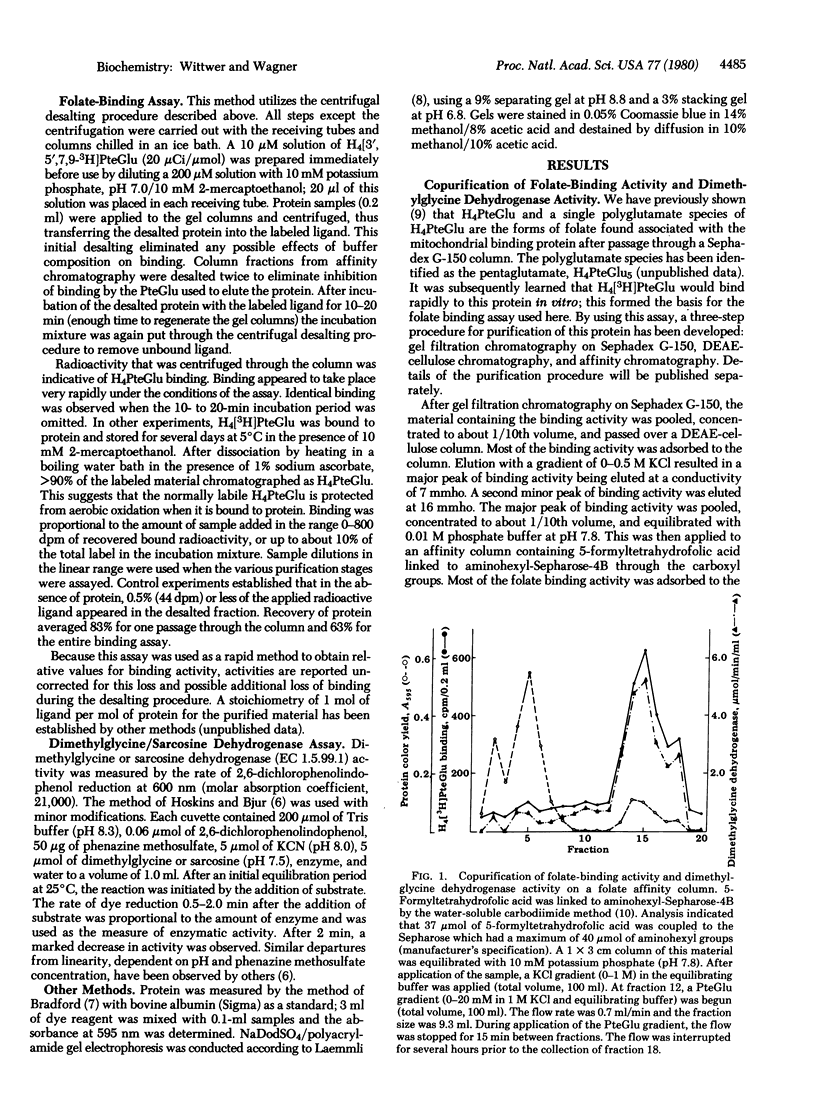
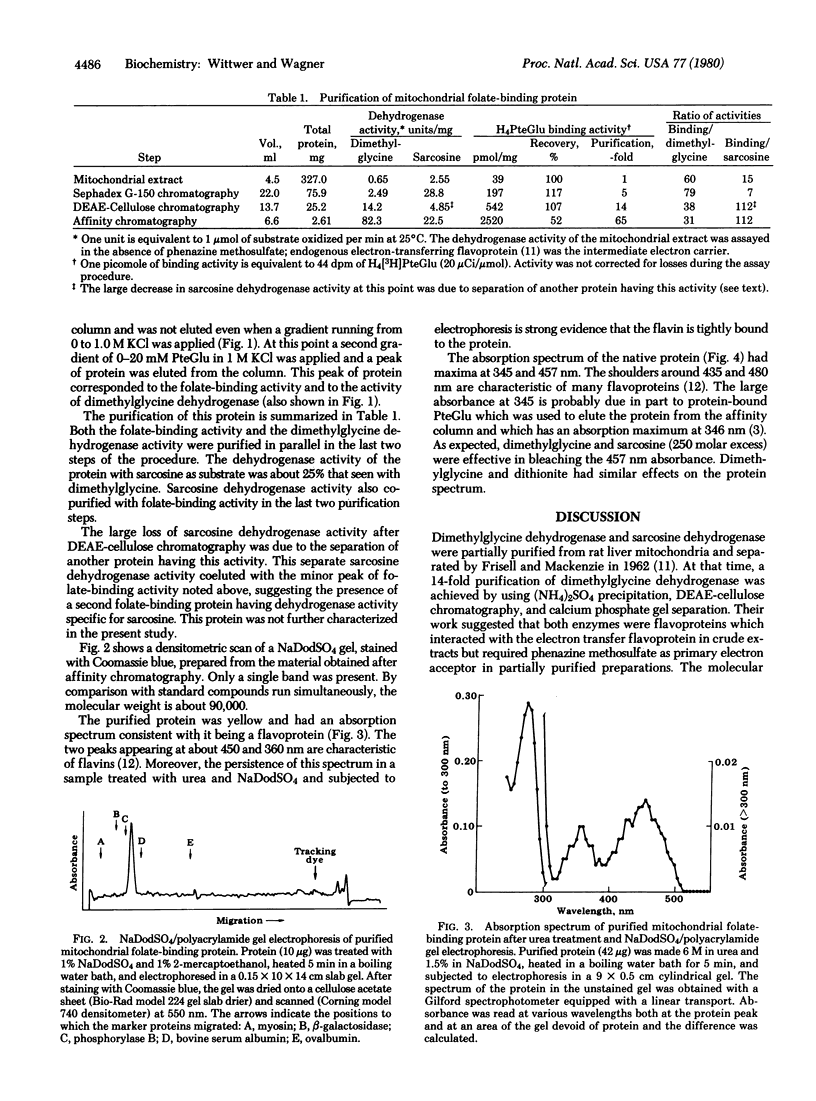
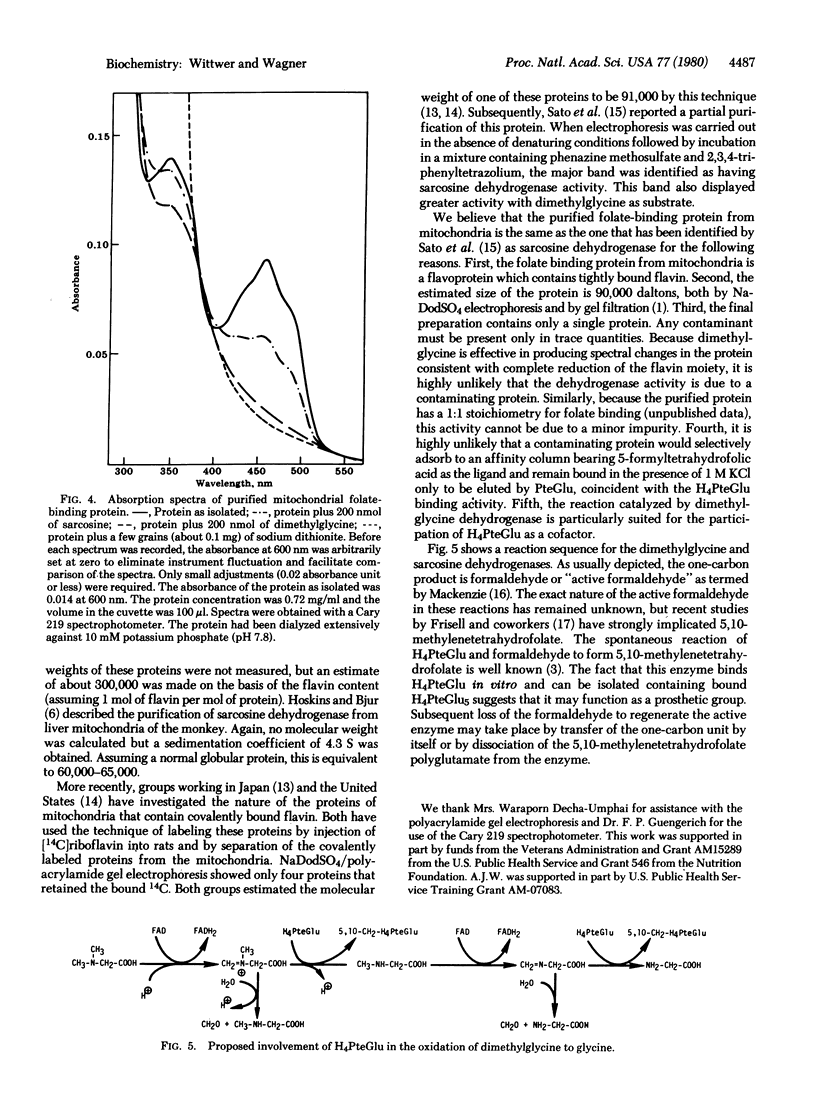
Abstract
The folate-binding protein of rat liver mitochondria [Zamierowski, M. & Wagner, C. (1977) J. Biol. Chem. 252, 933-938] has been purified to homogeneity by a combination of gel filtration, DEAE-cellulose, and affinity chromatography. This protein was assayed by its ability to bind tetrahydro[3H]folic acid in vitro. the purified protein contains tightly bound flavin and has a molecular weight of about 90,000 as determined by sodium dodecyl sulfate electrophoresis. This protein also displays dimethylglycine dehydrogenase [N,N-dimethylglycine: (acceptor) oxidoreductase (demethylating), EC 1.5.99.2] activity which copurifies with the folate-binding activity. It is suggested that the role of the tetrahydrofolic acid is to accept the formaldehyde produced during the course of the reaction.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addison R., McCormick D. B. Biogenesis of flavoprotein and cytochrome components in hepatic mitochondria from riboflavin-deficient rats. Biochem Biophys Res Commun. 1978 Mar 15;81(1):133–138. doi: 10.1016/0006-291x(78)91640-6. [DOI] [PubMed] [Google Scholar]
- Blair J. A., Saunders K. J. A convenient method for the preparation of dl-5-methyltetrahydrofolic acid (dl-5-methyl-5,6,7,8-tetrahydropteroyl-L-monoglutamic acid). Anal Biochem. 1970 Apr;34(2):376–381. doi: 10.1016/0003-2697(70)90122-3. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- FRISELL W. R., MACKENZIE C. G. Separation and purification of sarcosine dehydrogenase and dimethylglycine dehydrogenase. J Biol Chem. 1962 Jan;237:94–98. [PubMed] [Google Scholar]
- HOSKINS D. D., BJUR R. A. THE OXIDATION OF N-METHYLGLYCINES BY PRIMATE LIVER MITOCHONDRIA. ASSAY, PURIFICATION, AND CHARACTERIZATION OF SARCOSINE DEHYDROGENASE. J Biol Chem. 1964 Jun;239:1856–1863. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewis K. F., Randolph V. M., Nemeth E., Frisell W. R. Oxidation of one-carbon compounds to formate and carbon dioxide in rat liver mitochondria. Arch Biochem Biophys. 1978 Jan 30;185(2):443–449. doi: 10.1016/0003-9861(78)90187-x. [DOI] [PubMed] [Google Scholar]
- Neal M. W., Florini J. R. A rapid method for desalting small volumes of solution. Anal Biochem. 1973 Sep;55(1):328–330. doi: 10.1016/0003-2697(73)90325-4. [DOI] [PubMed] [Google Scholar]
- Penefsky H. S. Reversible binding of Pi by beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1977 May 10;252(9):2891–2899. [PubMed] [Google Scholar]
- Sato M., Ohishi N., Nishikimi M., Yagi K. Proteins with covalently-bound flavin in rat liver mitochondria. Biochem Biophys Res Commun. 1977 Oct 10;78(3):868–873. doi: 10.1016/0006-291x(77)90503-4. [DOI] [PubMed] [Google Scholar]
- Sato M., Ohishi N., Yagi K. Identification of a covalently bound flavoprotein in rat liver mitochondria with sarcosine dehydrogenase. Biochem Biophys Res Commun. 1979 Apr 13;87(3):706–711. doi: 10.1016/0006-291x(79)92016-3. [DOI] [PubMed] [Google Scholar]
- Waxman S., Schreiber C. The purification and characterization of the low molecular weight human folate binding protein using affinity chromatography. Biochemistry. 1975 Dec 16;14(25):5422–5428. doi: 10.1021/bi00696a007. [DOI] [PubMed] [Google Scholar]
- Zamierowski M. M., Wagner C. Identification of folate binding proteins in rat liver. J Biol Chem. 1977 Feb 10;252(3):933–938. [PubMed] [Google Scholar]



