Abstract
Aim:
The purpose of this study was to evaluate and compare the root surface changes subsequent to the application of citric acid, tetracycline, Ethylenediaminetetraacetic acid (EDTA), and the combination of citric acid and tetracycline, and its influence on the adhesion of a fibrin clot with and without mild disruptive forces.
Materials and Methods:
A total of 100 periodontally diseased root specimens were grouped into Saline (control Group I), 24% EDTA gel (Group-II), Citric acid (Group-III), Tetracycline (Group IV), and Citric acid + tetracycline (Group V) treatment groups containing 20 in each. After root conditioning, fresh human blood was applied to each root specimen and was allowed to clot. Ten specimens in each group were rinsed in phosphate-buffered saline and designated as ′Non-agitated′. The remaining ten specimens from each group were rinsed in phosphate-buffered saline on a rotary shaker and designated as ′Agitated′. The roots were processed for scanning electron microscopy (SEM) to assess and compare the clot adhesion on them. The scores were compared through standard statistical packages.
Results:
The highest mean blood clot adhesion score was observed in roots treated with a combination of citric acid and tetracycline, whereas, the least score was observed in roots treated with saline.
Conclusion:
The root specimens treated with the combination of citric acid and tetracycline as well as citric acid alone, best supported the fibrin clot. Tetracycline alone appeared to be less effective in supporting the clot. EDTA gel of 24% was least effective to promote the adhesion of a fibrin clot.
Keywords: Citric acid, EDTA, root conditioning, tetracycline
INTRODUCTION
Periodontitis is characterized by inflammation of various components of the periodontium and produces substantial changes in the tooth and root surface which is referred to as a ‘pathologically exposed’ root surface. In a ‘pathologically exposed’ root surface, the extrinsic and intrinsic fibers are destroyed by plaque-induced inflammation, allowing a downgrowth of the junction epithelium. Plaque, calculus and cytotoxic substances penetrate the pathologically exposed root surface which acts as a physical barrier, inhibiting a new attachment and provides a substrate for bacterial growth.[1]
The traditional treatment of pathologically altered root surfaces has relied on mechanical instrumentation[1,3] which results in the formation of a smear layer of organic and mineralized debris.[4] As a compensation for these limitations of mechanical root surface therapy; chemical root conditioning has been introduced. Chemical root conditioning may favor clot stabilization in the earlier stages of periodontal healing by increasing the adhesion of blood cells and fibrin to the root surface.[5] Root conditioning is intended to detoxify the root surface by removing the smear layer and demineralizing it by exposing the collagen matrix that supports migration and proliferation of the cells involved in periodontal healing.[6–9]
Many root conditioning agents like citric acid, phosphoric acid, EDTA, tetracycline, fibronectin, antiformin, and sodium deoxycholate have been used. Citric acid and tetracycline hydrochloride have been extensively researched and used clinically because of higher tissue tolerance and easy storage. Also, etching at neutral pH with agents like EDTA has been shown to have a comparable demineralizing potential.
In this study an attempt has been made to evaluate and compare the effects of root conditioning by citric acid, tetracycline hydrochloride, EDTA gel and a combination of tetracycline and citric acid on the adhesion of the fibrin clot with and without disruptive forces to the instrumented root surface.
MATERIALS AND METHODS
Fifty single-rooted human teeth extracted due to severe periodontal disease [Figure 1], were collected as specimens from the Department of Oral and Maxillofacial Surgery, Bangalore Institute of Dental Sciences & Hospital and Post Graduate Research Centre, Bangalore. The extracted teeth were required to meet the following inclusion and exclusion criteria.
Figure 1.
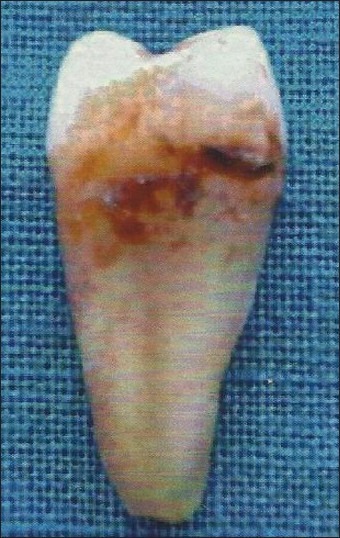
Diseased mandibular first premolar
Inclusion criteria
Extracted single-rooted teeth due to extensive loss of periodontal supporting tissues
Teeth extracted under local anesthesia in a non-traumatic fashion
Vital teeth at the time of extraction
Exclusion criteria
Patients with a history of systemic diseases
Patients who were under antibiotic treatment in the last four months
Patient who underwent periodontal debridement in the last six months
Teeth with periapical infection or non-vital teeth
The extracted teeth were immediately washed in distilled water and stored in 0.9% saline, until further study.
Preparation of teeth specimen
Two parallel grooves were made on the proximal root surface of each extracted tooth using a diamond-tapered fissure bur with an airotor hand piece under copious irrigation. The first groove was made at the cementoenamel junction and the other approximately 3 mm apical to it. The area between the two grooves was debrided and planed with 50 apicocervical strokes,[10] using a sharp Gracey curette No.5-6 (Hu-Friedy, USA) [Figure 2]. After root planing, each tooth was sectioned to obtain two dentin blocks [Figure 3] approximately 3×3×1 mm in size.[11] Thus, 100 root specimens obtained were stored individually in saline solution to avoid dehydration.
Figure 2.
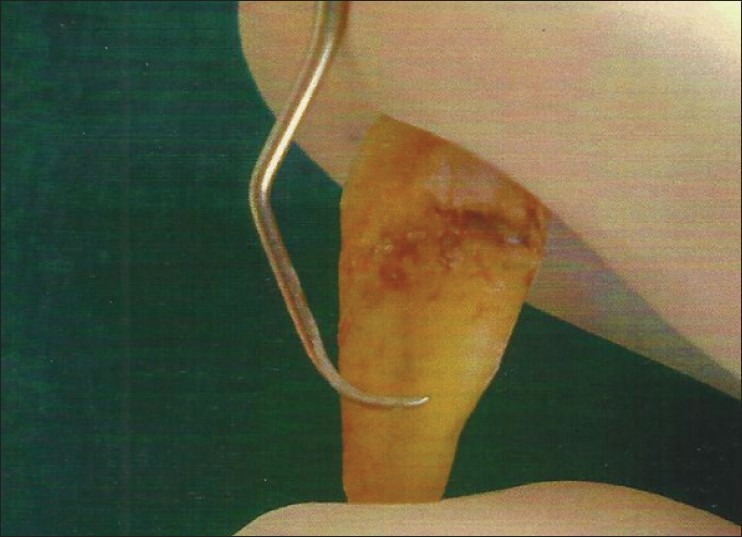
Root planing
Figure 3.
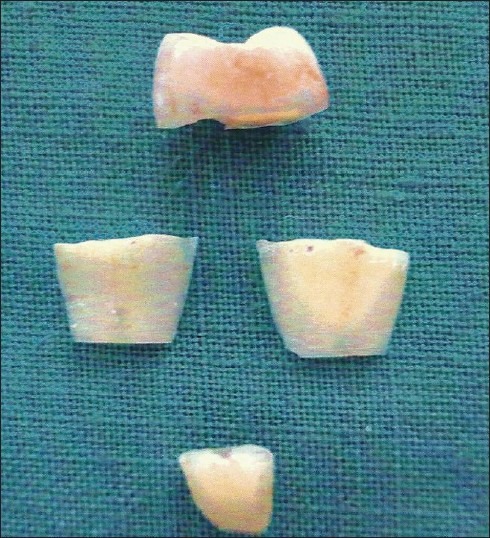
Sectioned root specimen
Chemical treatment of root surface
All 100 specimens were divided into five groups:
Group I: Twenty root specimens treated with saline for three minutes (control).
Group II: Twenty root specimens conditioned with 24% EDTA Gel by placing cotton pellets saturated with it and changed every twenty seconds for three minutes.
Group III: Twenty root specimens conditioned with saturated citric acid solution by placing cotton pellets saturated with it and changed every twenty seconds for three minutes.
Group IV: Twenty root specimens conditioned with tetracycline HCL solution by placing cotton pellets saturated with it and changed every twenty seconds for three minutes.
Group V: Twenty root specimens conditioned with a combination of citric acid and tetracycline hydrochloride solution in a 1 : 1 proportion. Cotton pellets saturated with this solution were changed every twenty seconds for three minutes [Figure 4].
Figure 4.
Procedure
In this study, citric acid of pH 1 (S.D Fine)[12] a concentration of 500 mg / 5 ml (100 mg / ml) tetracycline hydrochloride of pH 1.3 (Himedia)[13] and 24% EDTA of pH 7.2 (Saibliss pharmaceuticals)[14] were used. Root conditioning was done by burnishing soaked cotton pellets with light pressure.[15] Then the specimens were washed with 10 ml saline solution. Venous blood was collected from a healthy male volunteer and a drop of his blood was placed on each chemically treated root surface. The drop of blood was allowed to clot for 20 minutes in a humidified chamber at 37°C. Then ten specimens were subjected to five-minute rinses in phosphate-buffered saline, thrice and were designated as the ‘NONAGITATED’ group. The remaining ten specimens from each group were subjected to five minute rinses in phosphate-buffered saline on a rotary shaker table at a speed of 70 r.p.m (rotations per minute) [Figure 5] thrice and designated as the ‘AGITATED’ group. Thus, 50 agitated and 50 nonagitated root specimens were obtained.
Figure 5.
Rinsing of root specimen in phosphate buffered saline on Rotary Shaker (Agitated)
Preparation for scanning electron microscopy study
The blocks of specimens were fixed for 30 minutes in 2.5% glutaraldehyde. Subsequently, the blocks were subjected thrice in phosphate buffered saline and for five minutes in graded ethanol (70, 90 and 95%) for dehydration. Two final dehydrations of five minutes each were performed using hexamethyldisilazine. The samples were dried overnight in a dehydration jar, mounted on metallic stubs with adhesive tape, and sputter-coated with gold [Figure 6]. Finally the specimens were observed by using a SEM unit. The surfaces of the roots were scanned and representative photomicrographs were obtained on the computer screen at 500×, 1000× and 2000×magnifications.
Figure 6.
Root specimens after gold coating on brass stubs
Criteria for grading the scanning electron microscopic photomicrograph
The photomicrographs were identified and analyzed with scores, in order to verify the adhesion of the blood components and analyze the morphological characteristics of the root surface obtained by treating with various root conditioning agents.
Using a single-blind method, the photomicrographs obtained from the samples were examined thrice by a trained operator. It was again calibrated by two other operators. Among the three readings, a median score was considered for each sample.
Rating system
Score 0: Absence of fibrin network and blood cells[5]
Score 1: Scarce fibrin network and / or blood cells
Score 2: Moderate fibrin network and moderate quantity of blood cells
Score 3: Dense fibrin network and trapped blood cells
Statistical methods
Descriptive statistical analysis has been carried out in this study. Results on continuous measurements are presented on Mean±SD (Minimum - Maximum) and results on categorical measurements are presented in number (%). Significance is assessed at a 5% level of significance. Fisher Exact Test (2 × 4) has been used to find the significance intergroup analysis. Mann Whitney U test has been done to find inter- and intragroup analysis.
RESULTS
The nonagitated saline group did not show any exposure of the matrix collagen fibers. The agitated saline group showed the presence of a smear layer in most of the root surfaces. A majority of the samples from both the groups presented a blood clot adhesion score of 0. There was no significant difference in the fibrin clot adhesion between the nonagitated and agitated saline control groups (P=0.369) [Table 1, Figures 7 and 8].
Table 1.
Comparison of adhesion of blood components between the agitated and nonagitated groups
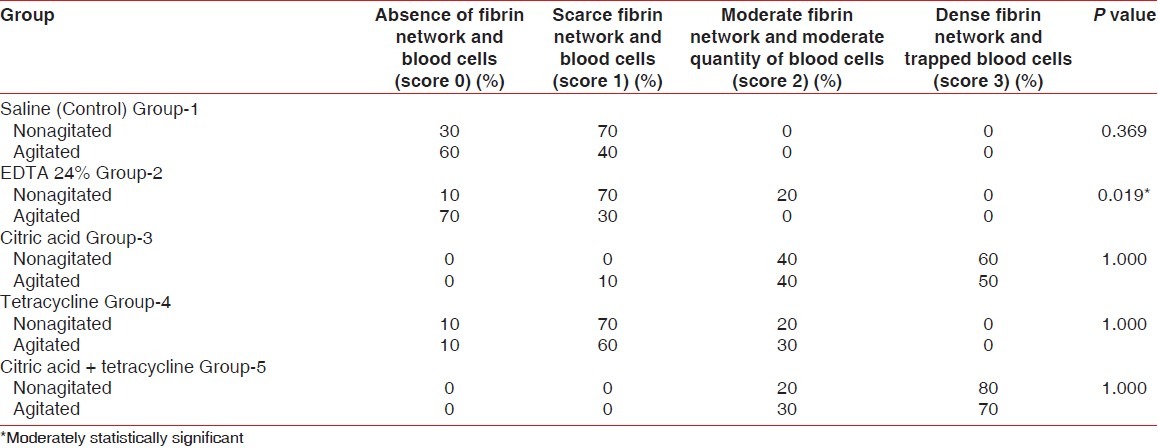
Figure 7.

Saline control group (Agitated)
Figure 8.

Saline control group (Nonagitated)
In 24% of the EDTA group, the dentinal tubular openings were clearly visible in the photomicrographs. There was a statistically significant difference observed in blood clot adhesion between the nonagitated and agitated EDTA treated roots (P=0.019) [Table 1, Figures 9 and 10].
Figure 9.
24% EDTA group (Agitated)
Figure 10.
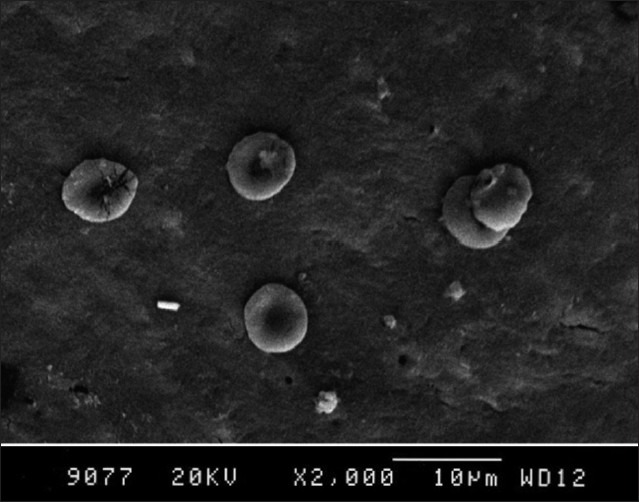
24% EDTA group (Nonagitated)
The citric acid group also showed dentinal tubular openings. Six samples in the nonagitated and five samples in the agitated group presented a blood clot adhesion score of 3, and four samples in each showed better adhesion and strength of blood clot on the root surfaces with a score of 2. The nonagitated and agitated citric acid groups did not show a significant difference in the blood clot adhesion scores (P=1.0) [Table 1, Figures 11 and 12].
Figure 11.
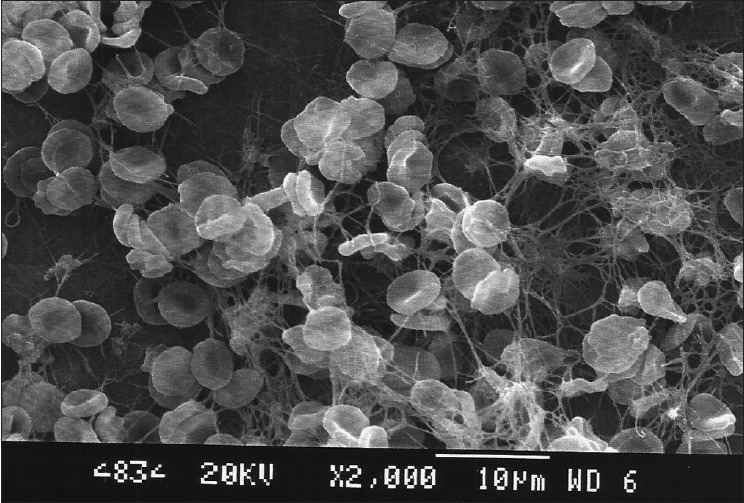
Citric acid group (Agitated)
Figure 12.
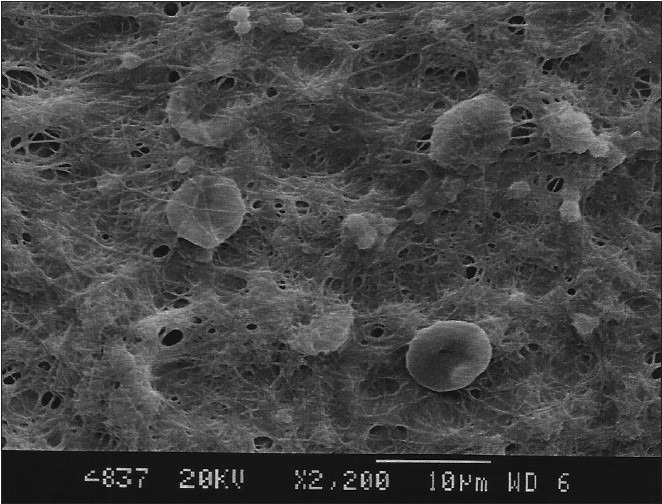
Citric acid group (Nonagitated)
A majority of the root specimens treated with tetracycline showed a blood clot adhesion score of 1 and very few showed a score of 2. There was no significant difference in the blood clot adhesion scores between the agitated and nonagitated tetracycline groups (P=1.0) [Table 1, Figures 13 and 14]. In the citric acid + tetracycline group, eight samples from the nonagitated and seven samples from the agitated group presented a blood component adhesion score of 3 characterized by a dense fibrin meshwork, with entrapped red blood cells on the root surfaces. The remaining samples presented a score of 2 showing a moderate fibrin network and blood cells. The combination of citric acid and tetracycline presented the best effect on blood clot adhesion and its strength, but there was no statistically significant difference in the blood clot adhesion scores between the agitated and nonagitated groups (P=1.0) [Table 1, Figures 15 and 16.
Figure 13.

Tetracycline group (Agitated)
Figure 14.

Tetracycline group (Nonagitated)
Figure 15.

Citric acid + tetracycline group (Agitated)
Figure 16.
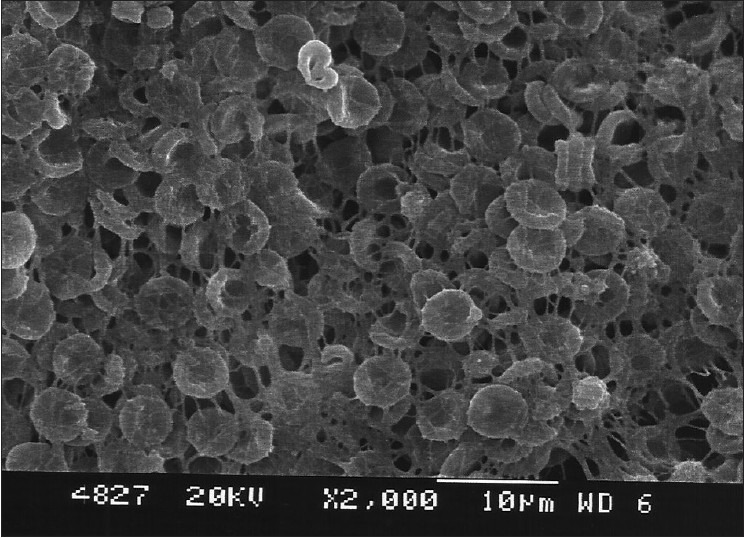
Citric acid + tetracycline group (Nonagitated)
Table 2 and Figure 17 show the comparison of the mean score of blood clot adhesion between the agitated and nonagitated groups of all chemical agents. Only the EDTA group showed a statistically significant difference in the mean score of blood clot adhesion between the agitated and nonagitated groups (P=0.011).
Table 2.
Comparison of mean score of fibrin clot adhesion between all agitated and nonagitated groups
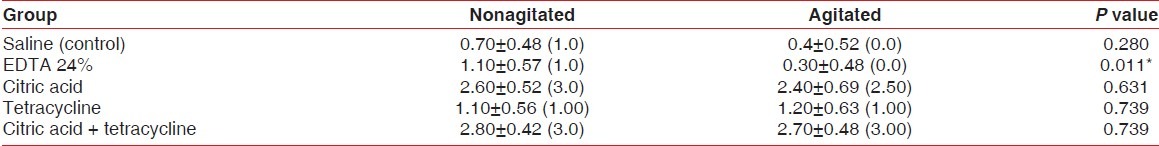
Figure 17.
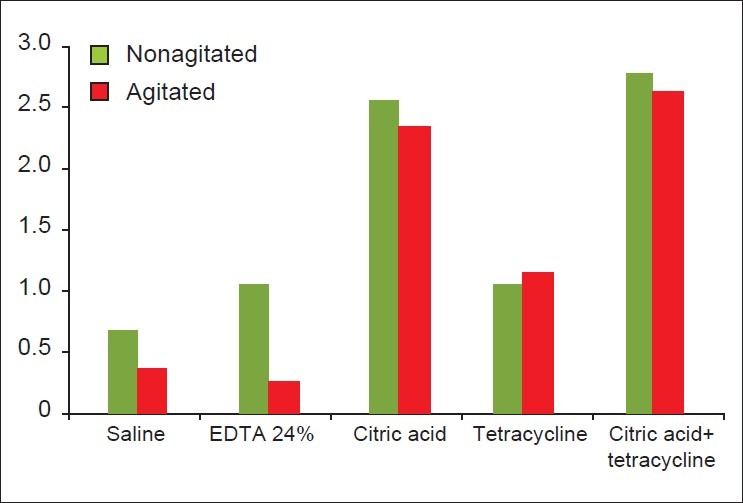
Comparison of mean score of fibrin clot adhesion between all agitated and nonagitated groups
Group V, a combination of citric acid and tetracycline (nonagitated) showed the highest mean score of blood clot adhesion and the EDTA group II (agitated) showed the least mean score of blood clot adhesion.
Intragroup analysis in the nonagitated specimens showed ‘statistically significant’ differences between the following groups [Table 3]:
Table 3.
Intragroup analysis
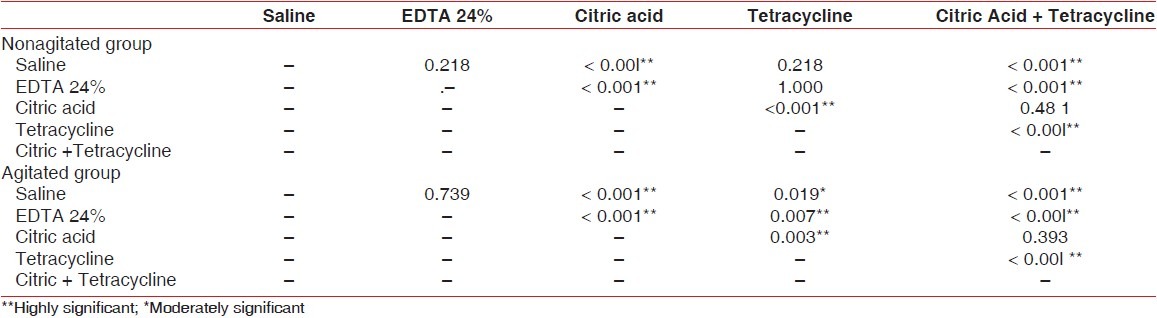
Saline and citric acid groups (P<0.001)
Saline and citric + tetracycline groups (P<0.001)
EDTA and citric acid groups (P<0.001)
EDTA and citric + tetracycline groups (P<0.001)
Citric acid and tetracycline groups (P<0.001)
Tetracycline and citric acid + tetracycline groups (P<0.001)
Intragroup analysis in agitated specimens showed ′statistically significant′ differences between the following groups [Table 3]:
Saline and citric acid groups (P<0.001)
Saline and citric + tetracycline groups (P<0.001)
EDTA and citric acid groups (P<0.001)
EDTA and tetracycline groups (P=0.007)
EDTA and citric + tetracycline groups (P <0.001)
Citric acid and tetracycline groups (P=0.003)
Tetracycline and citric acid + tetracycline groups (P<0.001)
DISCUSSION
The ultimate goal of periodontal therapy is the predictable regeneration of a periodontium previously destroyed by periodontitis. The root surfaces exposed by periodontitis show hypermineralization of the superficial layer and also demineralization areas caused by bacterial enzymes. Hence, the critical step is to achieve biomodification of the contaminated root surfaces to establish a more suitable environment for periodontal regeneration. Mechanical and chemical methods have been proposed to remove the root surface deposits.[16]
Scaling and root planing procedures produce a 2.15 μm thick smear layer of microcrystalline debris, which is intimately associated with the root surface and is virtually removed only by demineralizing agents.
In periodontal healing, the fibrin of the clot forms an initial attachment to the root surface, to establish a scaffold for the development of a cell and collagen fiber attachment and also prevent a downgrowth of the junctional epithelium.[5]
Root surface demineralization with various chemical agents has been proposed to eliminate cytotoxic materials from the affected root surface, clean exposed dentine surface and decalcify the planed root surface, exposing the dentine or cementum matrix collagen, and facilitating attachment between the root surface and the healing connective tissue of the flap. Thus root conditioning may compensate for the limitations inherent in mechanical root surface debridement.[5]
Although evidence to date suggests that the use of citric acid, tetracycline or EDTA to modify the root surface provides no benefit of clinical significance to regeneration in patients with chronic periodontitis, and varying results from histological and clinical studies have created a controversy about the clinical effectiveness of root surface decalcification, root conditioning as a treatment modality has not been abandoned yet.[17]
Hence, in this study an attempt has been made to evaluate and compare fibrin clot adhesion to dentine treated by various root conditioning agents such as citric acid, EDTA, tetracycline hydrochloride and a combination of citric acid and tetracycline.
This study consisted of 50 single-rooted mandibular first premolars, extracted due to severe periodontitis. The method of root planing and sectioning followed in this study was proposed by Leite et al.[11] Root planing was done in order to enhance the action of the root conditioning agents.
Normal saline was used for temporary storage of these sectioned teeth.[13] The method of application of the root conditioning agents[18] followed in this study resulted in root surface demineralization, exposing dentinal tubules and collagen of intra- and peritubular dentinal matrix[19] which helped in adhesion of the fibrin clot.
Baker et al.[20] used the in vitro modeling system to show a simulation of the earliest healing events in periodontal regeneration on a dentinal surface-like root instrumentation, dentin conditioning to remove a smear layer, formation of a fibrin clot and subjection of the fibrin clot to mild disruptive forces. It is conceivable that these conditions may promote or adversely affect fibrin clot adhesion in this model system may also produce similar effects in vivo.
Instrumentation of human dentin blocks that produced a surface smear layer has been shown in previous reports.[4,9] Conditioning of the instrumented root with saturated citric acid causes partial demineralization which appears to enhance mesenchymal cell adhesion possibly by a biochemical mechanism.[6] The drawback of citric acid conditioning is that it creates an extremely acidic pH in the surrounding tissues, which may result in unfavorable wound healing responses[21] and also denaturation of the collagen. Hence, its use has been discontinued. EDTA is preferred for use as it exerts its demineralizing effect through chelating divalent cations at neutral pH.[22] Tetracycline hydrochloride enhances the binding of matrix proteins to the dentin and stimulates fibroblast attachment and growth.[9] In this study, except saline, all root conditioning agents have removed the smear layer exposing the dentinal tubules and the intra- and intertubular collagenous matrix.[4,9,20,23]
The saline group displayed absence of scarce fibrin network in both agitated and nonagitated groups, showing no significant difference between them. Similar results were shown by Leite et al. in their study.[11]
The EDTA [group II] showed statistically significant difference in blood clot adhesion between the agitated and nonagitated groups. (P=0.019). Most of the samples in group-II inhibited blood element adsorption and adhesion to the root surface. The possible explanation for this could be incomplete removal of the gel from the root surface, and as EDTA is a calcium chelator; its residues might have inhibited or retarded the coagulation events.[11,22] In contrast, studies conducted by Blomlof and Lindskog[23] have shown that etching by EDTA appeared to promote early cell and tissue colonization, by providing a more biocompatible surface for cell and tissue attachment. Another study showed that root conditioning with EDTA gel improves β-TCP blended clot adhesion to periodontally involved root surfaces.[24]
In the specimens treated by citric acid, the agitated and nonagitated groups did not show any significant difference in the blood clot adhesion scores, but when compared with group I, II and IV, the differences were highly significant (P < 0.001, P<0.001, and P=0.003 respectively). Better smear layer removal property of citric acid exposed the funnel-shaped tubular orifices.[9,20,25,26] A similar effect of citric acid might have successfully removed the smear layer due to which blood clot adhesion was better when compared to other groups. Similar in vitro studies showed that CA surface demineralization removed a dentin surface smear layer to promote adhesion of a fibrin clot and connective tissue cell development.[27,28]
In contrast, animal studies conducted by Nyman et al[29] Gottlow et al.[30] and clinical trials by Stahl and Froum[31] Stahl et al.[32] and Smith et al.[33] who utilized citric acid for root conditioning failed to result in a new attachment. Speculative explanations for these inconsistent findings have included variations in animal models[29,34] like, inconsistent flap adaptation[34] inadequate demineralization of periodontitis-affected root surfaces[35,36] and repopulation of the root surface with inappropriate cell types.[29,30,37]
In the specimens treated with tetracycline hydrochloride (Group-IV) also, the agitated and nonagitated groups did not show any significant difference in the blood clot adhesion scores, but when compared with Group I agitated, the difference was moderately significant (P=0.019). Comparison with Group II agitated showed a highly significant difference (P = 0.007). Comparison of Group IV with Group III nonagitated and agitated also showed significant differences in the blood clot adhesion scores (P<0.001, and P=0.003 respectively). Comparative evaluation of the root surface alterations by using citric acid and oxytetracycline reported that the smear layer removal in the oxytetracycline-treated specimens was not as effective as in the citric acid-treated specimens, and it varied from specimen to specimen.[38] This result was similar to the result of our study.
There was no significant difference in blood clot adhesion between the agitated and nonagitated groups treated with a combination of citric acid and tetracycline (Group-V). Intragroup analysis showed highly significant differences in blood clot adhesion (P<0.001) when compared with Groups I, II and IV. Group V showed the highest mean score of the blood adhesion component possibly due to the synergistic effect of root conditioning and the antimicrobial property of citric acid and tetracycline, which would make the root surface more biocompatible for blood clot adhesion, an earlier event in periodontal healing.[39]
The differences in the extent of blood clot adhesion on the instrumented root surfaces may be related to the degree of demineralization and removal of smear layer achieved by root conditioning agents and the effect of the root conditioning agent may be related to the degree of hypermineralization of roots in periodontitis, extent of instrumentation and concentration of the root conditioning agents or a combination of these variables. The biochemical and morphological changes in the root surface, produced by the various mechanical techniques and conditioning agents, are yet to be understood.
From this study it can be concluded that the application of a combination of citric acid and tetracycline on instrumented periodontally diseased roots showed a better effect as a root conditioning agent enhancing the adhesion of the fibrin clot and could prove to be a useful tool in the establishment of a new connective tissue attachment in periodontal regenerative procedures.
CONCLUSION
In consideration of the inherent limitations of this in vitro study design, the following conclusions were drawn:
The root specimens treated with a combination of citric acid and tetracycline showed better adhesion of the fibrin clot than any other group, although the results were not statistically significant in comparison with significant groups
The fibrin clot adhesion was better supported and could not get disrupted by the forces produced by a rotary shaker in all the groups except in the EDTA group. Thus 24% EDTA gel was least effective to promote the adhesion of a fibrin clot
Conditioning of root surfaces appropriately is likely to be important for enhancing the predictability of regenerative therapies. Research has focused on identifying factors that can detoxify roots and also influence appropriate cell attachment that is needed to identify appropriate root conditioning therapies.
Randomized clinical trials with a sufficient statistical power supported by quantitative histological evaluation are necessary to confirm and validate these in vitro observations.
ACKNOWLEDGMENTS
The authors would like to thank Mr. Gurulingiah, Indian Institute of Sciences, Bangalore, for helping with the SEM analysis, and Mr. K.P. Suresh, Biostatistician National Institute of Animal Nutrition and Physiology, for the statistical analysis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Eide B, Lie T, Selvig KA. Surface coating on dental cementum incident to periodontal disease.A Scanning electron microscopic study. J Clin Periodontol. 1983;10:157–63. doi: 10.1111/j.1600-051x.1983.tb02204.x. [DOI] [PubMed] [Google Scholar]
- 2.Kelpic TJ, Oleary TJ, Kafrawy AH. Total calculus removal: An objectionable objective? J Periodontol. 1990;61:16–20. doi: 10.1902/jop.1990.61.1.16. [DOI] [PubMed] [Google Scholar]
- 3.Adrina PA, Edward CA, Deboever JA, Loeshe WJ. Ultra structural observations on bacterial invasion in cementum and radicular dentin of periodontally diseased human teeth. J Periodontol. 1998;59:493–503. doi: 10.1902/jop.1988.59.8.493. [DOI] [PubMed] [Google Scholar]
- 4.Polson A, Frederick G, Ladenheim S, Hanes P. The production of a root surface smear by instrumentation and its removal by citric acid. J Clin Periodontol. 1984;54:443–8. doi: 10.1902/jop.1984.55.8.443. [DOI] [PubMed] [Google Scholar]
- 5.Theodoro LH, Sampaio JE, Haypek P, Bachmann L, Zezell DM, Garcia VG. Effect of Er: YAG and Diode lasers on the adhesion of blood components and on the morphology of irradiated root surfaces. J Periodontal Res. 2006;41:381–90. doi: 10.1111/j.1600-0765.2005.00846.x. [DOI] [PubMed] [Google Scholar]
- 6.Boyko GA, Brunette DM, Melcher AH. Cell attachment to demineralized root surfaces in vitro. J Periodontal Res. 1980;15:297–303. doi: 10.1111/j.1600-0765.1980.tb00285.x. [DOI] [PubMed] [Google Scholar]
- 7.Pitaru S, Melcher AH. Orientation of and oriented fiber system in vitro by human gingival fibroblasts attached to dental tissue: Relationship between cells and mineralized and demineralized tissue. J Periodontal Res. 1987;22:6–13. doi: 10.1111/j.1600-0765.1987.tb01533.x. [DOI] [PubMed] [Google Scholar]
- 8.Fardal O, Lowenberg BF. A quantitative analysis of the migration, attachment, and orientation of human gingival fibroblasts to human dentinal root surfaces in vitro. J Periodontol. 1990;61:529–35. doi: 10.1902/jop.1990.61.8.529. [DOI] [PubMed] [Google Scholar]
- 9.Terranova VP, Franzetti LC, Hic S. A biochemical approach to periodontal regeneration: Tetracycline treatment of dentin promotes fibroblasts adhesion and growth. J Periodontal Res. 1986;21:330–7. doi: 10.1111/j.1600-0765.1986.tb01467.x. [DOI] [PubMed] [Google Scholar]
- 10.Jones WA, O’Leary TJ. The effectiveness of in vivo root planing in removing bacterial endotoxins from the roots of periodontally involved teeth. J Periodontol. 1978;49:337–42. doi: 10.1902/jop.1978.49.7.337. [DOI] [PubMed] [Google Scholar]
- 11.Leite F, Moreira C, Theodoro L. Blood cell attachment to root surfaces treated with EDTA gel. Braz Oral Res. 2005;19:88–92. doi: 10.1590/s1806-83242005000200003. [DOI] [PubMed] [Google Scholar]
- 12.Labahn R, Fahrenbach WH, Clark SM, Lie T, Adams DF. Root dentin morphology after different modes of citric acid and tetracycline hydrochloride conditioning. J Periodontol. 1992;63:303–9. doi: 10.1902/jop.1992.63.4.303. [DOI] [PubMed] [Google Scholar]
- 13.Laffertty T, Gher M, Gray J. Comparative SEM study on the effect of acid etching with tetracycline hydrochloride or citric acid on instrumented periodontally involved human root surfaces. J Periodontol. 1993;64:689. doi: 10.1902/jop.1993.64.8.689. [DOI] [PubMed] [Google Scholar]
- 14.Blomlof J, Blomlof LB, Lindskogs F. Smear layer formed in different root planing modalities and its removal by EDTA gel preparation. Int J Periodontics Restorative Dent. 1997;17:243–9. [PubMed] [Google Scholar]
- 15.Trombelli L. Effects of tetracycline hydrochloride on periodontally affected human root surfaces. J Periodontol. 1995;66:685–91. doi: 10.1902/jop.1995.66.8.685. [DOI] [PubMed] [Google Scholar]
- 16.Balos K, Bal B, Eren K. The effects of various agents on root surfaces: A scanning electron microscopic study. Newsl Int Acad Periodontol. 1991;1:13–21. [PubMed] [Google Scholar]
- 17.Mariotti A. Efficacy of chemical root surface modifiers in the treatment of periodontal disease: A Systematic Review. Ann Periodontol. 2003;8:205–26. doi: 10.1902/annals.2003.8.1.205. [DOI] [PubMed] [Google Scholar]
- 18.Mehta DS, Ashwini S. Comparative evaluation of surface alterations on periodontally diseased root subsequent to application of phosphoric acid, minocycline and combination of citric acid and tetracycline HCL- A Scanning electron microscopic study. J Indian Dent Assoc. 2001;72:10–14. [Google Scholar]
- 19.Sterrett J, Murphy H. Citric acid burnishing of dentinal root surfaces: A scanning electron microscopy report. J Clin Periodontol. 1989;16:98–104. doi: 10.1111/j.1600-051x.1989.tb01621.x. [DOI] [PubMed] [Google Scholar]
- 20.Baker P, Rotch H. An in vitro screening model to evaluate root conditioning protocols for periodontal regenerative procedures. J Periodontol. 2000;71:1139–43. doi: 10.1902/jop.2000.71.7.1139. [DOI] [PubMed] [Google Scholar]
- 21.Blomlof J, Blomlof LB, Lindskogs L. Smear removal and collagen exposure after non-surgical root planing followed by etching with EDTA gel preparation. J Periodontol. 1996;67:841–5. doi: 10.1902/jop.1996.67.9.841. [DOI] [PubMed] [Google Scholar]
- 22.Blomlof J, Bergman E, Forsgardh A, Foss L, Sjoberg A. A clinical study of root surface conditioning with an EDTA gel.I Non surgical periodontal treatment. Int J Periodontics Restorative Dent. 1998;20:561–5. [PubMed] [Google Scholar]
- 23.Blomlof J, Lindskog S. Root surface texture and early cell and tissue colonization after different etching modalities. Eur J Oral Sci. 1995;103:17–24. doi: 10.1111/j.1600-0722.1995.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 24.AY Enhanced β-tricalcium phosphate blended clot adhesion to EDTA biomodulated periodontally affected root surfaces: In vivo scanning electron microscopy evaluation. J Periodontol. 2011;82:1587–95. doi: 10.1902/jop.2011.110023. [DOI] [PubMed] [Google Scholar]
- 25.Frantz B, Polson A. Tissue interactions with dentin specimens after demineralization using tetracycline. J Periodontol. 1988;59:714–21. doi: 10.1902/jop.1988.59.11.714. [DOI] [PubMed] [Google Scholar]
- 26.Hanes PJ, O’Brien NJ, Garnick JJ. A morphological comparison of radicular dentin following root planing and treatment with citric acid or tetracycline HCI. J Clin Periodontol. 1971;18:660–8. doi: 10.1111/j.1600-051x.1991.tb00107.x. [DOI] [PubMed] [Google Scholar]
- 27.Leite AA, Sampajo JE, Zandim DL, Dantas AA, Leite ER, Leite AA. Influence of root surface conditioning with acid and chelating agents on clot adhesion. Quintessence Int. 2010;41:341–9. [PubMed] [Google Scholar]
- 28.Baker DL, Stanley Pavlow SA, Wikesio UM. Fibrin clot adhesion to dentin conditioned with protein constructs: An in vitro proof of principle study. J Clin Periodontol. 2005;32:561–6. doi: 10.1111/j.1600-051X.2005.00714.x. [DOI] [PubMed] [Google Scholar]
- 29.Nyman S, Lindhe J, Karring T. Healing following surgical treatment and root demineralization in monkeys with periodontal disease. J Clin Periodontol. 1981;8:249–58. doi: 10.1111/j.1600-051x.1981.tb02036.x. [DOI] [PubMed] [Google Scholar]
- 30.Gottlow J, Nyman S, Karring T. Healing following citric acid conditioning of roots implanted into bone and gingival connective tissue. J Periodontal Res. 1984;19:214–20. doi: 10.1111/j.1600-0765.1984.tb00811.x. [DOI] [PubMed] [Google Scholar]
- 31.Stahl SS, Froum S. Human clinical and histological repair responses following the use of citric acid in periodontal therapy. J Periodontol. 1984;48:261–8. doi: 10.1902/jop.1977.48.5.261. [DOI] [PubMed] [Google Scholar]
- 32.Stahl SS, Froum SJ, Kushner L. Healing responses of human intraosseous lesions following the use of debridement, grafting and citric acid root treatment. II. Clinical and histologic observations: One year post surgery. J Periodontol. 1983;54:325–38. doi: 10.1902/jop.1983.54.6.325. [DOI] [PubMed] [Google Scholar]
- 33.Smith BA, Smith JS, Kowalski CJ. Effect of citric acid and various concentrations of fibronectin on healing following periodontal flap surgery in dogs. J Periodontol. 1987;58:667–73. doi: 10.1902/jop.1987.58.10.667. [DOI] [PubMed] [Google Scholar]
- 34.Polson AM, Proye MP. Effect of root surface alterations on periodontal healing.II Citric acid treatment of the denuded root surface. J Clin Periodontol. 1982;9:441–54. doi: 10.1111/j.1600-051x.1982.tb02105.x. [DOI] [PubMed] [Google Scholar]
- 35.Garrett J, Crigger M, Egelberg J. Effects of citric acid on diseased root surfaces. J Periodontal Res. 1978;13:155–63. doi: 10.1111/j.1600-0765.1978.tb00164.x. [DOI] [PubMed] [Google Scholar]
- 36.Hanes PJ, Polson AM, Frederick T. Citric acid treatment of periodontitis affected cementum.A Scanning electron microscopic study. J Clin Periodontol. 1991;18:567–75. doi: 10.1111/j.1600-051x.1991.tb00090.x. [DOI] [PubMed] [Google Scholar]
- 37.Melcher AH. On the repair potential of periodontal tissues. J Periodontol. 1976;47:552–62. doi: 10.1902/jop.1976.47.5.256. [DOI] [PubMed] [Google Scholar]
- 38.Vandana D, Cherian G, George JP. Comparative evaluation of surface alterations on diseased roots subsequent to application of citric acid and oxytetracycline hydrochloride. J Indian Soc Periodontol. 1999;2:13. [Google Scholar]
- 39.Jeong SN, Han SB, Lee SW, Magnusson I. Effects of tetracycline-containing gel and a mixture of tetracycline and citric acid-containing gel on non-surgical periodontal therapy. J Periodontol. 1994;69:840–47. doi: 10.1902/jop.1994.65.9.840. [DOI] [PubMed] [Google Scholar]


