Abstract
To review the data with respect to prevalence and risk factors of metabolic syndrome (MetS) in bipolar disorder patients. Electronic searches were done in PUBMED, Google Scholar and Science direct. From 2004 to June 2011, 34 articles were found which reported on the prevalence of MetS. The sample size of these studies varied from 15 to 822 patients, and the rates of MetS vary widely from 16.7% to 67% across different studies. None of the sociodemographic variable has emerged as a consistent risk factor for MetS. Among the clinical variables longer duration of illness, bipolar disorder- I, with greater number of lifetime depressive and manic episodes, and with more severe and difficult-to-treat index affective episode, with depression at onset and during acute episodes, lower in severity of mania during the index episode, later age of onset at first manic episode, later age at first treatment for the first treatment for both phases, less healthy diet as rated by patients themselves, absence of physical activity and family history of diabetes mellitus have been reported as clinical risk factors of MetS. Data suggests that metabolic syndrome is fairly prevalent in bipolar disorder patients.
Keywords: Bipolar disorders, diabetes mellitus, metabolic syndrome, obesity, prevalence
INTRODUCTION
Metabolic syndrome (MetS) is of immense clinical relevance because it is associated with development of coronary heart disease, cerebrovascular disease, as well as type 2 diabetes mellitus. Available data suggests that cardiovascular disease is the most common cause of excess and premature mortality in bipolar disorder (BPAD) patients.[1] Hence, prevention, identification, and modification of the cardiovascular risk factor should be one of the important therapeutic objectives in the management of bipolar disorder.[2]
MetS and BPAD appear to share common risk factors, including endocrine disturbances and dysregulation of the sympathetic nervous system, and behaviour patterns, such as physical inactivity, smoking, and overeating.[3–6] In addition, many pharmacological medications used for BPAD cause weight gain and metabolic disturbances.[7,8] There is some evidence to suggest that metabolic disturbances and obesity are associated with a disease course, which is worse and are likely to contribute to the premature mortality in BPAD.[9,10] Metabolic disturbances have also been associated with treatment non-adherence and higher treatment costs.[8]
Information is available about the prevalence of obesity,[11–17] diabetes,[18–23] dyslipidemia,[18,24–26] and hypertension[27] in patients with BPAD, but few studies have evaluated the prevalence of MetS per se in patients of BPAD.
For this review, search of electronic databases and manual search of relevant publications or cross references were done. The electronic searches were done for articles published in English, but if the cross references yielded articles published in any other language, then these were also included. Electronic search included PUBMED, Google Scholar, and Science direct. Cross-searches of key references (both electronic and hand-search) often yielded other relevant material. The search terms used (in various combinations) were bipolar disorder, metabolic syndrome, prevalence, metabolic disturbances, obesity, correlates of metabolic syndrome, and risk factors of metabolic syndrome. From 2004 to June 2011, 34 articles were found which reported on the prevalence of MetS and another 3 articles although did not report on the prevalence, but reported about risk factors of MetS in BPAD. Additionally, we included the data of a manuscript in press. Data from these articles are reviewed here.
Studies which have evaluated the components of MetS in BPAD have not been included in this review.
DEFINITIONS OF METABOLIC SYNDROME
Competing criteria for defining MetS have been formulated by the World Health Organization (WHO),[28] the European Group for the Study of Insulin Resistance,[29] the International Diabetes Federation (IDF),[30] the National Cholesterol Education Program-Third Adult Treatment Panel,[31] the American Association of Clinical Endocrinology,[32] and the American Heart Association (AHA).[33]
Though there are minor differences between criteria in terms of the components of MetS, and the cut-offs required for these components to be considered abnormal, the central features are essentially similar. Most of these definitions require the presence of at least three abnormal parameters to characterize a person as having MetS. An advantage of the IDF and the NCEP ATP-III criteria is that unlike the WHO criteria, these are easily measurable and do not require specialized investigations. NCEP ATP-III is the most commonly used criteria-set for defining MetS. Some researchers have adapted or modified NCEP-ATP-III criteria for different ethnic populations to make this equivalent to definition of IDF, which gives different cut offs of waist circumference for different ethnic groups/countries. One fundamental difference between IDF and other criteria is that IDF requires fulfilment of waist circumference as a mandatory criterion along with presence of any two other criteria for making a diagnosis of MetS, whereas other criteria require presence of any of the three out of five criteria for making the diagnosis of MetS. Among various available criteria while evaluating MetS in BPAD patients 26 out of the 34 studies have used NCEP-ATP-III criteria.
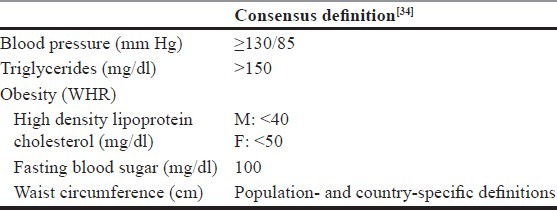
Recently there had been an effort to harmonise the definitions of MetS. For these there have been discussions between the representatives of IDF and AHA and National Heart, Lung, and Blood Institute. In a joint interim statement of the IDF Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; AHA; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity a consensus has been reached for defining MetS [Table 1].[34] According to this statement abdominal obesity is no more a pre-requite criteria for MetS and presence of any of three of five risk factors is sufficient for the diagnosis of MetS. Further for waist circumference, it has been agreed that population and country-specific definitions will be used for cutoffs.[34] In another recent development, WHO Consultation Group suggested that while defining MetS those with established diabetes mellitus and known cardiovascular disease should be excluded. The basic premise behind this recommendation is that MetS should be considered as a premorbid condition to predict the development of diabetes mellitus and cardiovascular disease in future.[35]
Table 1.
Definitions of MetS
PREVALENCE OF METS IN BPAD
Thirty-four studies[36–69] [Table 2] from different countries and ethnic backgrounds have reported the prevalence of MetS in patients with BPAD. Sample sizes of these studies have varied from 15 to 822 patients and the rates of MetS vary widely from 16.7% to 67%. Of the 34 studies shown in [Table 2], some authors have published the data of the same group of patients with varying sample size[65–68] and others have published the data separately for various definitions[45,46] of MetS. More than half of the available studies (18 out of 34) have included less than 100 patients.
Table 2.
Prevalence of MetS in BPAD
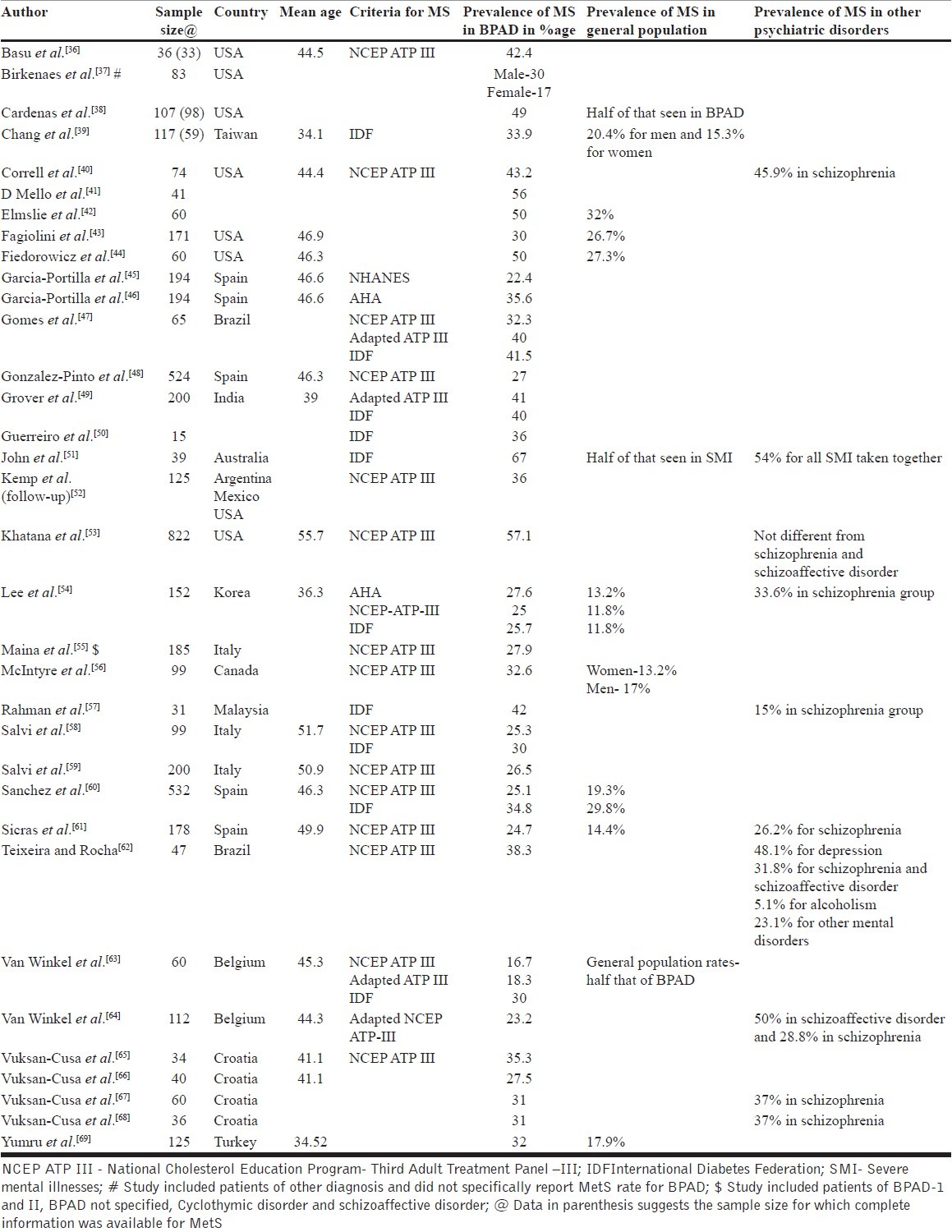
Some of the studies have included patients of other severe mental disorders along with BPAD and have not reported the prevalence of MetS specifically in BPAD.[37] Half of the studies (18 out of 34 studies) have employed a control group (either healthy control or a group of patients with other mental disorders) and suggest that the prevalence of MetS appears to be higher in BPAD than general population rates, and comparable to other disorders such as schizophrenia. Mean age of the patients in most of the studies (16 out of the 20 studies which have reported the same) have been above forty years. Further, some of the studies have used more than one definition for MetS and show comparable prevalence rates of MetS with more than one definition, but in general studies which have used both NCEP ATP-III criteria and IDF criteria suggest that the prevalence rates of MetS are higher with IDF criteria. This could possibly be due to different cut offs provided by IDF for waist circumference for different countries/ ethnic groups, whereas NCEP ATP–III does not provide such cut-offs, although recently some of the researchers have used adapted/modified NCEP ATP–III criterion for waist circumference.[47,49,64]
Except for one study, all of these are cross-sectional investigations and do not provide data on how the rates of MetS change over time. Kemp et al.[52] evaluated changes in the prevalence of MetS in patients receiving aripiprazole or placebo. Data were available for 94 patients at the baseline and the end point (week 26). At baseline, 34% of patients met the criteria for MetS. At the end point (week 26), 35.1% patients had MetS. Of the 94 patients, 45 received aripiprazole during the 26-week period; of these 14 patients had MetS at the baseline, whereas 18 out of 49 patients randomised to placebo had MetS at the baseline. At 26-week, out of the 14 patients randomised to aripiprazole who had MetS at baseline, 10 continued to meet the criteria of MetS, whereas 13 out of the 18 patients in the placebo group continued to meet the criteria of MetS. Additionally, in aripiprazole group, 6 patients who were not positive for MetS at baseline developed MetS at week 26 and in the placebo group, 4 patients developed MetS.
When one takes a closer look at these studies it is evident that at least 27 are from the Western countries, 2 from 2 from Brazil, 2 from Korea/Taiwan, 1 each from Turkey, Malaysia and India. This has important implications, because now there is a consensus to use ethnic specific definitions for waist circumference to define MetS. Hence, the prevalence reported in some of these studies without taking the ethnic cut-off into consideration may be misleading. Further, in some of these studies the sample size is of concern. Only 16 studies have included more than 100 patients.
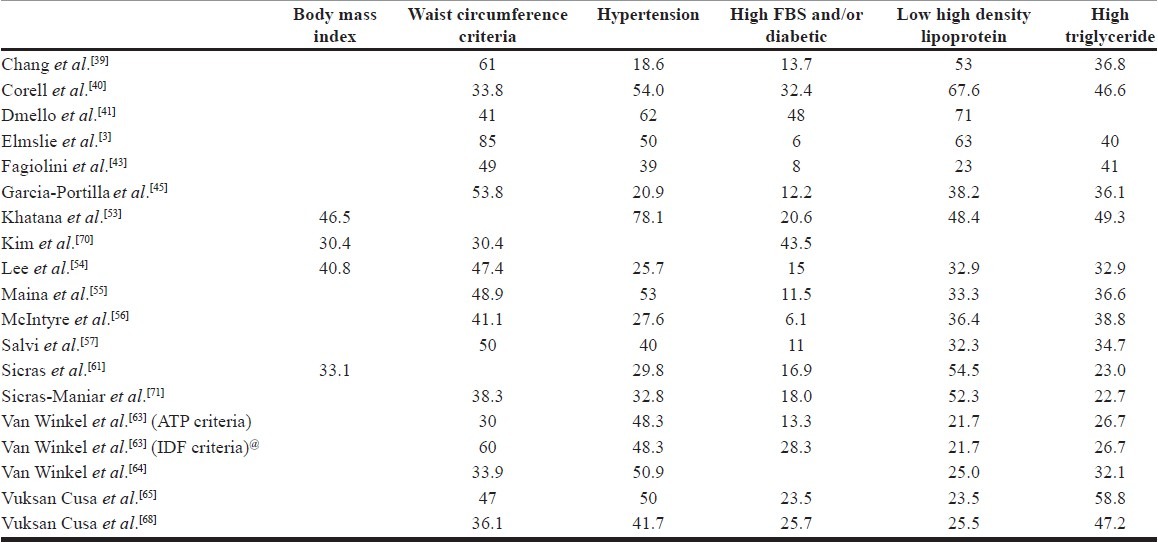
PREVALENCE OF COMPONENTS OF METS
All studies that have evaluated MetS in BPAD patients have not reported the prevalence of various components. The studies which have reported the prevalence of various components are shown in [Table 3]. The percentage of patients fulfilling the waist circumference criteria have spanned from 30-85%, that with raised blood pressure or on antihypertensive treatment have varied from 18.6-62%, raised fasting blood glucose levels have varied from 6-43.5%, low high density lipoprotein levels varied from 21.7-67.6% and that of high triglyceride levels have varied from 22.7 to 58.8%. High waist circumference and raised blood pressure are reported as the common abnormalities (each is reported as the most common abnormality in 7 out of the 19 studies) and raised fasting blood sugar or being on anti-diabetic treatment is least commonly reported abnormality across various studies (14 out of 19 studies). The lipid abnormalities are reported to have intermediate prevalence. From prevention and monitoring point of view, the commonness of prevalence of waist circumference and blood pressure indicates that monitoring them may be more useful and cost effective.
Table 3.
Prevalence of components of MetS in various studies
Socio-demographic and clinical factors associated with MetS in BPAD
Studies which have attempted to study the socio-demographic risk factors of MetS in BPAD have done so by comparing various parameters between the patients who have and those who do not have MetS. Few studies have carried out regression analysis to study the factors of MetS.
Although attempts have been made to study the sociodemographic factors of MetS in BPAD patients, but none of the sociodemographic variable has emerged as a consistent predictor of MetS. Studies have shown that patients with BPAD with MetS are older than those without MetS,[38,45,55,58,61] however, some studies have come up with negative findings.[40,51] One study reported that women had peak of prevalence of MetS in the ≥60 years group, while men displayed high rates even in the young age groups.[59] Some of the studies have reported that MetS is more common in females,[62] while others have reported no gender differences in the prevalence rates of MetS[38,51,56,58,61,63,65,69] and an occasional study has reported higher prevalence in males,[60] especially in younger age group.[37]
Some studies have also reported socio-demographic correlates of components of MetS. Salvi et al.[59] found that men had higher rates of hypertension and hypertriglyceridemia and, women had more abdominal obesity. Studies have reported that male patients have higher systolic blood pressures,[39,56] diastolic blood pressure,[56] waist-to-hip ratios,[39] and hypertriglyceridemia[56] compared to females. On the other hand, some studies have reported higher prevalence of obesity in females.[61] No difference has been noted with respect to years of education and occupational status.[58]
Longer duration of illness,[39,58] BPAD-I,[39] with greater number of lifetime depressive and manic episodes, and with more severe and difficult-to-treat index affective episode,[41,43] with depression at onset and during acute episodes,[9] lower in severity of mania during the index episode,[45] later age of onset at first manic episode,[56] later age at first treatment for the first treatment for both phases,[56] less healthy diet as rated by patients themselves,[51] absence of physical activity[55] and family history of diabetes mellitus[63,72] have been reported as clinical correlates of MetS. One study reported association of MetS with Cluster B personality disorders and less physical exercise in young patients.[59] One of the consistent findings across various studies is that patients with MetS are significantly more likely to be overweight or obese than patients that did not meet criteria for the MetS.[45,58,63] Some studies have found association between history of at least one suicide attempt and MetS,[43] others have reported no such association.[56] Similarly, comorbid substance use or smoking has been inconsistently associated with presence of MetS with some studies reporting higher prevalence of MetS in patients with comorbid substance use or smoking[9] and others have reported no such difference between those with MetS and those without MetS.[58] Studies have not found significant difference between patients with MetS and those without MetS with respect to presence of family history of lipid disorders or cardiovascular disease,[63] and rate of psychiatric comorbidity.[56]
Psychotropics and MetS in BPAD: Since the introduction of second-generation antipsychotic and their association with metabolic abnormalities, studies have evaluated the association of MetS and atypical antipsychotics. Few studies suggest that patients on a second-generation antipsychotic are significantly more likely to meet criteria for MetS compared to those receiving mood stabilizers alone,[43,63,69] though other studies have found no such association.[36,44] No significant differences have been noted between different antipsychotics in one study,[69] but one study reported that significantly higher percentage of patients on olanzapine or clozapine at the time of entry in the study met criteria for MetS.[38] Similarly, a study which evaluated the effect of second generation antipsychotics on the prevalence of MetS in patients with severe mental illness (which also included BPAD patients) reported higher prevalence of MetS in patients taking clozapine.[63] One study evaluated the differential effects of typical and atypical antipsychotics on the prevalence of MetS, however, it is important to note that this study did not evaluate this effect specifically for patients with BPAD, rather reported the findings for all the severe mental disorders taken together as a group.[51]
Studies that have compared the prevalence of MetS in BPAD with general population suggest that use of second generation antipsychotics is associated with higher risk of development of MetS.[40] One of the recent study which compared the metabolic effects of second generation antipsychotic in young patients of BPAD, other psychotic disorders and non psychotic disorders reported that 3 months treatment with second generation antipsychotics led to significant weight gain in more than 70% of patients across various diagnostic categories, but in the BPAD group additionally there was significant increased in the total cholesterol and LDL-cholesterol levels.[73] These findings again suggest that patients of BPAD are intrinsically more prone to develop MetS.
Basu et al.,[36] found no association between MetS and various mood stabilizers, however, it has been shown that valproate[74] and lithium[62] are associated with greater risk of metabolic disturbances. Association of higher serum valproate levels with presence of MetS has been documented.[39] Although studies which have evaluated adverse effects of lithium and valproate have reported excessive weight gain and insulin resistance related to long-term use of these medications,[7,75–81] study evaluating the prevalence of MetS has reported no difference in the duration of treatment with lithium, valproate or antipsychotics in those with or without MetS.[36] A study showed that simultaneous treatment with mood stabilizers and atypical antipsychotics is associated with significantly higher prevalences of metabolic abnormalities, hyperglycemia, higher triglyceride levels, and larger waist circumferences.[39] Similar association for concurrent use of two-three mood stabilizers and MetS has been reported.[45]
Biological correlates of MetS in BPAD: Some researchers have attempted to identify biological markers for MetS in BPAD patients and have shown that MetS is associated with high C-reactive protein levels (CRP >5 mg/l)[67], high interleukin-6 (IL-6)[82] and hyper-homocysteinaemia.[68] An investigation which evaluated the relationship of MetS with IL-6 in BPAD patients demonstrated that IL-6 levels correlated significantly with a number of criteria of MetS and suggested that it may be a diagnostic marker of MetS.[82]
Factors in regression analysis: Studies which have used regression analysis to evaluate the risk factors of MetS have reported increasing age,[38,58] obesity (i.e., higher BMI),[58] female gender,[62] and use of lithium[62] to predict MetS in BPAD.
Impact of MetS
Although studies have evaluated the prevalence of MetS in BPAD, there is a serious lack of data about its impact. Inconsistent evidence exists to suggest that MetS in BPAD patients is associated with significantly high rate of lifetime suicidal attempts.[43] A longitudinal study attempted to study the influence of MetS on rate of stabilization during the maintenance phase of treatment and reported no adverse effect of MetS on disease stabilization.[52]
Studies evaluating the impact of obesity suggest that subjects with obesity are more likely to develop an affective recurrence and, in particular, a depressive recurrence. Furthermore, it is reported that the time to depressive recurrence was shorter in those with obesity.[43] Preliminary findings suggest that obesity has a negative impact on functioning and leads to poor health-related quality of life.[83]
Data also suggest that BPAD patients with diabetes mellitus have higher rates of rapid cycling and chronic course poor level of functioning and higher level of disability, higher body mass index and increased frequency of hypertension and more life time psychiatric hospitalizations compared to non-diabetic BPAD patients.[19]
In some of the recent research it has been shown that obesity in BPAD patients is associated with reduced total brain volume and gray matter volume.[84]
Reasons for High prevalence of MetS in BPAD patients
It is suggested that bipolar disorder and MetS share common risk factors, including endocrine disturbances, dysregulation of the sympathetic nervous system, and unhealthy behaviors like physical inactivity, overeating, smoking, and use of alcohol. Additionally, psychotropics used for the treatment of BPAD lead to weight gain and metabolic disturbances, including alterations in lipid and glucose metabolism.
Studies suggest that compared to general population, impaired glucose intolerance and insulin resistance are more common in patients with BPAD.[10] There is some evidence to suggest higher rates of diabetes mellitus in patients with BPAD compared to schizophrenia, and this higher prevalence of diabetes mellitus is independent of the effects of BMI and psychotropic medication use.[20] It is suggested that stress pathway through hypothalamic-pituitary axis mediates insulin resistance, abdominal obesity, and dyslipidemia in BPAD patients.[9] Other factors which have been implicated in the development of obesity and MetS include genetic factors, although these have not been investigated thoroughly.
WHAT CAN BE CONCLUDED FROM THE DATA?
Review of the available data suggests that MetS is fairly prevalent in BPAD patients. The prevalence rate is similar to other severe mental illness like schizophrenia and is much higher than that seen in general population or healthy controls. Among the various components of MetS, increased waist circumference and raised blood pressure are the most common abnormalities reported by majority of the studies and abnormality of fasting blood sugar is the least common finding. Effect of psychotropics on prevalence of MetS is inconclusive. There is some evidence to suggest higher levels of inflammatory markers in patients of MetS in BPAD. However, the existing data is limited to 34 studies most of which have come from western countries. The existing data is also limited by smaller sample size, heterogenicity in reporting of the prevalence of components of MetS and longitudinal studies almost lacking. Similarly, data is lacking with respect to the impact of MetS on BPAD and reasons for higher prevalence of MetS in BPAD patients. Data is non-existent regarding intervention strategies to either prevent or treat the same.
FUTURE DIRECTION
There is a need to have studies with larger sample size from various ethnic backgrounds and from different countries to have a better estimate of the problem. Further with the current effort to unify the definition of MetS, it is important to use ethnic specific criteria to define MetS. A consistent reporting of the components of MetS may provide inside into the evolution of the each component and may guide the prevention and treatment strategies. The impact of MetS requires to be fully explored. The impact of MetS on treatment response, treatment adherence, quality of life, other side effects, etc are some of the areas that require further research. The risk factors for the development of metabolic abnormalities as well as their pathophysiology in BPAD need further research.
DO WE NEED TO MONITOR THE BPAD FOR METS?
Considering the high prevalence of MetS in BPAD patients, routine screening for MetS is indicated. Waist circumference and raised blood pressure should be routinely measured and depending on the cost involved, the laboratory investigations should be done. Attempts should be made to change unhealthy lifestyle like inactivity, overeating, smoking and use of alcohol, and appropriate psycho-educational programs in this regard need to be developed. Although the data with respect to association of MetS and psychotropics in BPAD remain inconclusive, nonetheless, a cautious approach in prescribing psychotropics is advisable. Studies in BPAD patients do suggest that lithium, valproate, and atypical antipsychotics are associated with weight gain, dyslipidemia, diabetes mellitus, insulin resistance, etc; hence, due consideration should be given to their potential to cause metabolic disturbances while prescribing an agent, and whenever used, after the acute phase the prescription should be revised and minimum number of medications should be prescribed.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Osby U, Brandt L, Correia N, Ekbom A, Sparen P. Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry. 2001;58:844–50. doi: 10.1001/archpsyc.58.9.844. [DOI] [PubMed] [Google Scholar]
- 2.Ng F, Mammen OK, Wilting I, Sachs GS, Ferrier IN, Cassidy F, et al. The International Society for Bipolar Disorders (ISBD) consensus guidelines for the safety monitoring of bipolar disorder treatments. Bipolar Disord. 2009;11:559–95. doi: 10.1111/j.1399-5618.2009.00737.x. [DOI] [PubMed] [Google Scholar]
- 3.Elmslie JL, Mann JI, Silverstone JT, Williams SM, Romans SE. Determinants of overweight and obesity in patients with bipolar disorder. J Clin Psychiatry. 2001;62:486–91. doi: 10.4088/jcp.v62n0614. [DOI] [PubMed] [Google Scholar]
- 4.Waxmonsky JA, Thomas MR, Miklowitz DJ, Allen MH, Wisniewski SR, Zhang H, et al. Prevalence and correlates of tobacco use in bipolar disorder: Data from the first 2000 participants in the systematic treatment enhancement program. Gen Hosp Psychiatry. 2005;27:321–8. doi: 10.1016/j.genhosppsych.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Lakka TA, Bouchard C. Physical activity, obesity and cardiovascular diseases. Handb Exp Pharmacol. 2005;170:137–63. doi: 10.1007/3-540-27661-0_4. [DOI] [PubMed] [Google Scholar]
- 6.Ford ES, Kohl HW, III, Mokdad AH, Ajani UA. Sedentary behavior, physical activity, and the metabolic syndrome among U.S. adults. Obesity Res. 2005;13:608–14. doi: 10.1038/oby.2005.65. [DOI] [PubMed] [Google Scholar]
- 7.Meyer JM. A retrospective comparison of weight, lipid, and glucose changes between risperidone- and olanzapine-treated inpatients: metabolic outcomes after 1 year. J Clin Psychiatry. 2002;63:425–33. doi: 10.4088/jcp.v63n0509. [DOI] [PubMed] [Google Scholar]
- 8.Guo JJ, Keck PE, Corey-Lisle PK, Li H, Jiang D, Jang R, et al. Risk of diabetes mellitus associated with atypical antipsychotic use among patients with bipolar disorder: A retrospective, population-based, case–control study. J Clin Psychiatry. 2006;67:1055–61. doi: 10.4088/jcp.v67n0707. [DOI] [PubMed] [Google Scholar]
- 9.Fagiolini A, Chengappa KN, Soreca I, Chang J. Bipolar disorder and the metabolic syndrome: causal factors, psychiatric outcomes and economic burden. CNS Drugs. 2008;22:655–69. doi: 10.2165/00023210-200822080-00004. [DOI] [PubMed] [Google Scholar]
- 10.Taylor V, MacQueen G. Associations between bipolar disorder and metabolic syndrome: A review. J Clin Psychiatry. 2006;67:1034–41. doi: 10.4088/jcp.v67n0704. [DOI] [PubMed] [Google Scholar]
- 11.Fagiolini A, Frank E, Houck PR, Mallinger AG, Swartz HA, Buysse DJ, et al. Prevalence of obesity and weight change during treatment in patients with bipolar I disorder. J Clin Psychiatry. 2002;63:528–33. doi: 10.4088/jcp.v63n0611. [DOI] [PubMed] [Google Scholar]
- 12.Fagiolini A, Kupfer DJ, Houck PR, Novick DM, Frank E. Obesity as a correlate of outcome in patients with bipolar I disorder. Am J Psychiatry. 2003;160:112–17. doi: 10.1176/appi.ajp.160.1.112. [DOI] [PubMed] [Google Scholar]
- 13.McElroy SL, Kotwal R, Malhotra S, Nelson EB, Keck PE, Nemeroff CB. Are mood disorders and obesity related? A review for the mental health professional. J Clin Psychiatry. 2004;65:634–51. doi: 10.4088/jcp.v65n0507. [DOI] [PubMed] [Google Scholar]
- 14.Keck PE, McElroy SL. Bipolar disorder, obesity, and pharmacotherapy-associated weight gain. J Clin Psychiatry. 2003;64:1426–35. doi: 10.4088/jcp.v64n1205. [DOI] [PubMed] [Google Scholar]
- 15.McElroy SL, Frye MA, Suppes T, Dhavale D, Keck PE, Jr, Leverich GS, et al. Correlates of overweight and obesity in 644 patients with bipolar disorder. J Clin Psychiatry. 2002;63:207–13. doi: 10.4088/jcp.v63n0306. [DOI] [PubMed] [Google Scholar]
- 16.Nemeroff CB. Safety of available agents used to treat bipolar disorder: focus on weight gain. J Clin Psychiatry. 2003;64:532–39. doi: 10.4088/jcp.v64n0506. [DOI] [PubMed] [Google Scholar]
- 17.Elmslie JL, Silverstone JT, Mann JI, Williams SM, Romans SE. Prevalence of overweight and obesity in bipolar patients. J Clin Psychiatry. 2000;61:179–84. doi: 10.4088/jcp.v61n0306. [DOI] [PubMed] [Google Scholar]
- 18.Kilbourne AM, Cornelius JR, Han X, Pincus HA, Shad M, Salloum I, et al. Burden of general medical conditions among individuals with bipolar disorder. Bipolar Disord. 2004;6:368–73. doi: 10.1111/j.1399-5618.2004.00138.x. [DOI] [PubMed] [Google Scholar]
- 19.Ruzickova M, Slaney C, Garnham J, Alda M. Clinical features of bipolar disorder with and without comorbid diabetes mellitus. Can J Psychiatry. 2003;48:458–61. doi: 10.1177/070674370304800705. [DOI] [PubMed] [Google Scholar]
- 20.Regenold WT, Thapar RK, Marano C, Gavirneni S, Kondapavuluru PV. Increased prevalence of type 2 diabetes mellitus among psychiatric inpatients with bipolar I affective and schizoaffective disorders independent of psychotropic drug use. J Affect Disord. 2002;70:19–26. doi: 10.1016/s0165-0327(01)00456-6. [DOI] [PubMed] [Google Scholar]
- 21.Cassidy F, Ahearn E, Carroll BJ. Elevated frequency of diabetes mellitus in hospitalized manic-depressive patients. Am J Psychiatry. 1999;156:1417–20. doi: 10.1176/ajp.156.9.1417. [DOI] [PubMed] [Google Scholar]
- 22.Russell JD, Johnson GF. Affective disorders, diabetes mellitus and lithium. Aust NZ J Psychiatry. 1981;15:349–53. doi: 10.3109/00048678109159459. [DOI] [PubMed] [Google Scholar]
- 23.Lilliker SL. Prevalence of diabetes in a manic-depressive population. Compr Psychiatry. 1980;21:270–75. doi: 10.1016/0010-440x(80)90030-9. [DOI] [PubMed] [Google Scholar]
- 24.Sobczak S, Honig A, Christophe A, Maes M, Helsdingen RW, De Vriese SA, et al. Lower high density lipoprotein cholesterol and increased omega-6 polyunsaturated fatty acids in first-degree relatives of bipolar patients. Psychol Med. 2004;34:103–12. doi: 10.1017/s0033291703001090. [DOI] [PubMed] [Google Scholar]
- 25.Atmaca M, Kuloglu M, Tezcan E, Ustundag B, Bayik Y. Serum leptin and cholesterol levels in patients with bipolar disorder. Neuropsychobiology. 2002;46:176–9. doi: 10.1159/000067809. [DOI] [PubMed] [Google Scholar]
- 26.Horrobin DF, Bennett CN. Depression and bipolar disorder: relationships to impaired fatty acid and phospholipid metabolism and to diabetes, cardiovascular disease, immunological abnormalities, cancer, ageing and osteoporosis. Possible candidate genes. Prostaglandins Leukot Essent Fatty Acids. 1999;60:217–34. doi: 10.1054/plef.1999.0037. [DOI] [PubMed] [Google Scholar]
- 27.Yates WR, Wallace R. Cardiovascular risk factors in affective disorder. J Affect Disord. 1987;12:129–34. doi: 10.1016/0165-0327(87)90004-8. [DOI] [PubMed] [Google Scholar]
- 28.Definition, diagnosis and classification of diabetes mellitus and its complications. Report of a WHO consultation. Geneva: World Health Organisation; 1999. World Health Organization. [Google Scholar]
- 29.Balkau B, Charles MA. Comment on the provisional report from the WHO consultation. Diabet Med. 1999;16:442–3. doi: 10.1046/j.1464-5491.1999.00059.x. [DOI] [PubMed] [Google Scholar]
- 30.Alberti KG, Zimmet PZ, Shaw J. Metabolic syndrome-a new worldwide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469–80. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 31.National Cholesterol Education Program. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 32.American College of Endocrinology Task Force on the Insulin Resistance Syndrome. Endocr Pract. 2003;9:236–52. [PubMed] [Google Scholar]
- 33.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. American Heart Association; National Heart, Lung, and Blood Institute. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–8. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 34.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 35.Simmons RK, Alberti KG, Gale EA, Colagiuri S, Tuomilehto J, Qiao Q, et al. The metabolic syndrome: Useful concept or clinical tool? Report of a WHO Expert Consultation. Diabetologia. 2010;53:600–5. doi: 10.1007/s00125-009-1620-4. [DOI] [PubMed] [Google Scholar]
- 36.Basu R, Brar JS, Chengappa KN, John V, Parepally H, Gershon S, et al. The prevalence of the metabolic syndrome in patients with schizoaffective disorder--bipolar subtype. Bipolar Disord. 2004;6:314–8. doi: 10.1111/j.1399-5618.2004.00126.x. [DOI] [PubMed] [Google Scholar]
- 37.Birkenaes AB, Søgaard AJ, Engh JA, Jonsdottir H, Ringen PA, Vaskinn A, et al. Sociodemographic characteristics and cardiovascular risk factors in patients with severe mental disorders compared with the general population. J Clin Psychiatry. 2006;67:425–33. doi: 10.4088/jcp.v67n0314. [DOI] [PubMed] [Google Scholar]
- 38.Cardenas J, Frye MA, Marusak SL, Levander EM, Chirichigno JW, Lewis S, et al. Modal subcomponents of metabolic syndrome in patients with bipolar disorder. J Affect Disord. 2008;106:91–7. doi: 10.1016/j.jad.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 39.Chang HH, Chou CH, Chen PS, Gean PW, Huang HC, Lin CY, et al. High prevalence of metabolic disturbances in patients with bipolar disorder in Taiwan. J Affect Disord. 2009;117:124–9. doi: 10.1016/j.jad.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 40.Correll CU, Frederickson AM, Kane JM, Manu P. Equally increased risk for metabolic syndrome in patients with bipolar disorder and schizophrenia treated with second-generation antipsychotics. Bipolar Disord. 2008;10:788–97. doi: 10.1111/j.1399-5618.2008.00625.x. [DOI] [PubMed] [Google Scholar]
- 41.D’Mello DA, Narang S, Agredano G. Prevalence and Consequences of Metabolic Syndrome in Bipolar Disorder. Psychiatric Times. 2007;24:1. [Google Scholar]
- 42.Elmslie JL, Porter RJ, Joyce PR, Hunt PJ, Shand BI, Scott RS. Comparison of insulin resistance, metabolic syndrome and adiponectin in overweight bipolar patients taking sodium valproate and controls. Aust NZ J Psychiatry. 2009;43:53–60. doi: 10.1080/00048670802534341. [DOI] [PubMed] [Google Scholar]
- 43.Fagiolini A, Frank E, Scott JA, Turkin S, Kupfer DJ. Metabolic syndrome in bipolar disorder: findings from the Bipolar Disorder Centre for Pennsylvanians. Bipolar Disord. 2005;7:424–30. doi: 10.1111/j.1399-5618.2005.00234.x. [DOI] [PubMed] [Google Scholar]
- 44.Fiedorowicz JG, Palagummi NM, Forman-Hoffman VL, Miller DD, Haynes WG. Elevated prevalence of obesity, metabolic syndrome, and cardiovascular risk factors in bipolar disorder. Ann Clin Psychiatry. 2008;20:131–7. doi: 10.1080/10401230802177722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garcia-Portilla MP, Saiz PA, Benabarre A, Sierra P, Perez J, Rodriguez A, et al. The prevalence of metabolic syndrome in patients with bipolar disorder. J Affect Disord. 2008;106:197–201. doi: 10.1016/j.jad.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 46.Garcia-Portilla MP, Saiz PA, Bascaran MT, Martínez S, Benabarre A, Sierra P, et al. Cardiovascular risk in patients with bipolar disorder. J Affect Disord. 2009;115:302–8. doi: 10.1016/j.jad.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 47.Gomes FA, Magalhaes PV, Kunz M, Da silveira LE, Weyne F, Andreazza AC, et al. Insulin resistance and metabolic syndrome in outpatients with bipolar disorder. Rev Psiq Clín. 2010;37:81–4. [Google Scholar]
- 48.González-Pinto A, Vieta E, Montes JM, Rejas-Gutiérrez J, Mesa F. Metabolic syndrome in patients with bipolar disorders (BD): Findings from the BIMET study European Psychiatry. 24(null):S600. [Google Scholar]
- 49.Grover S, Aggarwal M, Chakrabarti S, Dutt A, Avasthi A, Kulhara P, et al. Prevalence of Metabolic syndrome in Bipolar Disorder: an exploratory study from North India. Prog Neuropsychiatry Neuropsychopharmacol. 2012;36:141–6. doi: 10.1016/j.pnpbp.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 50.Guerreiro DF, Navarro R, Telles-Correia D, Martins P, Trigo E, Silva M. Effectivity of screening, concepts and attitudes towards metabolic syndrome: A study in bipolar patients followed in hospital Santa Maria psychiatric consultation. Acta Med Port. 2010;23:173–82. [PubMed] [Google Scholar]
- 51.John AP, Koloth R, Dragovic M, Lim SC. Prevalence of metabolic syndrome among Australians with severe mental illness. Med J Aust. 2009;190:176–9. doi: 10.5694/j.1326-5377.2009.tb02342.x. [DOI] [PubMed] [Google Scholar]
- 52.Kemp DE, Calabrese JR, Tran QV, Pikalov A, Eudicone JM, Baker RA. Metabolic syndrome in patients enrolled in a clinical trial of aripiprazole in the maintenance treatment of bipolar I disorder: A post hoc analysis of a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2010;71:1138–44. doi: 10.4088/JCP.09m05159gre. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khatana SA, Kane J, Taveira TH, Bauer MS, Wu WC. Monitoring and prevalence rates of metabolic syndrome in military veterans with serious mental illness. PLoS One. 2011;6:e19298. doi: 10.1371/journal.pone.0019298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee NY, Kim SH, Cho B, Lee YJ, Chang JS, Kang UG, et al. Patients taking medications for bipolar disorder are more prone to metabolic syndrome than Korea's general population. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1243–9. doi: 10.1016/j.pnpbp.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 55.Maina G, D’Ambrosio V, Aguglia A, Paschetta E, Salvi V, Bogetto F. Bipolar disorders and metabolic syndrome: A clinical study in 185 patients. Riv Psichiatr. 2010;45:34–40. [PubMed] [Google Scholar]
- 56.McIntyre RS, Woldeyohannes HO, Soczynska JK, Miranda A, Lachowski A, Liauw SS, et al. The rate of metabolic syndrome in euthymic Canadian individuals with bipolar I/II disorder. Adv Ther. 2010;27:828–36. doi: 10.1007/s12325-010-0072-z. [DOI] [PubMed] [Google Scholar]
- 57.Rahman AH, Asmara HA, Baharudin A, Sidi H. Metabolic syndrome in psychiatric patients with primary psychotic and mood disorders. Asean J Psychiatry. 2009;10:1–8. [Google Scholar]
- 58.Salvi V, Albert U, Chiarle A, Soreca I, Bogetto F, Maina G. Metabolic syndrome in Italian patients with bipolar disorder. Gen Hosp Psychiatry. 2008;30:318–23. doi: 10.1016/j.genhosppsych.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 59.Salvi V, D’Ambrosio V, Rosso G, Bogetto F, Maina G. Age-specific prevalence of metabolic syndrome in Italian patients with bipolar disorder. Psychiatry Clin Neurosci. 2011;65:47–54. doi: 10.1111/j.1440-1819.2010.02160.x. [DOI] [PubMed] [Google Scholar]
- 60.Gabriel-Sanchez R, Lorenzo-Carrascosa L, Arroyo MA, GonzRlez-Pinto A, Vieta E, Montes JM, et al. Metabolic syndrome in bipolar disorders in Spain: Findings from the population based case-control BIMET-VIVA study. Eur Neuropsychopharmacology. 2009;19:S466. [Google Scholar]
- 61.Sicras A, Rejas J, Navarro R, Serrat J, Blanca M. Metabolic syndrome in bipolar disorder: A cross-sectional assessment of a Health Management Organization database. Bipolar Disord. 2008;10:607–16. doi: 10.1111/j.1399-5618.2008.00599.x. [DOI] [PubMed] [Google Scholar]
- 62.Teixeira PJ, Rocha FL. The prevalence of metabolic syndrome among psychiatric inpatients in Brazil. Rev Bras Psiquiatr. 2007;29:330–6. doi: 10.1590/s1516-44462007000400007. [DOI] [PubMed] [Google Scholar]
- 63.Van Winkel R, De Hert M, Van Eyck D, Hanssens L, Wampers M, Scheen A, et al. Prevalence of diabetes and the metabolic syndrome in a sample of patients with bipolar disorder. Bipolar Disord. 2008;10:342–8. doi: 10.1111/j.1399-5618.2007.00520.x. [DOI] [PubMed] [Google Scholar]
- 64.Van Winkel R, Van Os J, Celic I, Van Eyck D, Wampers M, Scheen A, et al. Psychiatric diagnosis as an independent risk factor for metabolic disturbances: Results from a comprehensive, naturalistic screening program. J Clin Psychiatry. 2008;69:1319–27. doi: 10.4088/jcp.v69n0817. [DOI] [PubMed] [Google Scholar]
- 65.Vuksan-Cusa B, Nadj S, Marcinko D, Jakovljevic M. Metabolic syndrome in patients with bipolar disorder. Eur Psychiatry. 2008;23:S238–9. [Google Scholar]
- 66.Vuksan-Cusa B, Marcinko D, Nad S, Jakovljević M. Differences in cholesterol and metabolic syndrome between bipolar disorder men with and without suicide attempts. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:109–12. doi: 10.1016/j.pnpbp.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 67.Vuksan-Cusa B, Sagud M, Jakovljević M. C-reactive protein and metabolic syndrome in patients with bipolar disorder compared to patients with schizophrenia. Psychiatr Danub. 2010;22:275–7. [PubMed] [Google Scholar]
- 68.Vuksan-Ćusa B, Jakovljević M, Sagud M, Mihaljević Peleš A, Marčinko D, Topić R, et al. Metabolic syndrome and serum homocysteine in patients with bipolar disorder and schizophrenia treated with SGA. Psychiatry Res. 2011;189:21–5. doi: 10.1016/j.psychres.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 69.Yumru M, Savas HA, Kurt E, Kaya MC, Selek S, Savas E, et al. Atypical antipsychotics related metabolic Syndrome in bipolar patients. J Affect Disord. 2007;98:247–52. doi: 10.1016/j.jad.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 70.Kim B, Kim S, McIntyre RS, Park HJ, Kim SY, Joo YH. Correlates of metabolic abnormalities in bipolar I disorder at initiation of acute phase treatment. Psychiatry Investig. 2009;6:78–84. doi: 10.4306/pi.2009.6.2.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sicras-Mainar A, Blanca-Tamayo M, Rejas-Gutiérrez J, Navarro-Artieda R. Metabolic syndrome in outpatients receiving antipsychotic therapy in routine clinical practice: A cross-sectional assessment of a primary health care database. Eur Psychiatry. 2008;23:100–8. doi: 10.1016/j.eurpsy.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 72.Rasgon NL, Kenna HA, Reynolds-May MF, Stemmle PG, Vemuri M, Marsh W, et al. Metabolic dysfunction in women with bipolar disorder: The potential influence of family history of type 2 diabetes mellitus. Bipolar Disord. 2010;12:504–13. doi: 10.1111/j.1399-5618.2010.00839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moreno C, Merchán-Naranjo J, Alvarez M, Baeza I, Alda JA, Martínez-Cantarero C, et al. Metabolic effects of second-generation antipsychotics in bipolar youth: Comparison with other psychotic and nonpsychotic diagnoses. Bipolar Disord. 2010;12:172–84. doi: 10.1111/j.1399-5618.2010.00797.x. [DOI] [PubMed] [Google Scholar]
- 74.Chang HH, Yang YK, Gean PW, Huang HC, Chen PS, Lu RB. The role of valproate in metabolic disturbances in bipolar disorder patients. J Affect Disord. 2010;124:319–23. doi: 10.1016/j.jad.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 75.Corman CL, Leung NM, Guberman AH. Weight gain in epileptic patients during treatment with valproic acid: A retrospective study. Can J Neurol Sci. 1997;24:240–4. doi: 10.1017/s0317167100021879. [DOI] [PubMed] [Google Scholar]
- 76.Garland EJ, Remick RA, Zis AP. Weight gain with antidepressants and lithium. J Clin Psychopharmacol. 1988;8:323–30. [PubMed] [Google Scholar]
- 77.Isojärvi JI, Laatikainen TJ, Knip M, Pakarinen AJ, Juntunen KT, Myllylä VV. Obesity and endocrine disorders in women taking valproate for epilepsy. Ann Neurol. 1996;39:579–84. doi: 10.1002/ana.410390506. [DOI] [PubMed] [Google Scholar]
- 78.Nasrallah HA, Meyer JM, Goff DC, McEvoy JP, Davis SM, Stroup TS, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: Data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86:15–22. doi: 10.1016/j.schres.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 79.Pylvänen V, Pakarinen A, Knip M, Isojärvi J. Insulin-related metabolic changes during treatment with valproate in patients with epilepsy. Epilepsy Behav. 2006;8:643–8. doi: 10.1016/j.yebeh.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 80.Vestergaard P, Poulstrup I, Schou M. Prospective studies on a lithium cohort. 3. Tremor, weight gain, diarrhea, psychological complaints. Acta Psychiatr Scand. 1988;78:434–41. doi: 10.1111/j.1600-0447.1988.tb06363.x. [DOI] [PubMed] [Google Scholar]
- 81.Wu RR, Zhao JP, Jin H, Shao P, Fang MS, Guo XF, et al. Lifestyle intervention and metformin for treatment of antipsychoticinduced weight gain: A randomized controlled trial. JAMA. 2008;299:185–93. doi: 10.1001/jama.2007.56-b. [DOI] [PubMed] [Google Scholar]
- 82.Prossin A, McInnis M, Zalcman S, Ellingrod V. IL-6 is associated with metabolic syndrome in bipolar disorder. Brain Behavior Immunity. 2010;24:S1–71. [Google Scholar]
- 83.Kolotkin RL, Crosby RD, Corey-Lisle PK, Li H, Swanson JM. Performance of a weight-related measure of Quality of Life in a psychiatric sample. Qual Life Res. 2006;15:587–96. doi: 10.1007/s11136-005-4627-4. [DOI] [PubMed] [Google Scholar]
- 84.Bond DJ, Lang DJ, Noronha MM, Kunz M, Torres IJ, Su W, et al. The association of elevated body mass index with reduced brain volumes in first-episode mania. Biol Psychiatry. 2011;70:381–7. doi: 10.1016/j.biopsych.2011.02.025. [DOI] [PubMed] [Google Scholar]


