ghrab BE, Maatoug M, Kallel N, Khemakhem K, Chaari M, Kolsi K, et al. Does combination of intrathecal magnesium sulfate and morphine improve postcaesarean section analgesia? Ann Fr Anesth Reanim. 2009;28:454–9. doi: 10.1016/j.annfar.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Okutomi T, Saito M, Matsumoto Y, Shimizu M, Fukuoka M, Hoka S. Altered bupivacaine pharmacokinetics by MgSO4 in rats. Can J Anaesth. 2004;51:93–4. doi: 10.1007/BF03018565. [DOI] [PubMed] [Google Scholar]
- 22.Hung YC, Chen CY, Lirk P, Wang CF, Cheng JK, Chen CC, et al. Magnesium sulfate diminishes the effects of amide local anesthetics in rat sciatic-nerve block. Reg Anesth Pain Med. 2007;32:288–95. doi: 10.1016/j.rapm.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Witlin AG, Sibai BM. Magnesium sulfate therapy in preeclampsia and eclampsia. Obstet Gynecol. 1998;92:883–9. doi: 10.1016/s0029-7844(98)00277-4. [DOI] [PubMed] [Google Scholar]


