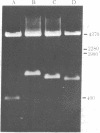
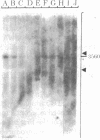
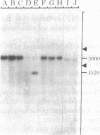
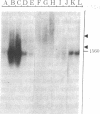
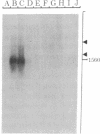
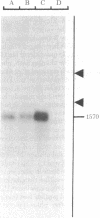
Abstract
To study the role of low-abundance, embryonic muscle-specific gene transcripts, we have developed a method to screen cDNA clones from embryonic muscle for such sequences. The protocol involves two stages: first, partial enrichment for cDNA clones carrying possible embryo-specific sequences by selecting clones of low-abundance sequences; and second, determination, by hybridization to RNA attached to diazobenzyloxymethyl-paper, which sequences from this category are regulated in an embryonic muscle-specific manner during development. At least three different clones were obtained which hybridized to sequences present in early muscle development but absent, or present at relatively low levels, at late embryonic and adult muscle stages. Two of these clones were not muscle-specific because they hybridized to poly(A)+RNA from liver or brain or both. The third clone, 106A4, did not detectably hybridize to total poly(A)+RNA at any stage of brain or liver development tested. This sequence also was not detectable in poly(A)+RNA from embryonic muscle progenitor cells. Thus, the 106A4 sequence is a likely candidate for an embryonic muscle-specific sequence. We have demonstrated that the 106A4 sequence is a mRNA, although the specific identity and function of the translated product is unknown. The method used to identify embryonic muscle-specific cDNA clones should be generally applicable for obtaining clones for low abundance transcripts regulated in a tissue-specific or developmental stage-specific manner.
Full text
PDF
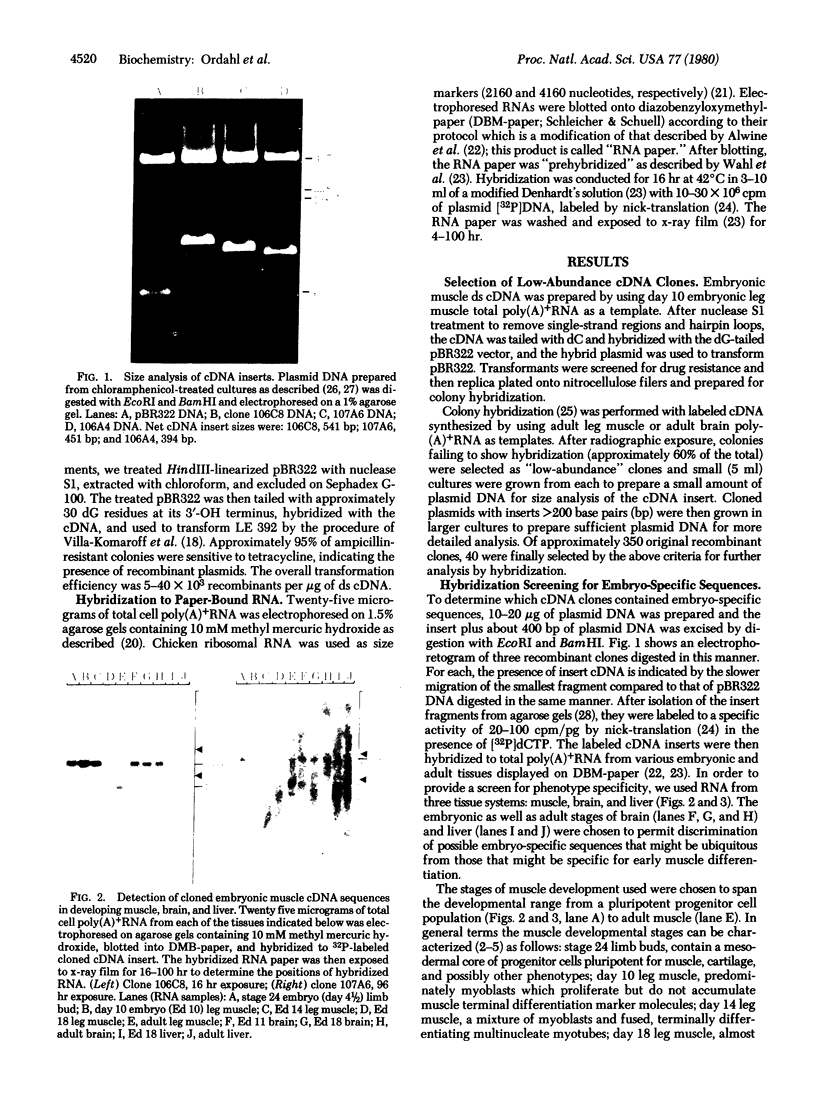

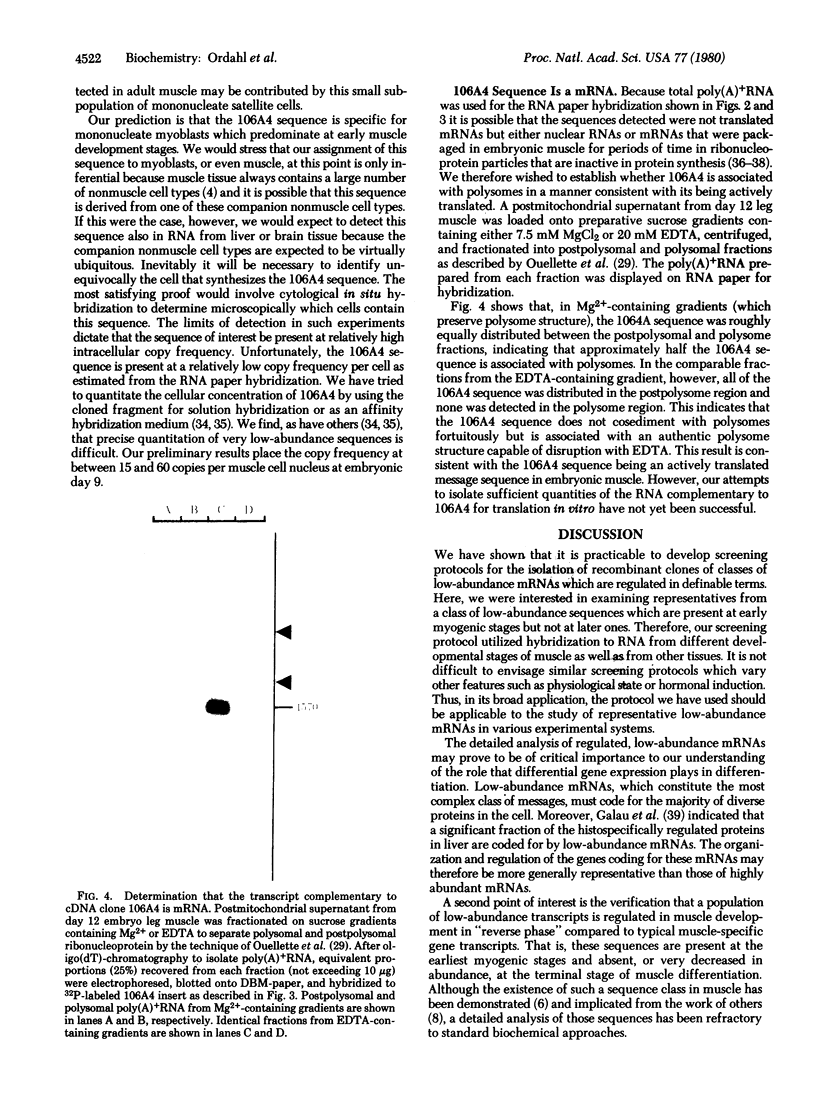

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahrens P. B., Solursh M., Reiter R. S., Singley C. T. Position-related capacity for differentiation of limb mesenchyme in cell culture. Dev Biol. 1979 Apr;69(2):436–450. doi: 10.1016/0012-1606(79)90303-8. [DOI] [PubMed] [Google Scholar]
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Bonner P. H., Hauschka S. D. Clonal analysis of vertebrate myogenesis. I. Early developmental events in the chick limb. Dev Biol. 1974 Apr;37(2):317–328. doi: 10.1016/0012-1606(74)90152-3. [DOI] [PubMed] [Google Scholar]
- Buckingham M. E., Cohen A., Gros F. Cytoplasmic distribution of pulse-labelled poly(A)-containing RNA, particularly 26 S RNA, during myoblast growth and differentiation. J Mol Biol. 1976 May 25;103(3):611–626. doi: 10.1016/0022-2836(76)90220-5. [DOI] [PubMed] [Google Scholar]
- Caplan A. I., Ordahl C. P. Irreversible gene repression model for control of development. Science. 1978 Jul 14;201(4351):120–130. doi: 10.1126/science.351805. [DOI] [PubMed] [Google Scholar]
- Caravatti M., Perriard J. C., Eppenberger H. M. Developmental regulation of creatine kinase isoenzymes in myogenic cell cultures from chicken. Biosynthesis of creatine kinase subunits M and B. J Biol Chem. 1979 Feb 25;254(4):1388–1394. [PubMed] [Google Scholar]
- Devlin R. B., Emerson C. P., Jr Coordinate accumulation of contractile protein mRNAs during myoblast differentiation. Dev Biol. 1979 Mar;69(1):202–216. doi: 10.1016/0012-1606(79)90286-0. [DOI] [PubMed] [Google Scholar]
- Galau G. A., Klein W. H., Britten R. J., Davidson E. H. Significance of rare m RNA sequences in liver. Arch Biochem Biophys. 1977 Mar;179(2):584–599. doi: 10.1016/0003-9861(77)90147-3. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann H., Heywood S. M., Marchok A. C. Reconstruction of muscle development as a sequence of macromolecular synthesis. Curr Top Dev Biol. 1970;5:181–234. [PubMed] [Google Scholar]
- Heywood S. M., Kennedy D. S., Bester A. J. Stored myosin messenger in embryonic chick muscle. FEBS Lett. 1975 Apr 15;53(1):69–72. doi: 10.1016/0014-5793(75)80684-3. [DOI] [PubMed] [Google Scholar]
- John H. A., Patrinou-Georgoulas M., Jones K. W. Detection of myosin heavy chain mRNA during myogenesis in tissue culture by in vitro and in situ hybridization. Cell. 1977 Oct;12(2):501–508. doi: 10.1016/0092-8674(77)90126-x. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibovitch M. P., Leibovitch S. A., Harel J., Kruh J. Changes in the frequency and diversity of messenger RNA populations in the course of myogenic differentiation. Eur J Biochem. 1979 Jul;97(2):321–326. doi: 10.1111/j.1432-1033.1979.tb13117.x. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchok A. C., Herrmann H. Studies of muscle development. I. Changes in cell proliferation. Dev Biol. 1967 Feb;15(2):129–155. doi: 10.1016/0012-1606(67)90010-3. [DOI] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Meagher R. B., Tait R. C., Betlach M., Boyer H. W. Protein expression in E. coli minicells by recombinant plasmids. Cell. 1977 Mar;10(3):521–536. doi: 10.1016/0092-8674(77)90039-3. [DOI] [PubMed] [Google Scholar]
- Meyuhas O., Perry R. P. Construction and identification of cDNA clones for mouse ribosomal proteins: application for the study of r-protein gene expression. Gene. 1980 Jul;10(2):113–129. doi: 10.1016/0378-1119(80)90129-8. [DOI] [PubMed] [Google Scholar]
- Noyes B. E., Mevarech M., Stein R., Agarwal K. L. Detection and partial sequence analysis of gastrin mRNA by using an oligodeoxynucleotide probe. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1770–1774. doi: 10.1073/pnas.76.4.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordahl C. P., Caplan A. I. High diversity in the polyadenylated RNA populations of embryonic myoblasts. J Biol Chem. 1978 Nov 10;253(21):7683–7691. [PubMed] [Google Scholar]
- Ordahl C. P., Caplan A. I. Transcriptional diversity in myogenesis. Dev Biol. 1976 Nov;54(1):61–72. doi: 10.1016/0012-1606(76)90286-4. [DOI] [PubMed] [Google Scholar]
- Ouellette A. J., Kumar A., Malt R. A. Physical aspects and cytoplasmic distribution of messenger RNA in mouse kidney. Biochim Biophys Acta. 1976 Apr 2;425(4):384–395. doi: 10.1016/0005-2787(76)90002-2. [DOI] [PubMed] [Google Scholar]
- Paterson B. M., Bishop J. O. Changes in the mRNA population of chick myoblasts during myogenesis in vitro. Cell. 1977 Nov;12(3):751–765. doi: 10.1016/0092-8674(77)90275-6. [DOI] [PubMed] [Google Scholar]
- Rowe D. W., Moen R. C., Davidson J. M., Byers P. H., Bornstein P., Palmiter R. D. Correlation of procollagen mRNA levels in normal and transformed chick embryo fibroblasts with different rates of procollagen synthesis. Biochemistry. 1978 May 2;17(9):1581–1590. doi: 10.1021/bi00602a001. [DOI] [PubMed] [Google Scholar]
- Roychoudhury R., Jay E., Wu R. Terminal labeling and addition of homopolymer tracts to duplex DNA fragments by terminal deoxynucleotidyl transferase. Nucleic Acids Res. 1976 Jan;3(1):101–116. doi: 10.1093/nar/3.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohman R. C., Moss P. S., Micou-Eastwood J., Spector D., Przybyla A., Paterson B. Messenger RNA for myosin polypeptides: isolation from single myogenic cell cultures. Cell. 1977 Feb;10(2):265–273. doi: 10.1016/0092-8674(77)90220-3. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. pBR322 restriction map derived from the DNA sequence: accurate DNA size markers up to 4361 nucleotide pairs long. Nucleic Acids Res. 1978 Aug;5(8):2721–2728. doi: 10.1093/nar/5.8.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilghman S. M., Kioussis D., Gorin M. B., Ruiz J. P., Ingram R. S. The presence of intervening sequences in the alpha-fetoprotein gene of the mouse. J Biol Chem. 1979 Aug 10;254(15):7393–7399. [PubMed] [Google Scholar]
- Villa-Komaroff L., Efstratiadis A., Broome S., Lomedico P., Tizard R., Naber S. P., Chick W. L., Gilbert W. A bacterial clone synthesizing proinsulin. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3727–3731. doi: 10.1073/pnas.75.8.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens M. P., Buell G. N., Schimke R. T. Synthesis of double-stranded DNA complementary to lysozyme, ovomucoid, and ovalbumin mRNAs. Optimization for full length second strand synthesis by Escherichia coli DNA polymerase I. J Biol Chem. 1978 Apr 10;253(7):2483–2495. [PubMed] [Google Scholar]