Abstract
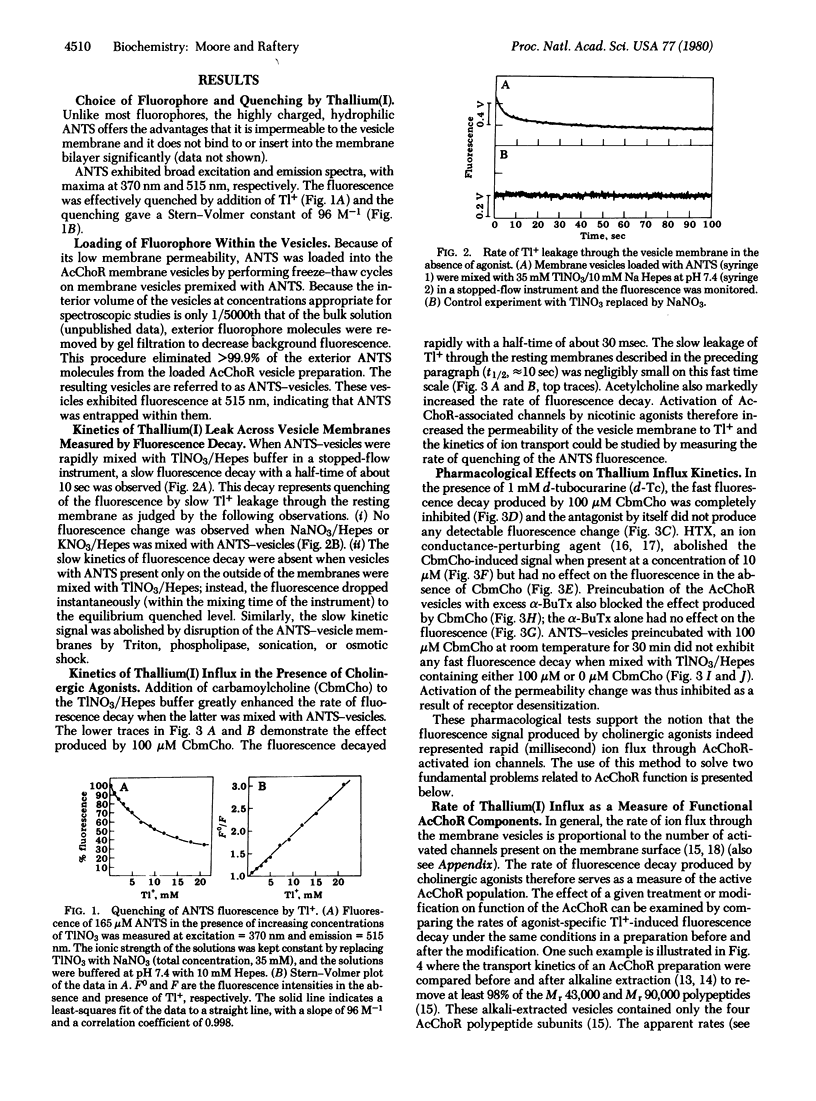
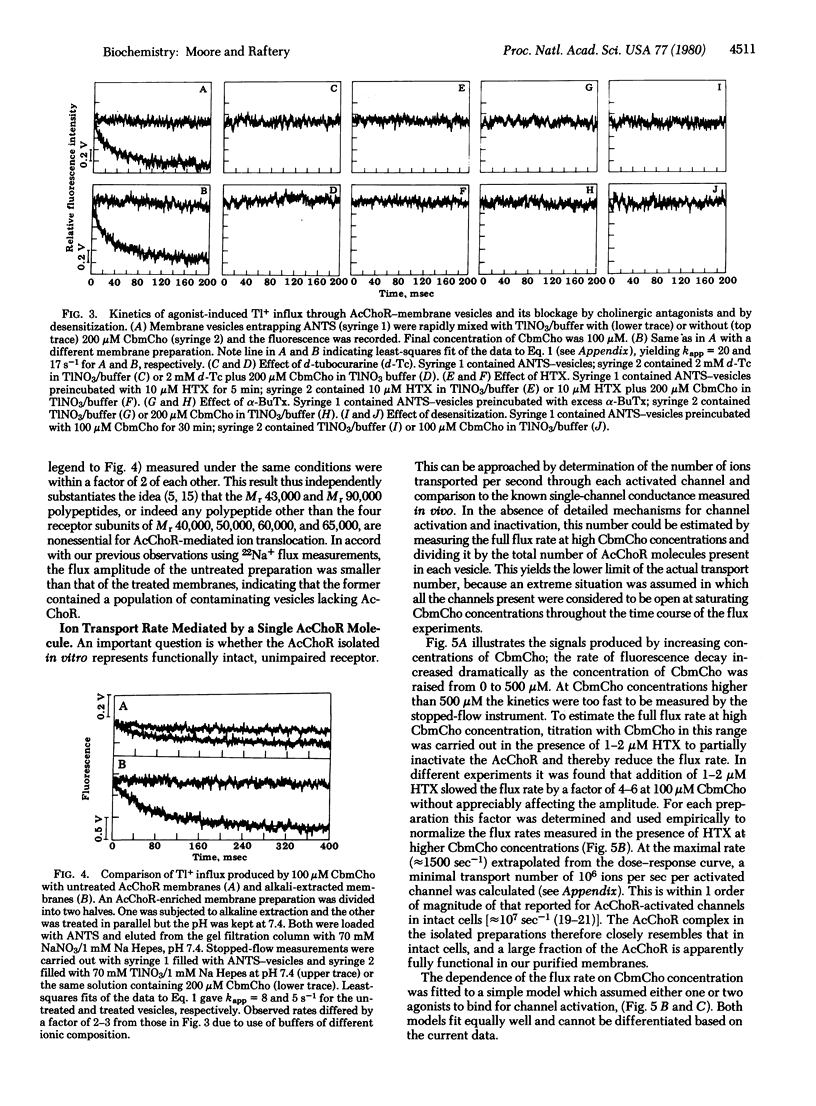
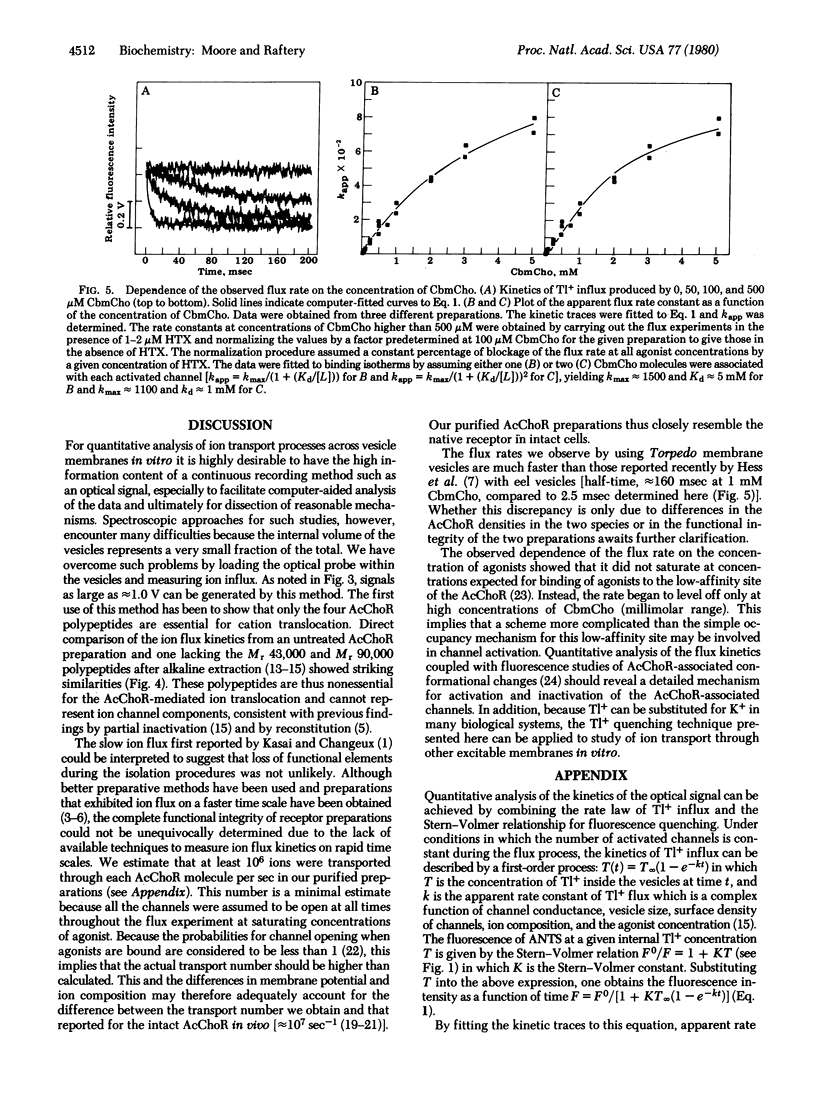
The kinetics of carbamoylcholine-mediated cation transport across the membrane of vesicles containing acetylcholine receptor have been measured on the physiologically relevant time scale of a few milliseconds. The stopped-flow spectroscopic approach utilizes thallium(I) as the cation transported into sealed vesicles containing a water-soluble fluorophore. Upon entry of thallium(I), fluorescence quenching occurs by a heavy atom effect. Rapid thallium translocation into the vesicles is mediated by cholinergic agonists and is blocked by antagonists and neurotoxins and by desensitization. The kinetics of thallium transport are used to demonstrate that the four polypeptides known to comprise the receptor are the only protein components necessary for cation translocation. The kinetics of thallium(I) transport at saturating agonist concentrations are also used to calculate the apparent ion transport rate for a single receptor. The minimal value obtained is close to that for a single activated channel determined in vivo. This demonstrates that the physiological receptor has been isolated in intact form.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albuquerque E. X., Barnard E. A., Chiu T. H., Lapa A. J., Dolly J. O., Jansson S. E., Daly J., Witkop B. Acetylcholine receptor and ion conductance modulator sites at the murine neuromuscular junction: evidence from specific toxin reactions. Proc Natl Acad Sci U S A. 1973 Mar;70(3):949–953. doi: 10.1073/pnas.70.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt J., Neumann E. Kinetic analysis of receptor-controlled tracer efflux from sealed membrane fragments. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3756–3760. doi: 10.1073/pnas.75.8.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne V. E., Steinbach J. H., Stevens C. F. An analysis of the dose-response relationship at voltage-clamped frog neuromuscular junctions. J Physiol. 1978 Aug;281:421–444. doi: 10.1113/jphysiol.1978.sp012431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliot J., Raftery M. A. Interactions of perhydrohistrionicotoxin with postsynaptic membranes. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1347–1353. doi: 10.1016/s0006-291x(77)80127-7. [DOI] [PubMed] [Google Scholar]
- Elliott J., Blanchard S. G., Wu W., Miller J., Strader C. D., Hartig P., Moore H. P., Racs J., Raftery M. A. Purification of Torpedo californica post-synaptic membranes and fractionation of their constituent proteins. Biochem J. 1980 Mar 1;185(3):667–677. doi: 10.1042/bj1850667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess G. P., Cash D. J., Aoshima H. Acetylcholine receptor-controlled ion fluxes in membrane vesicles investigated by fast reaction techniques. Nature. 1979 Nov 15;282(5736):329–331. doi: 10.1038/282329a0. [DOI] [PubMed] [Google Scholar]
- Hess G. P., Lipkowitz S., Struve G. E. Acetylcholine-receptor-mediated ion flux in electroplax membrane microsacs (vesicles): change in mechanism produced by asymmetrical distribution of sodium and potassium ions. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1703–1707. doi: 10.1073/pnas.75.4.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester H. A. The response to acetylcholine. Sci Am. 1977 Feb;236(2):106-16, 118. doi: 10.1038/scientificamerican0277-106. [DOI] [PubMed] [Google Scholar]
- MULLINS L. J., MOORE R. D. The movement of thallium ions in muscle. J Gen Physiol. 1960 Mar;43:759–773. doi: 10.1085/jgp.43.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. L., Moore H. P., Hartig P. R., Raftery M. A. Fast cation flux from Torpedo californica membrane preparations: implications for a functional role for acetylcholine receptor dimers. Biochem Biophys Res Commun. 1978 Nov 29;85(2):632–640. doi: 10.1016/0006-291x(78)91209-3. [DOI] [PubMed] [Google Scholar]
- Moore H. P., Hartig P. R., Raftery M. A. Correlation of polypeptide composition with functional events in acetylcholine receptor-enriched membranes from Torpedo californica. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6265–6269. doi: 10.1073/pnas.76.12.6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore H. P., Hartig P. R., Wu W. C., Raftery M. A. Rapid cation flux from Torpedo californica membrane vesicles: comparison of acetylcholine receptor enriched and selectively extracted preparations. Biochem Biophys Res Commun. 1979 May 28;88(2):735–743. doi: 10.1016/0006-291x(79)92109-0. [DOI] [PubMed] [Google Scholar]
- Neubig R. R., Krodel E. K., Boyd N. D., Cohen J. B. Acetylcholine and local anesthetic binding to Torpedo nicotinic postsynaptic membranes after removal of nonreceptor peptides. Proc Natl Acad Sci U S A. 1979 Feb;76(2):690–694. doi: 10.1073/pnas.76.2.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel E., Potter L. T. Ultrastructure of isolated membranes of Torpedo electric tissue. Brain Res. 1973 Jul 27;57(2):508–517. doi: 10.1016/0006-8993(73)90158-3. [DOI] [PubMed] [Google Scholar]
- Peper K., Dreyer F., Sandri C., Akert K., Moor H. Structure and ultrastructure of the frog motor endplate. A freeze-etching study. Cell Tissue Res. 1974 Jun 24;149(4):437–455. doi: 10.1007/BF00223024. [DOI] [PubMed] [Google Scholar]
- Quast U., Schimerlik M. I., Raftery M. A. Ligand-induced changes in membrane-bound acetylcholine receptor observed by ethidium fluorescence. 2. Stopped-flow studies with agonists and antagonists. Biochemistry. 1979 May 15;18(10):1891–1901. doi: 10.1021/bi00577a007. [DOI] [PubMed] [Google Scholar]
- Quast U., Schimerlik M., Lee T., Witzemann T. L., Blanchard S., Raftery M. A. Ligand-induced conformation changes in Torpedo californica membrane-bound acetylcholine receptor. Biochemistry. 1978 Jun 13;17(12):2405–2414. doi: 10.1021/bi00605a024. [DOI] [PubMed] [Google Scholar]
- Raftery M. A., Hunkapiller M. W., Strader C. D., Hood L. E. Acetylcholine receptor: complex of homologous subunits. Science. 1980 Jun 27;208(4451):1454–1456. doi: 10.1126/science.7384786. [DOI] [PubMed] [Google Scholar]
- Wu W. C., Raftery M. A. Carbamylcholine-induced rapid cation efflux from reconstituted membrane vesicles containing purified acetylcholine receptor. Biochem Biophys Res Commun. 1979 Jul 12;89(1):26–35. doi: 10.1016/0006-291x(79)90938-0. [DOI] [PubMed] [Google Scholar]



