Abstract
Objective:
Magnesium has been used as an adjuvant by various routes, including intravenous, intrathecal, and epidural in different dosage regimens. The effect of single bolus dose of magnesium as an adjuvant to fentanyl for postoperative analgesia has not been studied. This prospective randomized controlled trial was done to evaluate the efficacy of single bolus administration of magnesium epidurally as an adjuvant to epidural fentanyl for postoperative analgesia in patients undergoing total hip replacement under combined spinal epidural anesthesia.
Methods:
Sixty patients received combined spinal–epidural anesthesia with 2 mL of 0.5% hyperbaric bupivacaine intrathecally. After the surgery, patients were randomized into Group F [epidural fentanyl (1 μg/kg) in 10 mL saline] and Group FM [epidural magnesium (75 mg) along with fentanyl (1 μg/kg) in 10 mL saline]. Supplementary analgesia was provided by 50 mg intravenous tramadol if Verbal Rating Score (VRS) >4. Patient's first analgesic requirement and duration of analgesia were recorded.
Results:
The duration of analgesia was significantly longer for Group FM, 340±28.8 min, compared with Group F, 164±17.1 min (P=0.001). The frequency of rescue analgesics required in 24-h postoperative period in Group FM (2.3±0.5) was significantly less than that in Group F (4.3±0.5) (P=0.001). VRS was significantly lower in Group FM up to 4 h in the postoperative period (P=0.001). Bromage scale was statistically insignificant at all points of time.
Conclusions:
The administration of magnesium (75 mg) as an adjuvant to epidural fentanyl (1 μg/ kg) for postoperative analgesia results in significantly lower VRS with prolonged duration of analgesia as compared with epidural fentanyl (1 μg/kg) alone. Concomitant administration of magnesium also reduces the requirement of breakthrough analgesics with no increased incidence of side effects.
Keywords: Epidural, magnesium, N-methyl-d-aspartate receptor, post operative pain, rescue analgesia
INTRODUCTION
Providing postoperative pain relief is a challenge for anesthesiologists. Postoperative epidural analgesia after combined spinal epidural (CSE) anesthesia is one of the accepted techniques.[1] Various adjuvants in addition to opioids have been used epidurally to prolong analgesia and reduce the incidence of adverse events observed when opioids are used alone.[1]
N-methyl-d-aspartate (NMDA) receptors present in dorsal horn of spinal cord have a role in the modulation of central sensitization of noxious stimulus.[2] Calcium influx is thought to be the primary mechanism by which NMDA receptors act.[2] Calcium channel blockers and NMDA receptor antagonist have shown to be beneficial in preventing initiation of pain. Magnesium, a divalent cation, through noncompetitive mechanism blocks the NMDA receptor in a voltage-dependent manner and results in natural calcium antagonism.[3] Various animal and human studies have shown that magnesium possesses antinociceptive action. Magnesium has been used as an adjuvant by various routes, including intravenous, intrathecal, and epidural in different dosage regimens.[4,5]
We hypothesized that a single bolus dose of epidural magnesium will prolong the duration of postoperative analgesia. Thus, we planned a prospective randomized, double-blind study to evaluate the efficacy of single bolus administration of magnesium (75 mg) epidurally as an adjuvant to epidural fentanyl (1 μg/kg) for postoperative analgesia in patients undergoing total hip replacement.
METHODS
After obtaining ethical and research committee approval and written informed consent from all the patients, 60 American Society of Anesthesiologists physical status I or II patients, 18–65 years of age, undergoing total hip replacement under CSE anesthesia were enrolled for the study. Patients who had a past history of reaction to study drugs, on analgesic therapy and calcium channel blockers, major hepatic, renal, or cardiovascular dysfunction and any contraindication to central neuraxial blockade were excluded from the study. Patients were familiarized with the Verbal Rating Score (VRS) (0: no pain, 10: worst pain) a day before surgery. They were advised overnight fasting and premedicated with 0.5 mg oral alprazolam at night.
On arrival at the operating room, electrocardiogram, noninvasive automated blood pressure and pulse oximeter monitoring were started. Baseline pulse rate, blood pressure (systolic, diastolic, and mean), and oxygen saturation were noted. An intravenous access was established using 18-gauge intravenous cannula. All the patients were preloaded with lactated Ringer's solution (10 mL/kg body weight).
Under all aseptic precautions, patients received CSE anesthesia using CSE set (Portexâ Regional Anesthesia Tray, Smith Medical ASD, Inc., Keene, NH, USA) in sitting position. After local infiltration with 2% lignocaine at L3–L4 intervertebral space, CSE block was administered using a needle through needle technique. The epidural space was identified by loss of resistance using air with 18-gauge Touhy needle. Dural puncture was performed with 27-gauge Whitacre spinal needle. Subarachnoid space was identified by free flow of cerebrospinal fluid. Two milliliters of 0.5% hyperbaric bupivacaine was injected into the intrathecal space. Epidural catheter was threaded 4 cm in addition to the distance of epidural space and fixed with adhesive plaster on patient's back.
Sensory block was assessed bilaterally by using pin prick method with a short beveled needle. Motor block was evaluated using modified Bromage scale (0: no motor block, 1: inability to raise extended legs, 2: inability to flex knees, 3: inability to flex ankle joints).[6] Intraoperatively, sensory block was checked every 15 min. An epidural test dose of 45 mg lignocaine and 1:200000 adrenaline in a volume of 3 mL was administered. During the course of operation, epidural bupivacaine 0.5% was given in incremental dose of 5 mL every 10 min, if required, to achieve a block up to T8 level.
Hypotension was defined as systolic blood pressure <80 mmHg or >30% decrease from baseline, which was at first treated by intravenous bolus of 500 mL of lactated Ringer's solution, if required followed by 6 mg mephenteramine intravenously. Tachycardia was defined as heart rate >120 beats/min and bradycardia as <50 beats/min.
At the end of the surgery, patients were randomized by computer-generated random number assignment into 2 groups of 30 each. After the surgery, the patients were shifted to postanaesthesia care unit and Group F (n=30) patients received epidural fentanyl (1 μg/kg) in 10 mL isotonic saline, while patients in Group FM (n=30) received epidural magnesium (75 mg) along with fentanyl (1 μg/kg) diluted in isotonic saline to a total of 10 mL. The drug was prepared by an independent investigator who was not involved in the perioperative management of the patient. Fentanyl was prepared from an ampoule containing 50 μg/mL, whereas magnesium dose was prepared from an ampoule containing 50% magnesium (500 mg/mL), which was diluted to 5% in 10 mL normal saline and 1.5 mL was administered. Further observation was performed by an independent investigator who was unaware of the randomization. All hip replacements were done by the same surgeon.
VRS ≤ 4 was considered adequate pain relief. Supplementary analgesia was provided by 50 mg intravenous tramadol whenever patient has VRS >4. Patient's first analgesic requirement was recorded. The duration of postoperative epidural analgesia was defined as the time from the administration of epidural study drug postoperatively to the time to first demand of additional analgesia. The frequency of rescue analgesics during 24-h postoperative period was also noted.
Postoperative monitoring consisted of VRS (on rest), pulse rate, blood pressure, modified Bromage scale, and any complication, such as excessive sedation, pruritis, postoperative nausea vomiting (PONV), urinary retention, respiratory depression (respiratory rate <10 beats/min or oxygen saturation <95%). Sedation was assessed on a 4-point scale (0: awake and alert, 1: mildly sedated, easily awakened, 2: moderately sedated, awakened by shaking, 3: deeply sedated, difficult to be awakened by physical stimulation).[7] All the patients were observed for any neurologic complications until 24 h after surgery. Postoperative monitoring was recorded at 30 min, hourly for the first 6 h and 2 hourly for the 12-h period and then at 24 h.
Statistical analysis
The primary outcome variable was the duration of analgesia after administration of epidural study drug. Based on the study by Bilir and colleagues,[8] a sample size of 30 patients per group was needed (power 80% and a of 0.05). The secondary outcome variables included motor power, sedation, and hemodynamic variables. Statistical analysis was performed using Excel and SPSS package v15. The data were expressed as mean and standard deviation. Student's t test for equality of means was used to analyze demographic parameters. Duration of analgesia, VRS, modified Bromage scale, and hemodynamic parameters were analyzed by Student's t test. P<0.05 was considered significant.
RESULTS
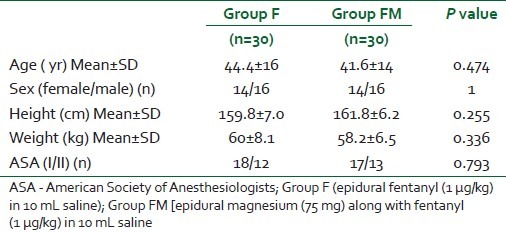
Seventy-two patients were recruited for the trial, of whom 12 were excluded as they did not fulfil the study criteria. Sixty patients were finally randomized into one of the 2 groups, including 30 patients each and no patients were subsequently excluded from the analysis [Figure 1]. The patients’ characteristics were comparable in both the groups [Table 1].
Figure 1.
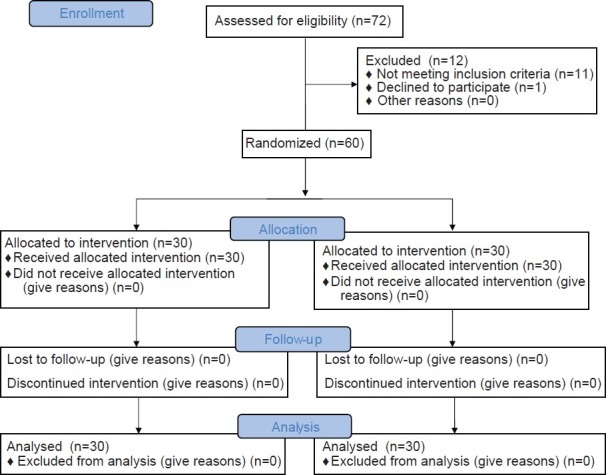
Consort flow diagram
Table 1.
Demographic profile of patients in the 2 groups
The duration of surgery was comparable in both the groups [159±22.5 min in Group F vs 163±20.6 min in Group FM (P=0.473)]. There was no incidence of failure of anesthesia technique and intraoperative period was uneventful with no difference in the level of sensory and motor block. Intraoperative epidural bupivacaine was required in one patient in each group (P=1). The patients in Group FM were administered epidural bupivacaine twice (boluses of 5 mL each), while the patients in Group F were administered single bolus of 5 mL of epidural bupivacaine. The sensory and motor block level was comparable prior to administration of epidural drugs in the 2 groups (P=0.7 and 0.52, respectively).
The mean±SD duration of analgesia after the epidural drug administration was significantly longer for Group FM, 340±28.8 min, compared with Group F, 164±17.1 min (P=0.001). The frequency of rescue analgesics (intravenous tramadol 50 mg) required in 24-h postoperative period in Group FM (2.3±0.5) was significantly less than in Group F (4.3±0.5) (P=0.001).
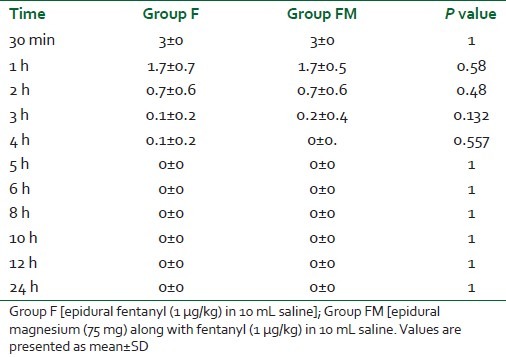
VRS was significantly lower for Group FM at 2, 3, and 4 h in the postoperative period (P=0.001) [Table 2]. After 6 h, VRS was statistically significantly lower for Group F as compared with Group FM, and was comparable at 24 h. Bromage scale was statistically insignificant at all points of time (at 30 min, hourly up to 6 h and 2 hourly up to 12 h and at 24 h in the postoperative period (P>0.05) [Table 3].
Table 2.
VRS (0–10) in the 2 groups in postoperative period
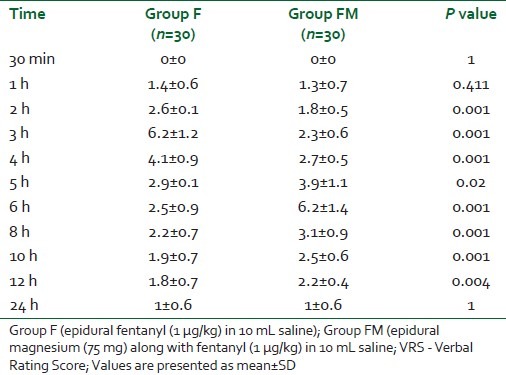
Table 3.
Assessment of motor power by modified Bromage scale in 2 groups
Postoperative pulse rate, systolic, diastolic, and mean blood pressure was comparable in both groups and was statistically insignificant (P>0.05). There was no incidence of hypotension or bradycardia in either of the group postoperatively. There was no incidence of respiratory depression in either of the group. No patients experienced excessive sedation (median sedation score 0 in both the groups), PONV, or pruritis. Two patients each in both the groups had urinary retention (P=1).
DISCUSSION
The results of the present investigation show that a single bolus of epidural magnesium (75 mg) as an adjuvant to epidural fentanyl (1 μg/kg) results in prolonged duration of analgesia as compared with epidural fentanyl (1 μg/kg) alone. Also, VRS was lower in the study group as compared with control group. Concomitant administration of magnesium also reduces the requirement of breakthrough analgesics with no increased incidence of side effects.
NMDA, an excitatory amino acid receptor has been implicated in transmission of noxious stimulus from periphery leading to central sensitization.[9] Magnesium, a noncompetitive NMDA receptor antagonist, has a role in prevention of central sensitization from peripheral noxious stimulus.[3] Whatever the route of administration, intravenous, intrathecal, or epidural, the true site of action of magnesium is probably at the spinal cord NMDA receptors.[10,11] The duration and intensity of postoperative analgesia is dependent on the degree of inhibition of NMDA receptor signal transmission.[9] So we studied the efficacy of single bolus administration of magnesium (75 mg) epidurally as an adjuvant to epidural fentanyl (1 μg/kg) for postoperative analgesia.
In our study, a 75 mg dose of epidural magnesium in Group FM resulted in lower VRS at 2, 3, and 4 h postoperatively. Bilir and colleagues demonstrated that 50 mg epidural bolus magnesium followed by 100 mg/day epidural infusion resulted in lower VAS score only at first hour postoperatively.[8] This difference of lower VRS score could be due to higher bolus dose of epidural magnesium in our study. Farouk has demonstrated that preoperative epidural administration of magnesium (50 mg bolus followed by infusion of 10 mg/h) till the end of surgery resulted in lowered pain score for 3 days as compared with postoperative epidural magnesium bolus (50 mg bolus).[5] So it appears that a single epidural bolus of magnesium potentiates analgesia for initial few hours only (4 h in our study). To prolong the analgesia either a repeat dose needs to be administered or magnesium infusion (as mentioned by Farouk) needs to be started.[5] We should also consider starting multimodal analgesia including oral analgesics after initial good analgesic action of epidural magnesium. This needs to be studied further.
The studies related to pain management have shown that magnesium potentiates the analgesic effects of opiates.[11,12] In our study the mean duration of analgesia was significantly prolonged in the fentanyl–magnesium group (340±28.8 min) as compared with fentanyl alone group (164±17.1 min). In contrast to our study, Bilir and colleagues observed that the time to first rescue analgesia demand when magnesium (50 mg) was administered epidurally was statistically insignificant as compared with the control group who received saline.[8] Similar limitation was observed by Kim and colleagues also.[13] In a clinical study by El-Kerdawy, patients received CSE followed by epidural infusion of magnesium (100 mg/h).[14] They concluded that time to first analgesic requirement was significantly prolonged in the magnesium group compared with the control placebo group. The duration of analgesia in their study was much less than in our study (79 vs 340 min). This may be explained by the fact that we administered epidural magnesium in addition to fentanyl, which could prolong the requirement of rescue analgesia. Recently this was also confirmed in patients scheduled for cesarean section. Yousef et al. administered 10 mL of 5% magnesium epidurally and concluded that the addition of magnesium to epidural bupivacaine and fentanyl in women undergoing elective cesarean section with combined spinal–epidural anesthesia improved intraoperative conditions and the quality of postoperative analgesia.[15]
The frequency of breakthrough analgesia requirement was lower in Group FM in the 24-h study period. Arcioni and colleagues studied the effect of combined intrathecal 94.5 mg and epidural 100 mg/h infusion of magnesium sulfate supplementation of spinal anesthesia on postoperative analgesic requirements in patients undergoing orthopedic surgery. They concluded that supplementation of spinal anesthesia with combined intrathecal and epidural magnesium significantly reduces patient's postoperative analgesic requirements.[12] Farouk observed in his study that administration of epidural magnesium reduces the rescue analgesic requirement in the postoperative period.[5] Bilir and colleagues also reported reduction in postoperative fentanyl consumption without any side effects.[8] Kim and colleagues conducted a trial to evaluate the effects of epidural magnesium on cumulative dose of ropivacaine in patients with patient-controlled epidural analgesia after a thoracotomy.[13] Magnesium group received 100 mg of magnesium in the initial dose and 4 mg in the demand dose. In contrast to our study they observed that the analgesic requirement at 3 and 6 h was not different as compared with the control group (received ropivacaine only) but was lower at 12, 24, and 48 h. This could emphasize the fact that magnesium may be used as an adjuvant to fentanyl to have late reduction of rescue analgesic requirement as well.
The concern of neuromuscular blockade after intravenous magnesium administration is emphasized in the literature but no impact was noted on motor function in our study (as adjudged by modified Bromage score) when magnesium was administered epidurally.[16] Also, the neurologic outcome after inadvertent administration of larger doses of intrathecal and epidural magnesium has been studied and no neurologic deficit has been reported.[17,18] There were no increased incidences of side effects in the magnesium group, although 2 patients in both the groups complained of urinary retention, which might have resulted from epidural administration of fentanyl.[15]
The study is limited by the absence of dose response of epidural magnesium for postoperative analgesia. In the absence of literature regarding bodyweight-based dosage of epidural magnesium, we had used a fixed dose of epidural magnesium in our study. The result of present randomized controlled trial raises the need for more clinical studies regarding the dosage regimen of epidural magnesium. Also, the effect of epidural magnesium on VRS on movement was not assessed as the study was limited for 24 h and as per our institute protocol, active movement of the operated limb is not allowed for the first 24 h.
CONCLUSION
We conclude that the administration of epidural magnesium (75 mg) as an adjuvant to epidural fentanyl (1 μg/kg) for postoperative analgesia results in prolonged duration of analgesia as compared with epidural fentanyl (1 μg/kg) alone. Concomitant administration of magnesium also reduces the requirement for breakthrough analgesics with no increased incidence of side effects.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Wheatley RG, Schug SA, Watson D. Safety and efficacy of postoperative epidural analgesia. Br J Anaesth. 2001;87:47–61. doi: 10.1093/bja/87.1.47. [DOI] [PubMed] [Google Scholar]
- 2.Ozalevli M, Cetin TO, Unlugenc H, Guler T, Isik G. The effect of adding intrathecal magnesium sulphate to bupivcaine–fentanyl spinal anaesthesia. Acta Anaesthesiol Scand. 2005;49:1514–9. doi: 10.1111/j.1399-6576.2005.00793.x. [DOI] [PubMed] [Google Scholar]
- 3.Begon S, Pickering G, Eschalier A, Dubray C. Magnesium increases morphine analgesic effect in different experimental models of pain. Anesthesiology. 2002;96:627–32. doi: 10.1097/00000542-200203000-00019. [DOI] [PubMed] [Google Scholar]
- 4.Buvanendran A, McCarthy RJ, Kroin JS, Leong W, Perry P, Tuman KJ. Intrathecal Magnesium prolongs fentanyl analgesia: A prospective, randomized, controlled trial. Anesth Analg. 2000;95:661–6. doi: 10.1097/00000539-200209000-00031. [DOI] [PubMed] [Google Scholar]
- 5.Farouk S. Pre-incisional epidural magnesium provides pre-emptive and preventive analgesia in patients undergoing abdominal hysterectomy. Br J Anaesth. 2008;101:694–9. doi: 10.1093/bja/aen274. [DOI] [PubMed] [Google Scholar]
- 6.Bromage PR. A comparison of the hydrochloride and carbon dioxide salts of lidocaine and prilocaine in epidural analgesia. Acta Anaesthesiol Scand. 1965;16:55–69. doi: 10.1111/j.1399-6576.1965.tb00523.x. [DOI] [PubMed] [Google Scholar]
- 7.Koinig H, Wallner T, Marhofer P, Andel H, Horauf K, Mayer N. Magnesium sulfate reduces intra and postoperative analgesic requirements. Anesth Analg. 1998;87:206–10. doi: 10.1097/00000539-199807000-00042. [DOI] [PubMed] [Google Scholar]
- 8.Bilir A, Gulec S, Erkan A, Ozcelik A. Epidural magnesium reduces postoperative analgesic requirement. Br J Anaesth. 2007;98:519–23. doi: 10.1093/bja/aem029. [DOI] [PubMed] [Google Scholar]
- 9.Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation: Implications for the treatment of post - injury pain and hypersensitivity states. Pain. 1991;44:293–9. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- 10.Ko SH, Lim HR, Kim DC, Han YJ, Choe H, Song HS, et al. Magnesium sulphate does not reduce postoperative analgesic requirements. Anesthesiology. 2001;95:640–6. doi: 10.1097/00000542-200109000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Xiao WH, Bennett GJ. Magnesium suppresses neuropathic pain responses in rats via a spinal site of action. Brain Res. 1994;666:168–72. doi: 10.1016/0006-8993(94)90768-4. [DOI] [PubMed] [Google Scholar]
- 12.Arconi R, Plamisani S, Tirano S, Santorsola C, Sauli V, Ramano S. Combined intrathecal and epidural magnesium sulphate supplementation of spinal anesthesia to reduce postoperative analgesic requirements: A prospective randomized double blind controlled trial in patients undergoing major orthopedic surgery. Acta Anaesthesiol Scand. 2007;51:482–9. doi: 10.1111/j.1399-6576.2007.01263.x. [DOI] [PubMed] [Google Scholar]
- 13.Kim SM, Cho SH, Kim SH, Lee DG, Chae WS, Jin HC. The effects of epidural magnesium on postoperative pain management in patients with patient controlled epidural analgesia after a thoracotomy. Korean J Anesthesiol. 2009;57:466–71. doi: 10.4097/kjae.2009.57.4.466. [DOI] [PubMed] [Google Scholar]
- 14.El-Kerdawy H. Analgesic requirements for patients undergoing lower extremity orthopedic surgery – the effect of combined spinal and epidural magnesium. Middle East J Anesthesiol. 2008;19:1013–26. [PubMed] [Google Scholar]
- 15.Yousef AA, Amr YM. The effect of adding magnesium sulphate to epidural bupivacaine and fentanyl in elective caesarean section using combined spinal–epidural anaesthesia: A prospective double blind randomised study. Int J Obstet Anesth. 2010;19:401–4. doi: 10.1016/j.ijoa.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 16.Levaux C, Bonhomme V, Dewandre PY, Brichant JF, Hans P. Effect of intra-operative magnesium sulphate on pain relief and patient comfort after major lumbar orthopaedic surgery. Anaesthesia. 2003;58:131–5. doi: 10.1046/j.1365-2044.2003.02999.x. [DOI] [PubMed] [Google Scholar]
- 17.Lejuste MJ. Inadvertent intrathecal administration of magnesium sulfate. S Afr Med J. 1985;68:367–8. [PubMed] [Google Scholar]
- 18.Goodman EJ, Haas AJ, Kantor GS. Inadvertent administration of magnesium sulfate through epidural catheter: Report and analysis of a drug error. Int J Obstet Anesth. 2006;15:63–7. doi: 10.1016/j.ijoa.2005.06.009. [DOI] [PubMed] [Google Scholar]


