Abstract
AML patients under the age of 60 whose blasts harbor a FLT3 internal tandem duplication (ITD) mutation have a higher relapse rate and inferior survival compared to those without this mutation. To determine if FLT3ITD also carries a negative prognostic impact in older adults receiving therapies commonly used in this age group, we retrospectively analyzed outcomes of patients ≥60 years with CN-AML according to FLT3 mutation status. We identified 91 newly diagnosed CN-AML patients, 55 with wild-type FLT3 and 36 with FLT3ITD. Of the 91 patients, 36 received supportive care and/or experimental therapies while the remaining 55 received induction chemotherapy, followed by allogeneic SCT in 17 of these patients. Based on univariate analysis, advanced age at diagnosis was significantly associated with shorter overall survival (OS) (p < .0001) while intensive therapies were associated with improved OS (p < .0001). In a multivariate analysis that accounted for type of treatment, patient age, gender, and WBC count, FLT3ITD was significantly associated with shorter OS compared to wtFLT3 [p = .001; hazard ratio (HR) = 2.23; 95% CI: 1.35–3.70]. Our data support the negative prognostic impact of FLT3ITD in older adults with CN-AML.
Keywords: FLT3ITD, AML, Elderly patients, Dose intensive therapy
1. Introduction
Acute myeloid leukemia (AML) is an intrinsically resistant myeloid malignancy that occurs in older adults, with a median age at diagnosis of 67 years [1]. Survival depends upon age, cytogenetics, primary or secondary nature of disease, and the presence of mutations within molecules such as FLT3 and nucleophosmin1 (NPM1). Age is the most important prognostic factor for AML, and adults over age 60 have a significantly shorter OS compared to adults under age 60 [2]. Karyotype and mutation analyses are used both for prognosis and therapeutic guidance in young adults with AML [2–4]. Patients over 60 generally have a poor prognosis but cytogenetics and genetic mutations can identify a small number of patients who might fare better with standard chemotherapy [5–7].
Approximately 40–50% of patients have normal cytogenetics (CN-AML). Within this group of patients, mutations in molecules such as FLT3, CEBPA, NPM1, and others provide additional prognostic information and suggest distinct outcomes among patients carrying mutations within these genes, particularly in adults <60 [2]. Their impact on the prognosis for those >60 was less clear until recently [7,8].
We focused our analysis on clinical outcomes according to mutational state of the fms-like tyrosine kinase 3 (FLT3) receptor, which is mutated in 30–40% of patients with AML [9]. ITD within the juxtamembrane domain of the FLT3 receptor represents one of the most common mutations found in AML. FLT3ITD is associated with an inferior disease free and overall survival in patients younger than 60 years and a higher WBC, LDH, and peripheral blood and bone marrow blast count compared to patients with wtFLT3 [10,11]. The ratio of wt to mutant FLT3 transcript as well as the location of the ITD also may have prognostic significance.
Most data concerning the relationship of FLT3 and prognosis are based on studies in patients less than age 60; the relevance of this acquired mutation in older adults is relatively unclear [12–17].
We analyzed the prognostic significance of FLT3ITD in patients ≥60 with newly diagnosed de novo or secondary CN-AML seen at our institutions over a seven year period and treated with a variety of regimens typically used to treat this population of patients. Our findings indicate that FLT3ITD is independently associated with inferior overall survival in older adults with AML.
2. Materials and methods
2.1. Patients
We retrospectively reviewed the outcomes of all newly diagnosed AML patients who presented to the Dana-Farber Cancer Institute/Brigham and Women’s Hospital and Massachusetts General Hospital between 2002 and 2008 who underwent testing for FLT3 mutations. Patients had consented to an IRB approved research protocol prior to obtaining samples for FLT3 analysis. All patients, without bias, suspected of having AML were offered testing for FLT3 mutations. Treatment decisions were made and treatment initiated before the FLT3 mutation status was known. In each case, treatment was determined as a consequence of discussions between the patient and physician and incorporated physician recommendation and patient preference. We analyzed all patients who had a normal karyotype, were ≥60 years and for whom pretreatment FLT3 mutation status was known. CR rates, OS and type of treatment received were assessed by IRB-approved medical record review.
2.2. Mutation analysis
FLT3 mutations, ITD sequence and allelic ratio, were determined as previously described [18]. NPM1 mutation status is not available for this cohort of patients.
2.3. End points and definitions
Complete remission (CR) was defined by the presence of less than 5% blasts in normocellular bone marrow showing trilineage maturation with an absolute neutrophil count of more than 1000/ul and a platelet count of more than 100,000/ul [19].
Overall survival was defined from the time of diagnosis of AML to the date of death or censored on the last known alive date if patients were still alive.
Treatment groups were categorized according to three broad methods by which older adults with AML are treated at our centers:
Supportive care and/or experimental therapy (n = 36): patients treated with transfusion support, anti-infectives, and low dose or clinical trial therapies not involving cytotoxic chemotherapy.
Induction chemotherapy group (n = 38): patients treated with standard induction chemotherapy such as an anthracycline with cytarabine or similar chemotherapy intended to achieve a CR after one or two cycles.
Allogeneic transplant group (n = 17): patients who received an allogeneic SCT at any time point in their treatment course. All patients in this group received induction chemotherapy. Additional treatment details are described in the supplementary information.
2.4. Statistics
Patient clinical characteristics were summarized as numbers and percentages for categorical variables and median and range for continuous variables. The primary endpoint for this study was OS. Median OS is summarized using the Kaplan–Meier method. A Cox proportional hazard model was used for assessing the associations of FLT3ITD, other clinical variables such as gender, age at diagnosis, WBC at diagnosis and types of treatment received in both univariate and multivariable analysis. Fisher’s exact test and Wilcoxon’s rank sum test were used to assess the associations between categorical and continuous variables and FLT3 mutation, respectively. Note that for the model, we categorized the continuous variables WBC and age at cutoff points described in Section 3. Allelic ratio was also categorized as high and low based on a cut point identified by recursive partitioning. Median follow up was calculated using the reverse Kaplan–Meier method.
3. Results
3.1. Patients and treatment
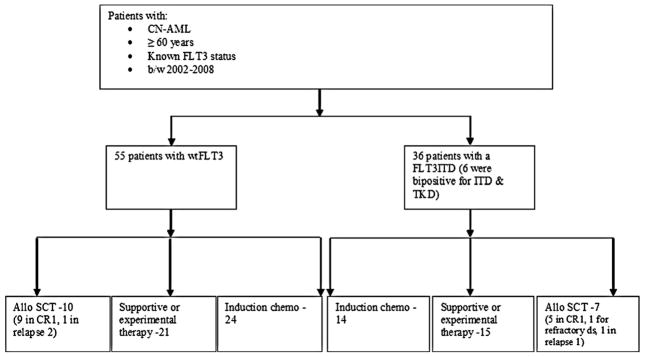
There were a total of 91 patients who were 60 years or older, had a normal karyotype and either a wtFLT3 or FLT3ITD at diagnosis [wtFLT3 (n = 55) and FLT3ITD (n = 36)]. Six patients with a FLT3ITD also had a FLT3TKD mutation and were included in the ITD group because such patients appear to behave in a biologically similar fashion to those with an isolated FLT3ITD mutation [17,20,21].
There were 36 patients who received experimental/supportive therapies and 38 patients who received induction chemotherapy without allogeneic SCT. Of these 38 patients, 15/24 with wtFLT3 and 5/14 with FLT3ITD received at least one cycle of consolidation. Patients with resistant disease, early relapse or serious infectious complications after CR were unable to receive consolidation chemotherapy. Lastly, there were 17 patients who received induction chemotherapy and who later underwent allogeneic SCT. Of these 17 patients, 10 had a wtFLT3 while the remaining 7 patients had a FLT3ITD. In the wtFLT3 group, 9/10 patients received the SCT in CR1 while 1 patient received SCT during second relapse. In the FLT3ITD group, 5/7 patients received an allogeneic SCT in CR1, 1 patient in setting of relapse disease, and 1 patient for primarily refractory disease. All patients received reduced intensity conditioning with the exception of the 1 patient in the FLT3ITD group with refractory disease, who received an ablative conditioning regimen (Fig. 1).
Fig. 1.
Distribution of therapy received according to FLT3 mutation status.
Considering all patients who received induction chemotherapy (Induction chemotherapy group + Allogeneic transplant group), CR rates were similar between wtFLT3 (28 of 34) and FLT3ITD (15 of 21 patients) groups (82.3% and 71.4%, respectively; p = .33). The distribution of type of therapy received in either patient group (wtFLT3 and FLT3ITD) is depicted in Fig. 1 and was not significantly different between the two groups (p = .91) (Table 1A).
Table 1A.
Clinical characteristics according to FLT3 mutation status.
| ITD | Wild type | p-Values | |
|---|---|---|---|
| Age | .86 | ||
| <70 | 18 | 29 | |
| 70–80 | 15 | 20 | |
| ≥80 | 3 | 6 | |
| WBC | .0003 | ||
| <20 | 12 | 42 | |
| 20–40 | 9 | 7 | |
| ≥40 | 14 | 6 | |
| Gender | .51 | ||
| Female | 17 | 21 | |
| Male | 19 | 34 | |
| Treatment type | .91 | ||
| Supportive care/experimental therapy | 15 | 21 | |
| Induction chemotherapy | 14 | 24 | |
| Allogeneic transplant | 7 | 10 |
We assessed the relationship between clinical characteristics at diagnosis and FLT3 mutation status. FLT3ITD was associated with elevated WBC (p = .0003), elevated serum LDH (p = .003) and higher peripheral and bone marrow blast percentage at presentation (p < .0001). There were no differences in age, gender distribution, and other clinical parameters including platelet count, prothrombin time, and partial thromboplastin time between patients with wtFLT3 and FLT3ITD (Tables 1A and 1B). Rates of secondary AML were similar between the two groups of patients (38.2% for wtFLT3 group and 33.33% for FLT3ITD group; p = .66).
Table 1B.
Clinical characteristics according to FLT3 mutation status.
| ITD
|
Wild Type
|
p-Values | |||
|---|---|---|---|---|---|
| N | Median (q1, q3) | N | Median (q1, q3) | ||
| Peripheral blasts | 33 | 57 (24, 75) | 53 | 5 (0, 15) | <.0001 |
| Platelets | 34 | 85 (48, 162) | 55 | 64 (32, 122) | .13 |
| PT (s) | 28 | 15 (14.26, 16.7) | 46 | 14.4 (13.9, 15.9) | .11 |
| PTT (s) | 28 | 31.6 (28.25) | 46 | 31.65 (28.3, 36.4) | .65 |
| LDH | 28 | 990 (601, 1570.5) | 47 | 586 (316, 816) | .003 |
| BM blasts (%) | 30 | 78.5 (57, 88) | 55 | 42 (33, 62) | <.0001 |
3.2. FLT3ITD is associated with worse overall survival in multivariable analysis
Advanced age at diagnosis was associated with poor OS (p < .0001), while both of intensive therapy groups (induction chemotherapy and allogeneic transplant) were associated with a better OS compared with supportive/experimental therapies in univariate analysis (p < .0001) (Table 2). Also, younger age (between 60 and 70 years) was significantly associated with intensive therapy treatment (induction chemotherapy and allogeneic transplant) (p < .0001). FLT3ITD was associated with a shorter median OS compared to wtFLT3, however, it was not statistically significant in univariate analysis [median OS 5.2 vs. 9.1 months, respectively; p = .21)].
Table 2.
Associations of WBC, age, gender, treatment, FLT3 mutation status and OS in univariate analysis.
| Total | Event | Median (months) | HR and 95% CI | p-Value | |
|---|---|---|---|---|---|
| FLT3 | .21 | ||||
| ITD | 36 | 28 | 5.2 | 1.34 (.84, 2.14) | |
| Wild Type | 55 | 49 | 9.1 | 1 (reference) | |
| Treatment type | <.0001 | ||||
| Induction chemotherapy | 38 | 36 | 8.6 | .41 (.25, .67) | |
| Allogeneic transplant | 17 | 6 | Not reached | .08 (.03, .19) | |
| Supportive care/experimental therapy | 36 | 35 | 3.0 | 1 (reference) | |
| Age at diagnosis | <.0001 | ||||
| <70 | 46 | 35 | 11.2 | .18 (.08, .41) | |
| 70–80 | 35 | 34 | 4.4 | .38 (.17, .87) | |
| ≥80 | 9 | 8 | 2.9 | 1 (reference) | |
| WBC | .31 | ||||
| <20 | 53 | 46 | 9.1 | .82 (.47, 1.43) | |
| 20–40 | 16 | 14 | 3.5 | 1.29 (.63, 2.62) | |
| ≥40 | 20 | 17 | 5.5 | 1 (reference) |
The patients in our study spanned a range of ages and treatment types typical of many leukemia treatment centers and both age and type of treatment were significantly associated with OS in univariate analysis. In order to account for differences in age, type of treatment, gender, and WBC we developed a multivariable model incorporating stepwise selection to determine the relationship between FLT3 mutation status and OS.
In our multivariable analysis accounting for these parameters, FLT3ITD was significantly associated with a shorter OS (p = .001; HR = 2.23; 95% CI: 1.35–3.70).
In addition, the type of therapy received was also significantly associated with OS, with both induction chemotherapy and allogeneic transplant correlated with improved OS (p < .0001). Age at diagnosis was not associated with OS in this model (Table 3).
Table 3.
Associations of FLT3 mutation, type of treatment patients received, gender, WBC, age at diagnosis and OS in a multivariable model (only significant results are shown).
| HR and 95% CI | p-Values | |
|---|---|---|
| FLT3 | .001 | |
| ITD | 2.23 (1.35, 3.70) | |
| Wild type | 1 (reference) | |
| Treatment type | <.0001 | |
| Induction chemotherapy | .4 (.24, .64) | |
| Allogeneic transplant | .05 (.02, .14) | |
| Supportive care/experimental therapy | 1 (reference) |
3.3. Higher allelic ratio is associated with a trend towards worse overall survival
The median mutant:wt FLT3 AR in our patient group was .355. Patients with a FLT3ITD allelic ratio (AR) > .4 (n = 16) had a trend towards worse OS compared to patients with an AR < .4 (n = 20) (median OS 3.3 months vs. 5.3 months; p = .06).
3.4. Median survival was not reached for patients with FLT3ITD receiving allogeneic stem cell transplantation
The median survival for patients with FLT3ITD (n = 7) who underwent allogeneic SCT was not reached, with median follow up of 37.2 months and the median survival of patients with wtFLT3 (n = 10) who underwent allogeneic SCT was 35.5 months (median follow up of 88 months).
4. Discussion
Older adults with AML have a worse prognosis than younger adults due to a variety of factors that include lower incidence of favorable cytogenetics, higher rates of secondary AML, lower CR rates, higher relapse rates and inability to tolerate intensive therapies [22]. Patients with AML ≥ 60 years are generally grouped together as “older adults.” However, they vary considerably with respect to disease biology and with respect to types of treatments they receive and can tolerate [7,15]. In the context of these known variables, we developed a multivariable model using stepwise selection to determine the prognostic significance of FLT3 status on outcome of older adults with AML.
Our study includes all patients ≥60 years with CN-AML seen at our institutions during the years specified for whom FLT3 mutation status was known. In addition our analysis was not limited by type of treatment received or onset of AML (secondary or de novo), with the aim of analyzing the significance of FLT3ITD across the entire spectrum of older adults with CN-AML.
Because of the broad range of patient age and types of treatments received, we were not surprised to find that FLT3 mutation status was not significantly associated with survival in univariate analysis. This illustrates the challenge in assessing the prognostic role of molecular markers within the broad category of “older adults” with AML. To overcome this problem, we developed a multivariable model and, when accounting for age, type of treatment, gender, and WBC count, we observed a significant relationship between FLT3 mutation status and survival.
Although there has been considerable debate about the prognostic significance of FLT3ITD in older adults with AML [12–15,17], our findings support the recent observation by Whitman et al. showing that FLT3ITD is associated with inferior prognosis in adults ≥ 60 years.
In addition, our findings provide insights into the treatment and outcome of AML in older adults. Specifically, in univariate analyses, we found that both age and type of treatment were significantly associated with survival. However, only type of treatment was significantly associated with OS in multivariable analysis. This raises the possibility that differences in age among patients with AML ≥ 60 may be less relevant than type of treatment received in impacting OS. In addition, we found that intensive treatment with induction chemotherapy and allogeneic SCT is associated with improved OS. This is particularly relevant at this time when low intensity therapies, such as the hypomethylating agents like decitabine and azacitidine, are under consideration as initial treatments for older adults with AML in an effort to reduce the treatment-related toxicities seen with aggressive induction chemotherapy. The patients in our study who did not receive induction therapy may have been sicker or less able to receive intensive therapy and though our study does not address the specific question of whether induction chemotherapy is superior to low intensity therapies in older adults with AML, our analysis does lend some support for the role of dose-intensive therapy in treatment of older adults with AML.
In summary, our data support the negative impact of FLT3ITD in adults ≥60 years with CN-AML and suggest that dose-intensive therapies, including induction chemotherapy and allogeneic SCT, have an important role in treating older adults with AML.
Supplementary Material
Acknowledgments
Funding source. H.S. is supported by the Judith Keen Postdoctoral Research Fellowship. This research is funded in part by the Leukemia Discovery and Treatment Fund at Massachusetts General Hospital.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.leukres.2011.05.032.
Footnotes
Conflict of interest statement
R.M.S. has served as a consultant for Novartis. There are no other relevant conflicts of interest.
Contributions. H.S., R.M.S. and E.C.A. provided the conception and design of the study, acquisition of data, analysis and interpretation of data, drafting the article, revised it critically for important intellectual content, and final approval of the version to be submitted; S.A., L.L.W., E.A.F. and D.S.N. acquisition of data, analysis and interpretation of data, revised it critically for important intellectual content, and final approval of the version to be submitted; D.J.D., K.K.B., P.C.A., M.W. and I.G.: acquisition of data, revised it critically for important intellectual content, and final approval of the version to be submitted.
References
- 1.Altekruse S, Kosary C, Krapcho M, Neyman M, et al. SEER incidence and NCHS mortality statistics. SEER Cancer Statistics Review, 1975–2007. 2010 [Google Scholar]
- 2.Estey E, Dohner H. Acute myeloid leukaemia. Lancet. 2006;368:1894–907. doi: 10.1016/S0140-6736(06)69780-8. [DOI] [PubMed] [Google Scholar]
- 3.Byrd JC, Mrozek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002;100:4325–36. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 4.Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–74. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 5.Frohling S, Schlenk RF, Kayser S, Morhardt M, Benner A, Dohner K, et al. Cytogenetics and age are major determinants of outcome in intensively treated acute myeloid leukemia patients older than 60 years: results from AMLSG trial AML HD98-B. Blood. 2006;108:3280–8. doi: 10.1182/blood-2006-04-014324. [DOI] [PubMed] [Google Scholar]
- 6.Farag SS, Archer KJ, Mrozek K, Ruppert AS, Carroll AJ, Vardiman JW, et al. Pre-treatment cytogenetics add to other prognostic factors predicting complete remission and long-term outcome in patients 60 years of age or older with acute myeloid leukemia: results from Cancer and Leukemia Group B 8461. Blood. 2006;108:63–73. doi: 10.1182/blood-2005-11-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becker H, Marcucci G, Maharry K, Radmacher MD, Mrozek K, Margeson D, et al. Favorable prognostic impact of NPM1 mutations in older patients with cytogenetically normal de novo acute myeloid leukemia and associated gene- and microRNA-expression signatures: a Cancer and Leukemia Group B study. J Clin Oncol. 2010;28:596–604. doi: 10.1200/JCO.2009.25.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becker H, Marcucci G, Maharry K, Radmacher MD, Mrozek K, Margeson D, et al. Mutations of the Wilms tumor 1 gene (WT1) in older patients with primary cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. Blood. 2010;116:788–92. doi: 10.1182/blood-2010-01-262543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meshinchi S, Appelbaum FR. Structural and functional alterations of FLT3 in acute myeloid leukemia. Clin Cancer Res. 2009;15:4263–9. doi: 10.1158/1078-0432.CCR-08-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frohling S, Schlenk RF, Breitruck J, Benner A, Kreitmeier S, Tobis K, et al. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood. 2002;100:4372–80. doi: 10.1182/blood-2002-05-1440. [DOI] [PubMed] [Google Scholar]
- 11.Thiede C, Steudel C, Mohr B, Schaich M, Schakel U, Platzbecker U, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99:4326–35. doi: 10.1182/blood.v99.12.4326. [DOI] [PubMed] [Google Scholar]
- 12.Ferrara F, Criscuolo C, Riccardi C, Izzo T, Pedata M, Copia C, et al. FLT3 mutations have no prognostic impact in elderly patients with acute myeloid leukemia and normal karyotype. Am J Hematol. 2009;84:532–5. doi: 10.1002/ajh.21458. [DOI] [PubMed] [Google Scholar]
- 13.Andersson A, Johansson B, Lassen C, Mitelman F, Billstrom R, Fioretos T. Clinical impact of internal tandem duplications and activating point mutations in FLT3 in acute myeloid leukemia in elderly patients. Eur J Haematol. 2004;72:307–13. doi: 10.1111/j.1600-0609.2004.00225.x. [DOI] [PubMed] [Google Scholar]
- 14.Stirewalt DL, Kopecky KJ, Meshinchi S, Appelbaum FR, Slovak ML, Willman CL, et al. FLT3 RAS, and TP53 mutations in elderly patients with acute myeloid leukemia. Blood. 2001;97:3589–95. doi: 10.1182/blood.v97.11.3589. [DOI] [PubMed] [Google Scholar]
- 15.Whitman SP, Maharry K, Radmacher MD, Becker H, Mrozek K, Margeson D, et al. FLT3 internal tandem duplication associates with adverse outcome and gene- and microRNA-expression signatures in patients 60 years of age or older with primary cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. Blood. 2010;116:3622–6. doi: 10.1182/blood-2010-05-283648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breccia M, Latagliata R, Carmosino I, Guarini A, Diverio D, De Cuia R, et al. Negative impact of FLT3 abnormalities in elderly acute myeloid leukemia patients. Leuk Lymphoma. 2008;49:994–7. doi: 10.1080/10428190801947567. [DOI] [PubMed] [Google Scholar]
- 17.Beran M, Luthra R, Kantarjian H, Estey E. FLT3 mutation and response to intensive chemotherapy in young adult and elderly patients with normal karyotype. Leuk Res. 2004;28:547–50. doi: 10.1016/j.leukres.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 18.Armstrong SA, Mabon ME, Silverman LB, Li A, Gribben JG, Fox EA, et al. FLT3 mutations in childhood acute lymphoblastic leukemia. Blood. 2004;103:3544–6. doi: 10.1182/blood-2003-07-2441. [DOI] [PubMed] [Google Scholar]
- 19.Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, et al. Revised recommendations of the international working group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol. 2003;21:4642–9. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 20.Moreno I, Martin G, Bolufer P, Barragan E, Rueda E, Roman J, et al. Incidence and prognostic value of FLT3 internal tandem duplication and D835 mutations in acute myeloid leukemia. Haematologica. 2003;88:19–24. [PubMed] [Google Scholar]
- 21.Bagrintseva K, Geisenhof S, Kern R, Eichenlaub S, Reindl C, Ellwart JW, et al. FLT3-ITD-TKD dual mutants associated with AML confer resistance to FLT3 PTK inhibitors and cytotoxic agents by overexpression of Bclx(L) Blood. 2005;105:3679–85. doi: 10.1182/blood-2004-06-2459. [DOI] [PubMed] [Google Scholar]
- 22.Godwin JE, Smith SE. Acute myeloid leukemia in the older patient. Crit Rev Oncol Hematol. 2003;48:S17–26. doi: 10.1016/j.critrevonc.2003.04.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.