Abstract
Objectives:
The aim of this study was to evaluate the efficacy of subgingivally administered xanthan-based chlorhexidine gel when used in the maintenance phase following scaling and root planing (SRP) in the treatment of chronic periodontitis.
Materials and Methods:
A randomized, controlled, single-center study was conducted involving 92 sites in 46 systemically healthy patients suffering from moderate to advanced chronic periodontitis with isolated pockets. The selected sites were randomized to two treatment arms: Group A (SRP alone) and Group B (SRP + insertion of chlorhexidine gel after 1 month). The gingival index, plaque index, probing pocket depth (PPD) and clinical attachment level (CAL) were recorded at baseline and subsequently after 1 month and 3 months.
Results:
Both the groups showed significant reductions in PPD and CAL at both follow-up visits when compared with the baseline values (P<0.001).
Conclusions:
The results suggest that the application of xanthan based chlorhexidine gel following SRP in the maintenance phase might be beneficial in treatment of the chronic periodontitis in comparison to SRP alone. Greater improvements may be achieved when antimicrobial agents are used following SRP.
Keywords: Attachment loss, chlorhexidine gel, periodontitis, regeneration
INTRODUCTION
The periodontal pockets provide a warm, moist, nutritious and anaerobic environment for microbial colonization and multiplication.[1] Highly organized bacterial populations form the advancing front of periodontal pockets.[2] At present, the main therapeutic approaches for periodontal disease include mechanical scaling and root planning (SRP), which removes the deposits from the tooth surface and shifts the pathogenic microbiota to one compatible with periodontal health.[3,4] However, the pocket anatomy is a significant limiting factor in mechanical access, and sufficient reduction of the bacterial load may not be provided.[5] Surgical intervention provides accessibility to previously inaccessible root surfaces and helps in reduction or elimination of pockets along with production of healthy dentogingival junction. However, certain systemic conditions do not warrant the use of such invasive procedures. Moreover, the refractory and aggressive forms of disease require the use of most intensive and comprehensive therapeutic modalities. This has led to a more direct approach using antimicrobial agents as an integral part of therapeutic armamentarium, to achieve enhanced clinical outcomes.
Success of any antimicrobial agent depends on its ability to achieve bacteriostatic or bactericidal concentrations at the base of the pocket. It must also facilitate retention of medicament long enough in the pocket.[6] Systemic antibiotics require the administration of large doses in order to gain sufficient concentrations at the disease sites, and suffer from potential for the development of bacterial resistance, side-effects, drug interactions or inconsistent patient compliance.[7] The use of supra- and subgingival irrigation devices has proven to be ineffective due to the inability to obtain biologically significant concentrations of drug for sufficient lengths of time.[8] The inherent limitations of systemic or topical chemotherapies led to the development of local delivery systems for the administration of antimicrobials directly into the periodontal pockets. This form of therapy offers little or no systemic drug uptake, reduced risk of drug resistance, reduced side-effects and high concentrations at the targeted site.[6] Numerous studies have evaluated the effect of this treatment modality either during the initial phase[9] or during the maintenance phase of periodontal therapy.[10]
A new xanthan-based syringable gel system: CHLOSITE® (GHIMAS, Italy), has been introduced. This contains a unique combination of two chlorhexidine formulations: 0.5% chlorhexidine digluconate and 1.0% chlorhexidine dihydrochloride.
The aim of the present study was to evaluate the efficacy of CHLOSITE® when delivered subgingivally in stable pockets during the maintenance phase following the treatment of chronic periodontitis following SRP.
MATERIALS AND METHODS
Forty-six systemically healthy patients with moderate to advanced chronic periodontitis were selected for the study from the Department of Periodontics, D.A.V. (C) Dental College, Yamuna Nagar, India. Patients were selected according to the following criteria: men and women, 30-65 years of age, in good general health, patients having at least two non-adjacent interproximal sites (to avoid carry-across or spillover effects) with 5-8 mm pockets, diseased sites that bled on probing at initial visit and patients who had not received definitive periodontal therapy or any antibiotic therapy in the last 6 months. The following patients were excluded from the study: patients allergic to chlorhexidine, requiring periodontal surgery, with a history of smoking or who were current smokers or receiving chronic medications known to be associated with altered periodontal tissue growth or repair.
At the screening visit, the periodontal examination of all the eligible patients was carried out and impressions were taken for fabrication of acrylic stents. Acrylic stent was used to ensure reproducibility of measurements during the subsequent examinations. Based on this charting, the target sites were identified. The selected sites in each patient were randomized to two treatment groups:
Control Group (A): Scaling and root planing alone.
Test Group One (B): Scaling and root planing + insertion of chlorhexidine gel after 1 month.
At the baseline visit, an oral examination was undertaken and the following clinical parameters associated with the selected sites: plaque index,[11] gingival index,[12] probing depth (PD) and clinical attachment level (CAL) were measured using the UNC-15 (University of North Carolina) probe.
Treatment procedures
At the baseline visit, all the patients received supra- and subgingival SRP using hand and ultrasonic scalers and periodontal curettes. The patients were given oral hygiene instructions including twice-daily tooth brushing and interdental plaque control. No use of antimicrobial mouth rinses was allowed during the study period.
Following SRP, further evaluation of clinical parameters was carried out after 1 month and 3 months. No SRP procedures were performed in any of the selected sites at the recall visits. No dietary limitations were imposed during or after treatment. Normal oral hygiene procedures were permitted.
At the 1-month recall visit, the selected sites in Group B were irrigated with normal saline and, following measurement of clinical parameters, dried with paper points. CHLOSITE® was then applied directly from the syringe into the pocket. The gel was injected first into the deepest part of the pocket and then, while continuing to extrude the material, the needle was slowly withdrawn till it reached the superior portion of the pocket. The patients were instructed not to use floss or interdental aids for the next 10 days.
The xanthan-based chlorhexidine gel is supplied with a special needle having a blunt tip and a lateral opening. This facilitates the application of the gel without traumatizing or damaging the periodontal tissues. Xanthan is a naturally occurring, biocompatible saccharidic polymer that forms a three-dimensional pseudoplastic reticulum when in contact with water. Swelling-controlled erosional process allows for sustained release of the drug at zero-order kinetics from xanthan. Chlorhexidine digluconate is liberated in the first day, and achieves a concentration greater than 100 μg/mL. This concentration is maintained for an average of 6-9 days, and is much more than the minimum inhibitory concentration (MIC) for chlorhexidine (0.10 μg/mL). Chlorhexidine dihydrochloride is released in the following days, and maintains the bacteriostatic and bactericidal concentrations for at least 2 weeks and prevents re-colonization.
Data analysis
The statistical analysis was performed using the Student t-test for the unpaired observations, i.e. the inter-group comparison; and Paired t-test for the paired observations. Mean values and standard deviations were calculated for each variable and examination interval. Probabilities less than 0.05 (P<0.05) were considered significant.
RESULTS
Plaque indices and gingival indices
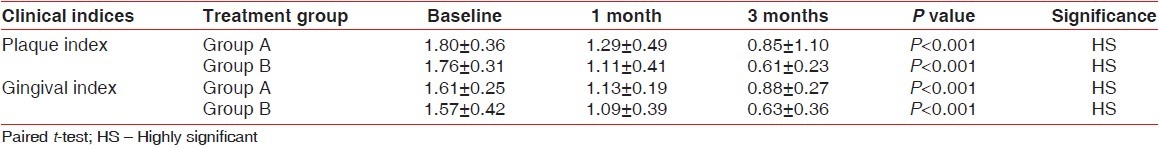
The mean changes overtime in plaque and gingival indices are given in Table 1. All the patients showed statistically and clinically significant improvements in gingival and plaque indices at both follow-up visits when compared with the baseline levels. The mean reduction in the plaque index score from baseline to 1 month and 3 months for group B was 0.65 and 1.15, respectively, and the mean reduction in the gingival index score for the same group was 0.48 and 0.94, respectively, which was highly significant (P<0.001). The control group showed higher index scores at the 3-month time interval.
Table 1.
Plaque and gingival indices at different time intervals
Probing pocket depth
Tables 2 and 3 show changes in Probing pocket depth (PPD) for each treatment group. At baseline, there was no statistically significant difference between the PPD of the selected sites. Both the groups showed a significant change in PPD after 1 and 3 months. The mean pocket depth reduction from baseline to 1 month and 3 months was found to be 0.87±0.62 mm and 1.22±0.73 mm, respectively, for Group A, which was highly significant (P<0.001) and 0.91±0.63 mm and 2.15±0.92 mm, respectively, for Group B, which was highly significant (P<0.001) [Figure 1].
Table 2.
Probing pocket depth and clinical attachment levels at different time intervals

Table 3.
Comparison of mean probing pocket depth reduction and mean clinical attachment gain at different time intervals
Figure 1.
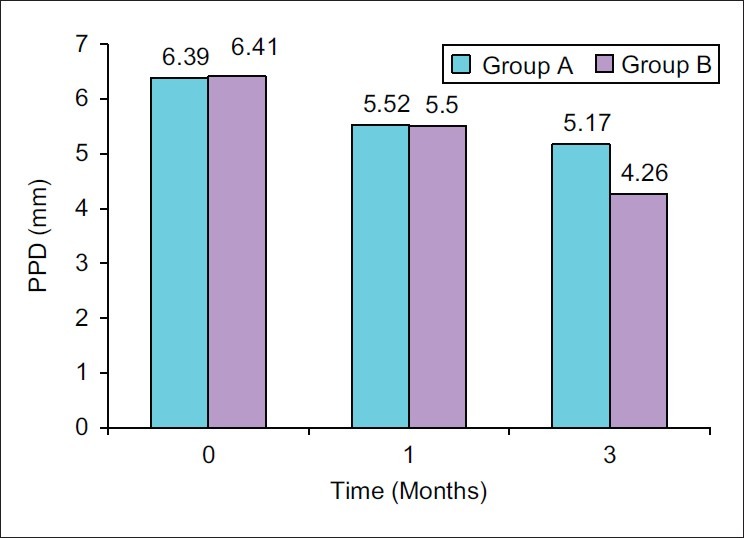
Mean probing pocket depth (PPD) at different time intervals
The mean pocket depth reduction, when compared from 1 month to 3 months, was higher in Group B (1.24±0.82) as compared with Group A (0.35±0.67), which was highly significant (P<0.001) [Figure 2].
Figure 2.
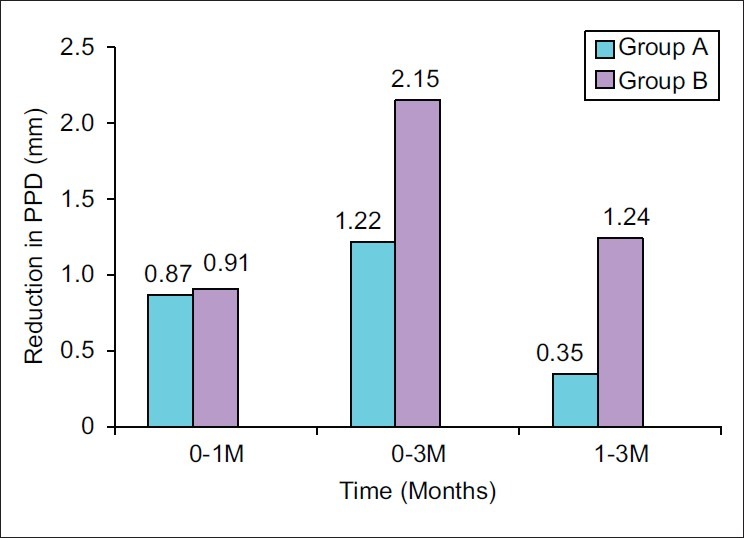
Mean probing pocket depth (PPD) reduction at different time intervals
Clinical attachment level
Alterations in Clinical attachment level (CAL) are reported in Tables 2 and 3. Statistically significant clinical attachment gains were reported for all the treatment groups at 1 month and 3 months. The mean clinical attachment gain from baseline to 1 month and 3 months was found to be 0.67±0.56 mm and 0.89±0.64 mm, respectively, for Group A, which was highly significant (P<0.001) and 0.67±0.60 mm and 1.52±0.72 mm, respectively, for Group B, which was highly significant (P<0.001) [Figure 3].
Figure 3.
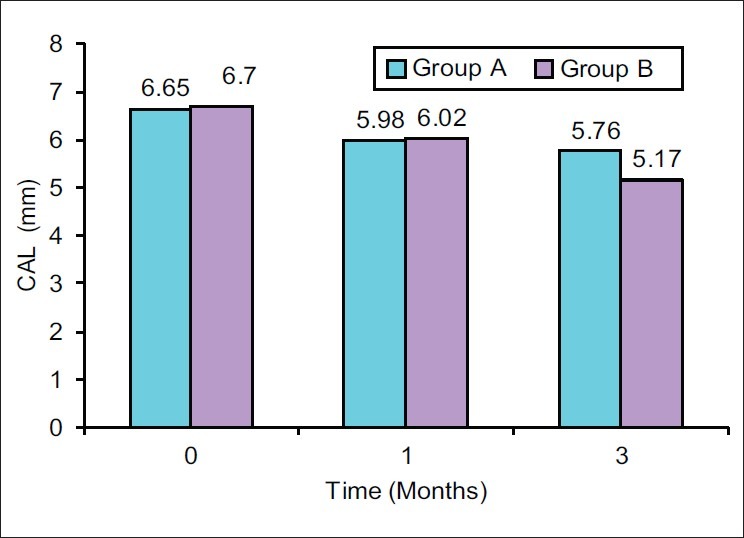
Mean clinical attachment level (CAL) at different time interval
The mean attachment gain, when compared from 1 month to 3 months, was higher in Group B (0.85±0.63) as compared with Group A (0.22±0.42), which was highly significant (P<0.001) [Figure 4].
Figure 4.
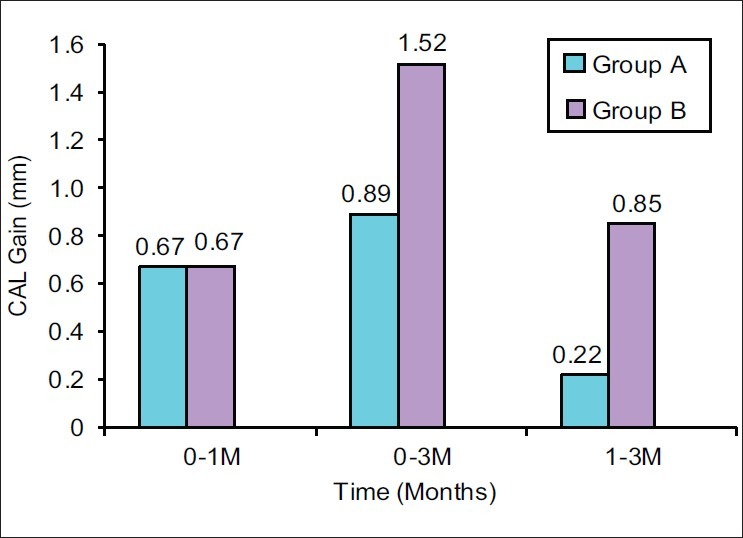
Mean clinical attachment level (CAL) gain at different time intervals
DISCUSSION
The results of this study show that the treatment of periodontal pockets with chlorhexidine in the maintenance phase provides a significantly greater improvement in clinical parameters when compared with the improvement obtained with SRP alone. No adverse effects were reported by the patients at any given time during the study duration.
The significant reductions observed in plaque and gingival scores from baseline could be attributed to the recording of these parameters before SRP at the baseline visit. Lower plaque and gingival index scores observed in the test group may also be the result of the antiplaque and antibacterial role of chlorhexidine that may have leaked out of the pockets.
Intergroup comparison showed that at the termination of the study, Group B had an additional pocket depth reduction of 0.93±0.16 mm over the control group, which is statistically significant. The additional pocket depth reduction in Group B could be attributed to the bactericidal concentrations achieved following administration of chlorhexidine gel at the selected sites, and these higher concentration levels were maintained for 2 weeks thereafter. As SRP was carried out at baseline, the sites in Group B could be assumed to be stable at the 1-month recall visit. The subsequent change in PPD, between the 1 month and 3 months interval, could be attributed to absence of microbial interference during the maturation phase of healing. Therefore, further improvement in PPD can be obtained following subgingival administration of antimicrobial agents after clinical parameters have been stabilized following SRP. This finding is supported by the studies conducted by Sosklone et al.[13] using PerioChip and by Steenberghe et al.[14] using minocycline-containing gel.
The results, however, are in contrast to the studies conducted by Azmak et al.,[15] Daneshmand et al.[16] and Grisi et al.[17] These studies failed to show any statistical improvement in the clinical parameters by the use of chlorhexidine chip along with SRP. However, Rusu et al.[18] observed a reduction of around 4 mm in the PPDs over a period of 1 month, following insertion of xanthan-based chlorhexidine gel, which is also used in the present study. Soskolne et al.[19] reported that eight of 10 patients showed a reduction of 3 mm over a maintenance phase of 24 months after insertion of chlorhexidine chip. In comparison, only one patient had pockets showing a similar reduction following routine maintenance. Improved results with xanthan-based gel have been reported by Dinca et al.,[20] Rajan et al.,[21] Paolantonio et al.,[22] Kranti et al.[23] and Vaidya et al.[24]
The overall gain in CAL was similar in Group B at the 1-month interval. The application of gel at 1 month in Group B produced additional small positive changes, which were statistically significant when compared with Group A at 3 months. This could be attributed to the absence of bacterial challenge during the phase of healing following SRP.
The reason for not achieving optimal improvement in the attachment level in the test group as compared with changes in pocket depth may be due to the presence of gel during the stages of healing, which may have provided the mechanical interference or may be due to gingival recession seen in some of the cases. Similar effects were seen by Unsal et al.[25] using gel in their study, Grisi et al.[17] using PerioChip and Cosyn et al.[5] using varnish.
CONCLUSION
The results in the present study thus favor the use of combination therapy over SRP in order to achieve greater pocket depth reduction and greater attachment level gain. The local drug delivery is effective in improving the clinical parameters even after 1 month following SRP, when the pockets are stabilized.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Slots J, Rams TE. Antibiotics in periodontal therapy: Advantages and disadvantages. J Clin Periodontol. 1990;17:473–9. doi: 10.1111/j.1365-2710.1992.tb01220.x. [DOI] [PubMed] [Google Scholar]
- 2.Rams TE, Slot J. Local delivery of antimicrobial agents in the periodontal pockets. Periodontol 2000. 1996;10:139–59. doi: 10.1111/j.1600-0757.1996.tb00072.x. [DOI] [PubMed] [Google Scholar]
- 3.Ramjford S, Caffesse R, Morrison E. Four modalities of periodontal treatment compared over five years. J Clin Periodontol. 1987;14:445–52. doi: 10.1111/j.1600-051x.1987.tb02249.x. [DOI] [PubMed] [Google Scholar]
- 4.Claffey N, Nylund K, Kiger R, Garrett S, Egelberg J. Diagnostic predictability of scores of plaque, bleeding, suppuration and probing depth for probing attachment loss. 3.5 years observation following initial periodontal therapy. J Clin Periodontol. 1990;17:108–14. doi: 10.1111/j.1600-051x.1990.tb01071.x. [DOI] [PubMed] [Google Scholar]
- 5.Cosyn J, Wyn I, Rouck TD. A chlorhexidine varnish implemented treatment strategy for chronic periodontitis. J Clin Periodontol. 2005;32:750–6. doi: 10.1111/j.1600-051X.2005.00751.x. [DOI] [PubMed] [Google Scholar]
- 6.Goodson JM. Pharmacokinetic principles controlling the efficacy of oral therapy. J Dent Res. 1989;68:1625–32. [Google Scholar]
- 7.Somayaji EF, Jariwala U, Jayachandran P. Evaluation of antimicrobial efficacy and release pattern of tetracycline and metronidazole using a local delivery system. J Periodontol. 2002;69:409–13. doi: 10.1902/jop.1998.69.4.409. [DOI] [PubMed] [Google Scholar]
- 8.Greenstein G. Effects of subgingival irrigation on periodontal status. J Periodontol. 1987;58:827–36. doi: 10.1902/jop.1987.58.12.827. [DOI] [PubMed] [Google Scholar]
- 9.Jeffcoat MK, Bray KS, Ciancio SG, Dentino AR, Fine DH, Gordon JM, et al. Adjunctive use of a subgingival controlled release chlorhexidine chip reduces probing depth and improves attachment level compared with scaling and root planing alone. J Periodontol. 1998;69:989–97. doi: 10.1902/jop.1998.69.9.989. [DOI] [PubMed] [Google Scholar]
- 10.Reip B, Purucker P, Bernimoulin JP. Repeated local metronidazole therapy as adjunct to scaling and root planing in maintenance patients. J Clin Periodontol. 1999;26:710–5. doi: 10.1034/j.1600-051x.1999.t01-2-261101.x. [DOI] [PubMed] [Google Scholar]
- 11.Silness P, Loe H. Periodontal disease in pregnancy. Acta Odontol Scand. 1964;22:21–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 12.Loe H. The gingival index, the plaque index and the retention index systems. J Periodontol. 1967;38:610–6. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 13.Soskolne WA, Proskin HM, Stabholz A. Probing depth changes following 2 years of periodontal maintenance therapy including adjunctive controlled release of chlorhexidine. J Periodontol. 2003;74:420–7. doi: 10.1902/jop.2003.74.4.420. [DOI] [PubMed] [Google Scholar]
- 14.Van Steenberghe D, Rosling B, Soder PO. A 15 months evaluation of the effects of repeated subgingival minocycline in chronic adult periodontitis. J Periodontol. 1999;70:657–67. doi: 10.1902/jop.1999.70.6.657. [DOI] [PubMed] [Google Scholar]
- 15.Azmak Z, Atilla G, Luoto H. The effect of subgingival controlled release delivery of chlorhexidine chip on clinical parameters and matrix Metalloproteinase-8 levels in gingival crevicular fluid. J Periodontol. 2002;73:608–15. doi: 10.1902/jop.2002.73.6.608. [DOI] [PubMed] [Google Scholar]
- 16.Daneshmand N, Jorgensen MG, Nowzari H, Morrison JL, Slots J. Initial effect of controlled release chlorhexidine on subgingival microorganisms. J Periodontal Res. 2002;37:375–9. doi: 10.1034/j.1600-0765.2002.01003.x. [DOI] [PubMed] [Google Scholar]
- 17.Grisi DC, Salvador SL, Figueiredo LC. Effect of a controlled release chlorhexidine chip on clinical and microbiological parameters of periodontal syndrome. J Clin Periodontol. 2002;29:875–81. doi: 10.1034/j.1600-051x.2002.291001.x. [DOI] [PubMed] [Google Scholar]
- 18.Rusu D, Benta A, Necker A. Non-surgical periodontal therapy using a novel chlorhexidine based xanthan gel: A split mouth study. Int Poster J Dent Oral Med. 2005;7:Poster–286. [Google Scholar]
- 19.Soskolne WA, Heasman PA, Stabholz A. Sustained local delivery of chlorhexidine in the treatment of periodontitis: A multi center study. J Periodontol. 1997;68:32–8. doi: 10.1902/jop.1997.68.1.32. [DOI] [PubMed] [Google Scholar]
- 20.Dinca A. Periodontal Non-surgical Therapy with the Chlorhexidine Xanthan-based Gel Chlositeâ: A Randomized Split-mouth Study on 51 Cases. Int Poster J Dent Oral Med. 2007;9:Poster–341. [Google Scholar]
- 21.Gupta R, Pandit N, Aggarwal S, Verma A. Comparative evaluation of subgingivally delivered 10% doxycycline hyclate and xanthan-based chlorhexidine gels in the treatment of chronic periodontitis. J Contemp Dent Pract. 2008;9:25–32. [PubMed] [Google Scholar]
- 22.Paolantonio M, D’Ercole S, Pilloni A, D’Archivio D, Lisanti L, Graziani F, Femminella B, et al. Clinical, microbiologic, and biochemical effects of subgingival administration of a Xanthan-based chlorhexidine gel in the treatment of periodontitis: A randomized multicenter trial. J Periodontol. 2009;80:1479–92. doi: 10.1902/jop.2009.090050. [DOI] [PubMed] [Google Scholar]
- 23.Kranti K, Hema S, Sameer Z. Clinical evaluation of topical subgingival application of biodegradable xanthan based 1.5% Chlorhexidine gel for treatment of periodontal pockets. J Adv Dent Res. 2010;1:48. [Google Scholar]
- 24.Vaidya P, Thomas M, Dixit A. Treatment of Chronic Periodontitis using Chlorhexidine Gel as an Adjunct to Scaling and Root Planing - A Clinical Study. J Indian Dent Association. 2011;5:962–5. [Google Scholar]
- 25.Unsal E, Akkaya M, Walsh TF. Influence of a single application of subgingival chlorhexidine gel or tetracycline paste on the clinical parameters of adult Periodontitis patients. J Clin Periodontol. 1994;21:351–5. doi: 10.1111/j.1600-051x.1994.tb00725.x. [DOI] [PubMed] [Google Scholar]


