Abstract
Aims:
The present study was undertaken to evaluate the effectiveness of the combination of hydroxyapatite and β-tricalcium phosphate bone alloplast with bioresorbable guided tissue regeneration membrane for the treatment of mandibular grade II furcation defects.
Settings and Design:
A total of eight patients, four females and four males, in the age group of 18 to 65 years, with bilateral buccal grade II furcation defects in the mandibular molars, participated in the study.
Materials and Methods:
The following clinical measurements were recorded at baseline as well as three and six months post surgery: The Turesky-Gilmore-Glickman modification of the Quigley Hein plaque index, the Loe and Silness gingival index, Relative Clinical Attachment Level Vertical Probing Depth in the mid-furcation area, and Horizontal Probing Depth in the mid-furcation area.
Statistical analysis:
Pairwise comparisons within the groups were done by applying the independent student t test. Comparisons were also drawn between the test and the control groups by applying the independent student t test.
Results:
The mean gain in the relative clinical attachment levels in the test and control groups, at the end of six months, were 2.50 and 1.63 mm, respectively. The mean change in the horizontal probing depth values at the end of six months in the test and control groups were 2.88 and 1.63 mm, respectively. The mean reduction in the vertical probing depth values in the test and control groups were 1.50 and 1.38 mm, respectively.
Conclusions:
The resorbable GTR membrane with bone material was more effective than open debridement alone, in the treatment of furcation defects.
Keywords: GTR, Grade II furcation, Hydroxyapatite, resorbable membrane, β-tricalcium phosphate
INTRODUCTION
The management of furcation involvement presents one of the greatest challenges in periodontal therapy. Furcation-involved molar teeth respond less favorably to conventional periodontal therapy, and molars are lost more often than any other tooth type.[1]
Among the factors that make molars particularly susceptible to periodontal disease include accumulation of bacterial plaque, as a result of difficult access to oral hygiene procedures. Access to the furcation areas is further complicated by the posterior location of the molars, the disparity between root and furcation anatomies, and the shape and dimension of the debriding instruments.[2] Root debridement is consequently very difficult and inefficient in furcations.
Roughly over the past 10 years, the outcomes have changed in part because of the new knowledge about the disease process and wound healing, and in part because of the availability of new materials.[3] Recent data clearly show that regeneration of the previously destroyed periodontal attachment tissues is biologically possible, and regeneration has become the goal of therapy since the 1990s. Use of osteoconductive and osteoinductive graft materials, under favorable conditions, can induce roughly 60 to 70% regeneration of the bone lesion's height or volume, with concomitant improvement in the clinical conditions.[4]
A variety of calcium phosphate ceramics have become available as artificial grafting materials for the restoration of periodontal osseous defects, including β-tricalcium phosphate and hydroxyapatite. These materials have been shown to be osteoconductive, that is, they can promote the growth of bone into areas that they would not normally occupy.[5]
Regeneration by grafting may be further enhanced by the use of barrier membranes that exclude gingival fibroblasts and epithelium from the healing site. It has also been shown that the guided tissue regeneration procedure, using membranes, holds promise for increasing the success of bone grafting.[5] However, if we consider a furcation closure as the main end-point of furcation therapy, then the results obtained with guided tissue regeneration have been found to be inconsistent.
Therefore, it was postulated that combining osseous grafting with guided tissue regeneration may enhance the response to membrane-only therapy, with bone restoration via the conductive effects of the graft, and supporting the membrane to a more optimal position in selective sites.[6]
With the premise of possible synergism of combining osseous grafting and barrier membrane placement, management of grade II furcation defects via a combination therapy was assessed for feasibility and evidence of predictability. The present study was therefore undertaken to evaluate the effectiveness of a combination of hydroxyapatite and β-tricalcium phosphate bone alloplast with a bioresorbable GTR membrane for the treatment of mandibular grade II furcation defects.
MATERIALS AND METHODS
A total of eight patients, four females and four males, in the age group of 18 - 65 years with bilateral buccal grade II furcation defects in the mandibular molars participated in the study. The criteria for selection were: no complicated medical history, no use of antibiotics or anti-inflammatory drugs in the last six months, and radiographic evidence of bilateral furcation defects in the mandibular molars.
The subjects received detailed information regarding their condition and the treatment plan. Oral hygiene instructions were given. After being informed about the aim of the project, a signed consent was taken from the patient.
Clinical data collection
The following clinical measurements were recorded at baseline as well as at three and six months post surgery — Plaque index (PI) — Turesky-Gilmore-Glickman modification of Quigley Hein[7] plaque index, Gingival index (GI) — Loe and Silness gingival index,[8] relative clinical attachment level (CAL) in millimeters - from the groove on the stent to the base of the pocket, Vertical probing depth, in millimeters, in the mid-furcation area (VPD) [Figures 1 and 2], and Horizontal probing depth in millimeters in the mid-furcation area (HPD) [Figures 3 and 4]. One site in each patient was randomly allocated to the test group and was treated by GTR and Hydroxyapatite bone grafting. The GTR membrane used was ProGideTM (Equinox). The contralateral site was treated using open flap debridement (OFD) alone. The test and the control sites were chosen with the toss of a coin.
Figure 1.

Presurgical vertical measurement at the control site with the stent
Figure 2.

Presurgical vertical measurements at the test site with the stent
Figure 3.
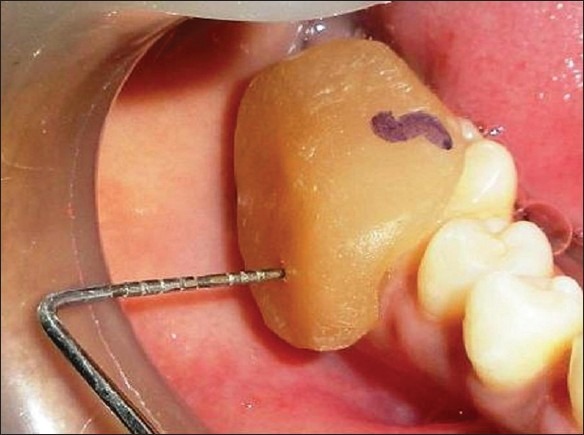
Presurgical horizontal measurement at the control site with the stent
Figure 4.

Presurgical horizontal measurements at the test site with the stent
Presurgical procedure
Prior to the surgical procedure, thorough scaling and root planing were performed. The subjects were recalled after four to six weeks for surgery. Intraoral periapical radiovisiographs were taken at both the test and control sites [Figures 5 and 6]. This was carried out at the baseline and at six months postoperatively. Two occlusal stents, one of clear resin and the other of pink auto polymerizing resin were fabricated by the sprinkle-on method for both the test and control sites.[9] Clinical measurements were obtained using a UNC-15 periodontal probe at the initial visit.
Figure 5.

Pre operative IOPA of the test site
Figure 6.

Six months postoperative IOPA of the test site
Surgical management
Four to six weeks after scaling and root planing and just prior to the surgical procedure, each subject was re-examined and baseline data were recorded. After local anesthesia (2% xylocaine, 1:200000), a sulcular incision was given on the buccal and lingual aspect of one tooth distal and mesial to the involved tooth. Full thickness mucoperiosteal flap (envelope) was then elevated by blunt dissection using a periosteal elevator [Figures 7 and 8].[10]
Figure 7.

Furcation defect upon flap elevation (control)
Figure 8.

Furcation defect upon flap elevation (test)
The granulation tissue was removed in the furcation defect and thorough debridement was carried out with curettes and an ultrasonic scaler, to ensure a clean site for incorporation of the bone graft material and membrane. The appropriate amount of the bone graft was taken in a container and transferred to a sterilized dappendish, to which a few drops of saline were added. The contents were then mixed with the blunt instrument and transferred to the defect with a plastic filling instrument and condensed.
Before placing the membrane at the test site, a sterile surgical template was applied and approximated for extensions and trimmed accordingly. After the defect was filled with the bone graft [Figure 9], the membrane was removed from the sterile package and was compared with the surgical template and reduced to the template dimensions. The membrane was soaked in normal saline solution to improve its adhesion properties as recommended by the manufacturer. It was subsequently adapted over the defect extending 2 - 3 mm apical to the crest of the existing bone, so as to provide a broad base during the placement. The coronal portion of the membrane was tightly secured to the cementoenamel junction (CEJ) of the tooth, with chromic catgut 5-0 sutures [Figure 10]. The flap was secured with direct loop interrupted black silk 4-0 sutures to obtain primary closure [Figures 11 and 12].
Figure 9.

Furcation defect with the bone graft (test)
Figure 10.

Furcation defect with the GTR membrane in place (test)
Figure 11.

Flap sutured at the desired position (control)
Figure 12.

Flap sutured at the desired position (test)
The patients were seen at the end of the first week when the sutures were removed and were instructed to use 10 ml of 0.2% chlorhexidine mouth wash twice daily for six weeks. They were asked not to brush their teeth in the surgical area for a period of three weeks. Clinical measurements were assessed at baseline, and three- and six-month intervals [Figures 13–16].
Figure 13.

Six months postsurgical vertical measurements at the control site with the stent
Figure 16.

Six months postsurgical horizontal measurements at the test site with the stent
Figure 14.

Six months postsurgical horizontal measurements at the control site with the stent
Figure 15.
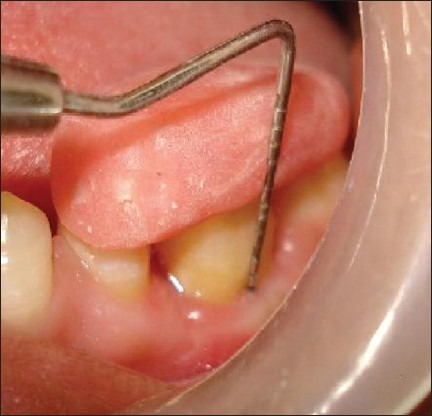
Six months postsurgical vertical measurements at the test site with the stent
Data analysis
Pairwise comparisons within the groups were done by applying the independent student t test. Comparisons were also drawn for the changed mean plaque and gingival scores, relative clinical attachment levels, horizontal probing values, and the vertical probing values between the test (GTR + BG) and the control (OFD) groups by applying the independent student t test.
RESULTS
All the eight subjects completed the study. No adverse event of any kind occurred during the course of the study. Membrane exposure was not seen in any of the cases.
The mean change in the gingival scores for both the test and the control groups were 1.38 and 1.38, respectively, at the end of six months, with a difference of 0.00, which was not statistically significant (P>0.05). The mean changes in the plaque scores for both the test and the control groups were 2.06 and 2.31, respectively, at the end of six months, with a difference of 0.25, which was not statistically significant (P>0.05) [Table 1].
Table 1.
Changes in gingival and plaque scores

The mean gain in the relative clinical attachment levels in the test and control groups at the end of six months were 2.50 mm and 1.63 mm, respectively, with a difference of 0.87 mm, which was statistically significant (P<0.05) [Table 2].
Table 2.
Changes in clinical attachment level in millimeters

The mean reduction in vertical probing depth values in the test and control groups were 1.50 mm and 1.38 mm, respectively, with a difference of 0.12 mm, which was not statistically significant (P>0.05) [Table 3].
Table 3.
Changes in vertical probing depth in millimeters

The mean change in horizontal probing depth values at the end of six months in the test and control groups were 2.88 mm and 1.63 mm, respectively, with a difference of 1.25 mm, which was statistically significant (P<0.05) [Table 4].
Table 4.
Changes in horizontal probing depth in millimeters*

DISCUSSION
The introduction of resorbable membrane materials brings clear advantages in the clinical management of guided tissue regeneration procedures: mainly, in the avoidance of a second surgical intervention, and thus, prevention from exposure of the newly formed tissue underneath the membrane.
In this study, selection of mandibular grade II defects was done based on the observation by Sanz and Givannoli,[11] who stated that, "placement of a barrier membrane should not be indicated in the treatment of maxillary molars with furcation involvement." In a study conducted by Metzler et al,[12] the GTR procedure showed limited application as a therapeutic modality for Class II furcations of the maxillary molars. Both vertical and horizontal attachment gains were of the magnitude of within 1 mm, and in none of the cases was the furcation closed or any significant difference between the guided tissue regeneration and open flap debridement seen.
All measurements were made using the UNC-15 probe, with the help of a custom-made acrylic stent, which served as a fixed reference point. The theoretical advantage of the stent system was demonstrated in the study by Sivertson and Burgett.[13]
The bone graft material and the bioabsorbable collagen membrane used in the study appeared to be biocompatible and safe. Membrane exposure was not observed in any of the cases in the study. This may be explained by the biocompatibility of the collagen membrane and its hemostatic and chemotactic functions.[14] The hemostatic function enhances early wound stabilization and clot formation. This indirectly promotes better flap adaptation, thus resulting in less membrane exposure. Furthermore, the chemotactic function of the collagen membrane promotes fibroblast migration that ensures primary wound coverage.
The mean gingival and plaque scores were significantly reduced at the end of three months and six months in both the test and the control groups. Comparison between the groups did not show any statistical difference in the mean gingival and plaque scores at the end of three months and at the end of six months. This demonstrates that the oral hygiene compliance of the subjects in both the groups, over the observation period, was statistically significant.
The mean relative clinical attachment values between the test and the control groups at the baseline were not statistically significant. The mean gain in the relative clinical attachment level in the test group was statistically significant at the end of three months and also at the end of six months. On the other hand, the mean gain in clinical attachment in the control group was not statistically significant at the end of either three or six months. The mean gain in the relative attachment levels in test group at the end of six months was 2.50 mm. Houser et al[15] showed a mean difference of 2.0 mm after using an organic bovine bone xenograft with resorbable membranes, which was similar to the reduction obtained in this study. However, Lekovic et al[16] demonstrated that the use of the bone graft did not enhance the effect of the membrane with respect to the level of clinical attachment in furcation defects.
The mean vertical probing depth values at the mid furcation area between the test and control groups at the baseline were not statistically significant. Between the two groups, the mean reduction in the vertical probing depth in the test group was 1.50 mm and in the control group it was 1.38 mm, which failed to reach statistical significance. This was less favorable than the conclusion from the systematic review reported by Murphy and Gunsolley,[17] which showed that GTR had more reduction in vertical probing depth compared to the Open Flap Debridement (OFD) controls. However, the vertical probing depth data obtained in this study was in agreement with the studies of Andersson et al,[18] Couri et al,[19] Cury et al[20] and Tsao et al,[21] which demonstrated that the changes in the vertical probing depth at the furcation sites between the baseline and six months were not statistically significant in all the groups.
The primary response variable in the treatment of furcation defects is the attachment level in the horizontal direction. Both the treatment modalities resulted in the reduction of horizontal probing depth values. These results were in agreement with Lekovic et al[16] and Anderegg et al.[4] However, combined therapy did not yield a significant change in the horizontal probing depth values in the studies conducted by and Calongne et al[22] and Palioto et al.[23]
The clinical effects of GTR therapy include reduction of furcation involvement from grade II to grade I. These changes reflect reduction of the horizontal inter-radicular probe penetration. The reason for this effect can either be the formation of new connective tissue attachment or of a long junctional epithelium between the root surfaces and the newly formed dense soft tissues. A radiographic examination revealed that no complete furcation closure was achieved in any of the defect sites. However, reduction in the mean horizontal probing depth suggested a considerable change from grade II to grade I in all of the defects studied.
Quantitative radiographic evaluation was not undertaken in the present study; however, comparisons between the initial and six-month postoperative radiographs revealed better radiographic bone level improvement in the GTR group, in which an increased radio-opacity around the furcation area appeared as a pattern, at the six-month postoperative evaluation.
Furcation involvement is probably the most difficult type of defect to standardize. Along with the variables associated with the osseous defect itself, aspects associated with the tooth, and more specifically with furcation morphology,[24] obviously play a significant role in the outcome of GTR.[25] The results obtained in this study confirm that many variables may render the treatment of grade II furcation defects unpredictable.
CONCLUSIONS
The following conclusions were drawn from the study:
The resorbable collagen membrane had excellent handling characteristics and biological acceptance.
The use of the collagen membrane and the bone material was not associated with any local or systemic adverse reaction or clinically detectable allergic reaction.
The resorbable collagen GTR membrane could be effectively used in the treatment of human grade II furcation defects.
The resorbable GTR membrane with bone material was more effective in the treatment of furcation defects than open debridement alone.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.McFall W. Tooth loss in 100 treated patients with periodontal disease. J Periodontol. 1982;53:539–49. doi: 10.1902/jop.1982.53.9.539. [DOI] [PubMed] [Google Scholar]
- 2.Loos B, Nylund K, Claffey N, Egelberg J. Clinical effect of root debridement in molar and non-molar teeth.A 2-year follow-up. J Clin Periodontol. 1989;16:498–504. doi: 10.1111/j.1600-051x.1989.tb02326.x. [DOI] [PubMed] [Google Scholar]
- 3.Bernard GW. Healing and repair of osseous defects. Dent Clin North Am. 1991;35:469–77. [PubMed] [Google Scholar]
- 4.Yukna RA, Harrison BG, Caudill RF, Evans GH, Mayer ET, Miller S. Evaluation of durapatite ceramica as an alloplastic implant in periodontal osseous defects. II. Twelve-month reentry results. J Periodontol. 1985;56:540–7. doi: 10.1902/jop.1985.56.9.540. [DOI] [PubMed] [Google Scholar]
- 5.Anderegg CR, Martin SJ, Gray JL, Mellonig JT, Gher ME. Clinical Evaluation of the Use of Decalcified Freeze-Dried Bone Allograft with Guided Tissue Regeneration in the Treatment of Molar Furcation Invasions. J Periodontol. 1991;62:264–8. doi: 10.1902/jop.1991.62.4.264. [DOI] [PubMed] [Google Scholar]
- 6.Pamela K, McClain PK, Schallhorn RG. Focus on furcation defects-guided tissue regeneration in combination with bone grafting. Periodontol 2000. 2000;22:190–212. doi: 10.1034/j.1600-0757.2000.2220112.x. [DOI] [PubMed] [Google Scholar]
- 7.Turesky S, Gilmore ND, Glickman I. Reduced plaque formation by the chloromethyl analogue of vitamin C. J Periodontol. 1970;41:41–3. doi: 10.1902/jop.1970.41.41.41. [DOI] [PubMed] [Google Scholar]
- 8.Loe H, Silness J. Periodontal disease in pregnancy. Acta Odontol Scand. 1963;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 9.Clark DC, Chin Quee T, Bergeron MJ, Chan EC, Lemay C, de Grucy K. Reliability of attachment measurements using the cementoenamel junction and a plastic stent. J Periodontol. 1987;58:115–8. doi: 10.1902/jop.1987.58.2.115. [DOI] [PubMed] [Google Scholar]
- 10.Carranza FA, McClain P, Schallhorn R. Regenerative Osseous Surgery. In: Carranza FA, Newman MG, editors. Clinical Periodontology. 9th ed. Philadelphia: W.B. Saunders Co; 1996. pp. 804–24. [Google Scholar]
- 11.Sanz M, Givannoli JL. Focus on furcation defects: Guided tissue regeneration. Periodontol 2000. 2000;22:169–89. doi: 10.1034/j.1600-0757.2000.2220111.x. [DOI] [PubMed] [Google Scholar]
- 12.Metzler DG, Seamons BD, Mellonig JT, Gher ME, Gray JL. Clinical evaluation of guided tissue regeneration in the treatment of maxillary class II molar furcation invasions. J Periodontol. 1991;62:353–60. doi: 10.1902/jop.1991.62.6.353. [DOI] [PubMed] [Google Scholar]
- 13.Sivertson JF, Burgett FG. Probing of pockets related to the attachment level. J Periodontol. 1976;47:281–6. doi: 10.1902/jop.1976.47.5.281. [DOI] [PubMed] [Google Scholar]
- 14.Bunyaratavej P, Wang HL. Collagen membranes: A review. J Periodontol. 2001;72:215–29. doi: 10.1902/jop.2001.72.2.215. [DOI] [PubMed] [Google Scholar]
- 15.Houser BE, Mellonig JT, Brunsvold MA, Cochran DL, Meffert RM, Alder ME. Clinical evaluation of anorganic bovine bone xenograft with a bioabsorbable collagen barrier in the treatment of molar furcation defects. Int J Periodontics Restorative Dent. 2001;21:161–9. [PubMed] [Google Scholar]
- 16.Lekovic V, Kenney EB, Carranza FA, Danilovic V. Treatment of Class II Furcation Defects Using Porous Hydroxylapatite in Conjunction with a Polytetrafluoroethylene Membrane. J Periodontol. 1990;61:575–8. doi: 10.1902/jop.1990.61.9.575. [DOI] [PubMed] [Google Scholar]
- 17.Murphy KG, Gunsolley JC. Guided tissue regeneration for the treatment of periodontal intrabony and furaction defects.A systematic review. Ann Periodontol. 2003;8:266–302. doi: 10.1902/annals.2003.8.1.266. [DOI] [PubMed] [Google Scholar]
- 18.Andersson B, Bratthall G, Kullendorff B, Grondahl K, Rohlin M, Attstrom R. Treatment of furcation defects.Guided tissue regeneration versus coronally positioned flap in mandibular molars; a pilot study. J Clin Periodontol. 1994;21:211–6. doi: 10.1111/j.1600-051x.1994.tb00306.x. [DOI] [PubMed] [Google Scholar]
- 19.Couri CJ, Maze GI, Hinkson DW, Collins BH III, Dawson DV. Medical grade calcium sulfate hemihydrates versus expanded polytetrafluoroethylene in the treatment of mandibular Class II furcations. J Periodontol. 2002;73:1352–9. doi: 10.1902/jop.2002.73.11.1352. [DOI] [PubMed] [Google Scholar]
- 20.Cury PR, Sallum PA, Nociti FH, Jr, Nociti FH, Jr, Sallum AW, Jeffcoat MK. Long term results of guided tissue regeneration therapy in the treatment of class II furcation defects: A randomized clinical trial. J Periodontol. 2003;74:3–9. doi: 10.1902/jop.2003.74.1.3. [DOI] [PubMed] [Google Scholar]
- 21.Tsao YP, Neiva R, Al-Shammari K, Oh TJ, Wang HL. Effects of a mineralized human cancellous bone allograft in regeneration of mandibular Class II furcation defects. J Periodontol. 2006;77:416–25. doi: 10.1902/jop.2006.050109. [DOI] [PubMed] [Google Scholar]
- 22.Calongne KB, Aichelmann-Reidy ME, Yukna RA, Mayer ET. Clinical comparison of microporous biocompatible composite of PMMA, PHEMA and calcium hydroxide grafts and expanded polytetrafluoroethylene barrier membranes in human mandibular molar Class II furcations.A case series. J Periodontol. 2001;72:1451–9. doi: 10.1902/jop.2001.72.10.1451. [DOI] [PubMed] [Google Scholar]
- 23.Palioto DB, Joly JC, de Lima AF, Mota LF, Caffesse R. Clinical and radiographic treatment evaluation of class III furcation defects using GTR with and without inorganic bone matrix. J Clin Periodontol. 2003;30:1–8. doi: 10.1034/j.1600-051x.2003.300101.x. [DOI] [PubMed] [Google Scholar]
- 24.Gher ME, Vernino AR. Root morphology-clinical significance in pathogenesis and treatment of periodontal disease. J Am Dent Assoc. 1980;101:627–33. doi: 10.14219/jada.archive.1980.0372. [DOI] [PubMed] [Google Scholar]
- 25.Matia J, Bissada N, Maybury J, Ricchetti P. Efficiency of scaling the molar furcation area with and without surgical access. Int J Periodontics Restorative Dent. 1986;5:25–35. [PubMed] [Google Scholar]


