Abstract
Background:
Cyclic nucleotide Phosphodiesterases (PDEs) are ubiquitously distributed in mammalian tissues and play a major role in cell signaling by hydrolyzing cyclic Adenosine Monophosphate (cAMP) and cyclic Guanosine Monophosphate (cGMP). Impairments in signal transduction have been implicated as possible mechanism of reduced plasticity and neuronal survival in major depressive disorders. PDE inhibitors possess a potentially powerful means to manipulate secondary messengers involved in learning, memory and mood. Cilostazol is an antiplatelet agent indicated for the treatment of intermittent claudication with peripheral artery occlusion and for the prevention of ischemic stroke worldwide. Various animal studies have reported neuroprotective, anti apoptotic, cognition and cerebral blood flow improvement properties of cilostazol.
Materials and Methods:
In this study, the antidepressant and anxiolytic effects of cilostazol were evaluated in mice using behavioral tests sensitive to clinically effective antidepressant compound.
Results:
Cilostazol, administered intraperitoneally (20 mg/kg), decreased immobility time of mice when subjected to forced swim test and tail suspension test as compared to standard fluoxetine (20 mg/kg). Cilostazol also produced significant decrease in the number of marbles buried as compared to fluoxetine in marble burying model.
Conclusion:
The present study suggests that cilostazol possesses potential antidepressant and anxiolytic activity, which could be of therapeutic interest for use in patients with depressive disorders.
Keywords: Cilostazol, forced swim test, marble burying behavior model, PDE3 inhibitor, tail suspension test
INTRODUCTION
According to WHO, depression is the leading cause of disability leading to suicide. Patients with cardiovascular disease are at increased risk of developing depression and, when depression develops, cardiovascular risk is exacerbated further.[1,2] Depression is associated with an approximately two-fold increase in cardiac morbidity and mortality for various coronary heart disease (CHD) populations, including patients with recent acute myocardial infarction (AMI), patients awaiting coronary artery bypass graft (CABG) surgery, and patients post revascularization.[3] Cilostazol has been used as an antiplatelet agent and in intermittent claudication in patients with peripheral vascular disease.[4,5] Moreover, it has antithrombotic, vasodilatory, lipid lowering, and anti proliferative effects.[6] PDE3 are found in hippocampus, striatum, and other sites of brain and may affect of calcium ions and electroshock may modify their activity.[7] Furthermore, studies on CNS by Cilostazol have tried to explore about amelioration of neuronal damage, neuroprotection, improvement of cognitive function, prevention of cerebral ischemia through various mechanisms in preclinical studies.[8–10] Phosphodiesterase (PDE) inhibitors present a potentially powerful means to manipulate second messengers involved in learning, memory, and mood.[11–14] PDE inhibitors are currently being investigated as possible memory enhancers, antidementia drugs, antidepressants, and antipsychotic agents due to location of PDEs in brain at discrete sites.[15–19] Cilostazol served as an alternative to milnacipran for the treatment of patients with post stroke depression as it leads to decrease in Hamilton rating scale for depression (HAM-D) after switching from milnacipran to cilostazol (100mg/day).[20] Thus, cilostazol is being evaluated for antidepressant and anxiolytic activity in mice.
MATERIALS AND METHODS
Animals and environment
Experiments were performed on Swiss albino mice (20-25 gm.) of either sex obtained from Torrent Research Centre, Ahmedabad, Gujarat, India. The animals were maintained in a well ventilated room with 12:12 hour light/dark cycle in polypropylene cages under well controlled temperature (25±2°C) and humidity (55%±5%). Commercially available standard pellet diet (Pranav Agro, Vadodara, India) and drinking water was provided ad libitum. Animals were acclimatized to laboratory conditions one week prior to initiation of experiments. The guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India, were followed and prior permission was granted by the Institutional Animal Ethics Committee of Shri Sarvajanik Pharmacy College, Mehsana with approval number 06/2011 for conducting the study.
Materials
Fluoxetine and Cilostazol were gifted samples from Cadila Pharmaceuticals, Ahmedabad, Gujarat, India.
Grouping of animals and treatment
Animals were divided into three groups of six animals each. Each group was given treatment as shown in [Table 1].
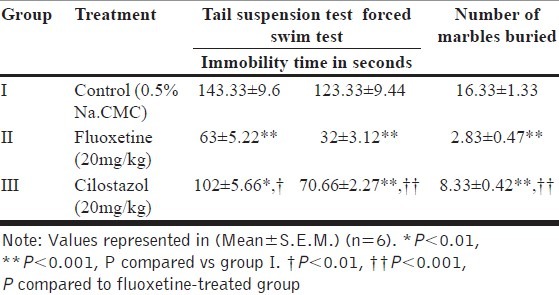
Table 1.
Grouping of animals

Cilostazol was dissolved in 0.5% Na CMC and administered at a daily dose of 20mg.kg-1 to group 3 for 15 days. Fluoxetine was dissolved in distilled water and administered at a dose of 20mg.kg-1 for 7 days.[21] Control animals were administered 0.5% Na CMC solution for 15 days. All treatments were given intraperitoneally. Dosing of animals was carried out every day between 10 am-11:30 am IST. After the dosing period of 7 days (fluoxetine) and 15 days (sodium carboxy methyl cellulose and cilostazol) all animals were moved from their housing colony to laboratory conditions for 1 hr and then exposed to marble burying test, tail suspension test and forced swim test.
Parameters assessed
Antidepressant activity
Tail suspension test
Tail suspension test (TST).[22] was employed for screening antidepressant activity in mice. Sodium carboxy methyl cellulose (Na CMC) and Cilostazol were administered for 15 days. Fluoxetine was administered for 7 days. Treatment drugs were administered 1 hr prior to testing on last day of dosing. Each animal was individually suspended on the edge of a shelf 50cm above a table top with the help of adhesive tape. The total period of immobility was recorded through a video recorder for 6 min and then subsequently analyzed. Mice were considered to be immobile in absence of body movement, hung passively, and were completely motionless. The test was conducted in a dim lighted room and each mouse was used only once in the test.[21,23]
Forced swim test
Forced swim test (FST)[24] was used for screening of antidepressant activity in mice. Na CMC and Cilostazol were administered for 15 days. Fluoxetine was administered for 7 days. Treatment drugs were administered 1 hr prior to testing on last day of dosing. Mice were individually forced to swim in an open circular glass chamber of diameter 15 cm and height 25 cm containing fresh water to a height of 15 cm and maintained at 25±1°C. At this height of water, animals were not able to support themselves by touching the bottom or the side walls of the chamber with their hind paws or tail. Water in the chamber was changed after subjecting each animal to FST since used water alters the behavior. Animal shows vigorous movements during initial 2 min swimming session. The duration of immobility was recorded for a period of next 4 min of the total 6 min swimming session with the help of video recorder and subsequently analyzed.[24]
Mice were considered immobile when they ceased struggling and remained floating motionless in water, making only those movements necessary to keep their head above water. Following swimming session, mice were towel dried, and returned to their housing condition.[23]
Anxiolytic activity
Marble-burying behavior model
In this method, animals were individually placed in a transparent poly carbonate cages (22 × 32 ×13.5 cm), containing 5 cm layer of saw dust and twenty four glass marbles (1.5 cm in diameter) that were evenly distributed on the saw dust in the cages. Sodium CMC and Cilostazol were administered for 15 days. Fluoxetine was administered for 7 days. Treatment drugs were administered 1 hr prior to testing on last day of dosing. Animals were kept in the cages for a period of 30 minutes and the number of marbles at least two-third buried in the saw dust was recorded.[25,26]
Statistical analysis
All the data represent Mean±S.E.M values for immobility time and number of marbles buried as shown in [Table 2]. The data were analyzed by using Graph Pad in Stat by one way analysis of variance (ANOVA). The test was followed by Tukey-kramer multi comparison test, P values less than 0.05 were considered as statistically significant. Analysis was performed using Graph Pad in Stat software (version 3.01, GraphPad Software Inc.).
Table 2.
Effects of fluoxetine and cilostazol on tail suspension test, forced swim test and marble burying behavior
RESULTS
Tail suspension test
In this test [Figure 1], animals treated with cilostazol (20 mg/kg) showed decrease in immobility time, which was significant (102±5.66; P<0.01) when compared with control (143.33±9.6). Animals treated with Fluoxetine (20 mg/kg), also showed a significant decrease in the immobility time (63±5.22; P<0.001) as compared to control (143.33±9.6). Similarly animals treated with Cilostazol showed significant decrease in immobility time (102±5.66; P<0.01) and thus effective antidepressant activity when compared to Fluoxetine (63±5.22).
Figure 1.
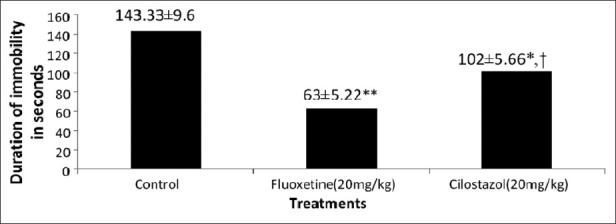
Effects of cilostazol and fluoxetine on duration of immobility in the TST. Note: Results are expressed as Mean±S.E.M (n=6). *P<0.01 and **P<0.001 as compared to control group. †P<0.01 as compared to fluoxetine
Forced swim test
In this test [Figure 2], after intraperitoneal administration of cilostazol (20 mg/kg) there was extremely significant decrease in immobility time (70.66±2.27; P<0.001) as compared to control (123.33±9.44). Animals treated with fluoxetine (20 mg/kg), showed significant decrease in the immobility time (32±3.12; P<0.001) when compared to control (123.33±9.44). Cilostazol also showed significant decrease in immobility time (63±5.22; P<0.001) and thus marked antidepressant activity when compared to Fluoxetine (32±3.12).
Figure 2.
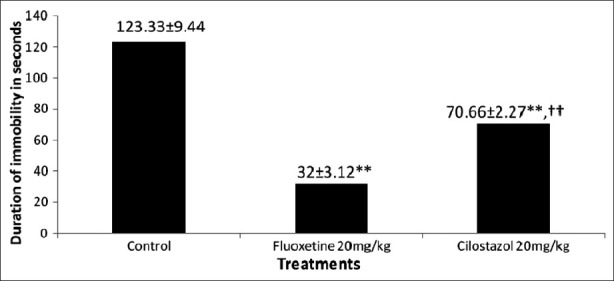
Effects of Cilostazol and fluoxetine on duration of immobility in the FST. Note: Results are expressed as Mean±S.E.M (n = 6). **P<0.001 as compared to control group. ††P<0.001 as compared to fluoxetine
Marble burying model
In this test [Figure 3], a significant decrease in the number of marbles buried (8.33±0.42; P<0.001) was observed with intraperitoneal administration of cilostazol (20 mg/kg) as compared to control (16.33±1.33). Similarly, animals treated with fluoxetine (20 mg/kg) showed significant decrease in the number of marbles buried (2.83±0.47; P<0.001) when compared to control (16.33±1.33). Cilostazol also showed significant decrease in marble burying (8.33±0.42; P<0.001) and thus marked anxiolytic activity when compared to fluoxetine (2.83±0.47).
Figure 3.

Effects of cilostazol and fluoxetine on number of marbles buried in the marble burying model. Note: Results are expressed as Mean±S.E.M (n=6). **P<0.001 as compared to control group. ††P<0.001 as compared to fluoxetine
DISCUSSION AND CONCLUSION
This study was initiated with an objective to evaluate the preclinical antidepressant activity of cilostazol at a dose equivalent to clinically therapeutic antiplatelet dose (50 mg BD) of cilostazol and the duration of treatment was also same as that for clinical antiplatelet response.[27] In the present study, the tail suspension test and forced swim test were carried out to assess antidepressant activity of cilostazol and the marble burying behavior model was done to assess the anxiolytic activity of cilostazol.
In forced swim test and tail suspension test, a normal animal submitted to a non-soluble aversive situation alternate between agitation and immobility. The reason of agitation is searching, it is highly energy consuming, while the purpose of immobility is energy conservation. Animals after antidepressant treatment struggle more even in desperate situation, and they spend less time with immobility.[28]
Fluoxetine and cilostazol treated animals showed significant reduction in immobility time as compared to control group in forced swim test and tail suspension test, which is indicative of antidepressant activity of cilostazol. Moreover, clinical study has shown that cilostazol possesses antidepressant effect as evidenced through decrease in Montgomery-Asberg Depression rating Scale scoring compared to baseline ratings. Cilostazol when used in cardiovascular patients with depression who underwent angioplasty and on adjuvant dual antiplatelet therapy has shown prominent results for treatment of mild to moderate depression.[29]
Anxiety is a feeling of apprehension, worry, or uneasiness that may or may not be based on reality.[30] The co-occurrence of anxiety and depression represents more severe and chronic illness and anxiety has been shown to increase the risk of suicidality in older patients with depression. Marble burying behavior is thought to be an expression of defensiveness, some forms of anxiety, obsessiveness, or compulsiveness.[31] In the present study, cilostazol was also evaluated for its anxiolytic activity through marble burying behavior model. Cilostazol showed a significant decrease in number of marbles buried as compared to control suggesting the anxiolytic activity of cilostazol.
It can be concluded that cilostazol possesses antidepressant and anxiolytic like activity by increasing cAMP in hippocampus through PDE3 inhibition, which could be of therapeutic interest for use in patients with depressive disorders. Moreover, cilostazol could serve as dual antiplatelet and antidepressant in patient with cardiovascular disease and depression. Although it has been established that cilostazol improves cognitive function in mice by increasing the production of insulin-like growth factor-I in the hippocampus and that the peripheral and central production of insulin-like growth factor-I is responsible for antidepressant like behavior.[10,32] Further studies are required to confirm the exact mechanism for antidepressant and anxiolytic activity of cilostazol.
ACKNOWLEDGMENT
The authors thank Cadilla Pharmaceuticals Ltd. Ahmedabad, for providing free gift sample of the drugs for research.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Lett HS, Blumenthal JA, Babyak MA, Sherwood A, Strauman T, Robins C, et al. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med. 2004;66:305–15. doi: 10.1097/01.psy.0000126207.43307.c0. [DOI] [PubMed] [Google Scholar]
- 2.Frasure-Smith N, Lesperance F, Habra M, Talajic M, Khairy P, Dorian P, et al. Atrial Fibrillation and Congestive Heart Failure Investigators. Elevated depression symptoms predict long-term cardiovascular mortality in patients with atrial fibrillation and heart failure. Circulation. 2009;120:134–40. doi: 10.1161/CIRCULATIONAHA.109.851675. [DOI] [PubMed] [Google Scholar]
- 3.Lett H, Ali S, Whooley M. Depression and Cardiac Function in Patients with Stable Coronary Heart Disease: Findings from the Heart and Soul Study. Psychosom Med. 2008;70:444–9. doi: 10.1097/PSY.0b013e31816c3c5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kimura Y, Tani T, Kanbe T, Watanabe K. Effect of cilostazol on platelet aggregation and experimental thrombosis. Arzneimittelforschung. 1985;35:1144–9. [PubMed] [Google Scholar]
- 5.Dawson DL, Cutler BS, Meissner MH, Strandness DE., Jr Cilostazol has beneficial effects in treatment of intermittent claudication: Results from a multicenter, randomized, prospective, double-blind trial. Circulation. 1998;98:678–86. doi: 10.1161/01.cir.98.7.678. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Shakur Y, Yoshitake M, Kambayashi Ji J. Cilostazol (Pletal): A dual inhibitor of cyclic nucleotide phosphodiesterase type 3 and adenosine uptake. Cardiovascular Drug Review. 2001;19:369–86. doi: 10.1111/j.1527-3466.2001.tb00076.x. [DOI] [PubMed] [Google Scholar]
- 7.Nandhakumar J, Raja AK, Tyagi MG. Characterization of effects of phosphodiesterase (PDE) isozyme inhibitors in animal models of epilepsy. Life Sci Med Res. 2010:1–9. [Google Scholar]
- 8.Lee JH, Kim KY, lee YK, Park SY, Kim CD, Lee WS, et al. Cilostazol Prevents Focal Cerebral Ischemic Injury by Enhancing Casein Kinase 2 Phosphorylation and Suppression of Phosphatase and Tensin Homolog Deleted from Chromosome 10 Phosphorylation in rats. J Pharmacol Experiment Thera. 2004;308:896–903. doi: 10.1124/jpet.103.061853. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe T, Zhang N, Liu M, Tanaka R, Mizuno Y, Urabe T. Cilostazol protects against brain white matter damage and cognitive impairment in a rat model of chronic cerebral hypoperfusion. Stroke. 2006;37:1539–45. doi: 10.1161/01.STR.0000221783.08037.a9. [DOI] [PubMed] [Google Scholar]
- 10.Zhao J, Harada N, Kurihara H, Nakagata N, Okajima K. Cilostazol improves cognitive function in mice by increasing the production of insulin-like growth factor-I in the hippocampus. Neuropharmacology. 2009;58:774–83. doi: 10.1016/j.neuropharm.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Hebb AL, Robertson HA. Role of phosphodiesterases in neurological and psychiatric disease. Curr Opin Pharmacol. 2007;7:86–92. doi: 10.1016/j.coph.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 12.Houslay MD, Schafer P, Zhang YJ. Keynote review: phosphodiesterase-4 as a therapeutic target. Drug Discov Today. 2005;10:1503–19. doi: 10.1016/S1359-6446(05)03622-6. [DOI] [PubMed] [Google Scholar]
- 13.Houslay MD, Baillie GS, Maurice DH. cAMP-specific phosphodiesterase-4 enzymes in the cardiovascular system: A molecular toolbox for generating compartmentalized cAMP signaling. Circ Res. 2007;100:950–66. doi: 10.1161/01.RES.0000261934.56938.38. [DOI] [PubMed] [Google Scholar]
- 14.O’Donnell JM, Zhang HT. Antidepressant effects of inhibitors of cAMP phosphodiesterase (PDE4) Trends Pharmacol Sci. 2004;25:158–63. doi: 10.1016/j.tips.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Zhang HT, Huang Y, Jin SL, Frith SA, Suvarna N, Conti M, et al. Antidepressant-like profile and reduced sensitivity to rolipram in mice deficient in the PDE4D phosphodiesterase enzyme. Neuropsychopharmacology. 2002;27:587–95. doi: 10.1016/S0893-133X(02)00344-5. [DOI] [PubMed] [Google Scholar]
- 16.Maxwell CR, Kanes SJ, Abel T, Siegel SJ. Phosphodiesterase inhibitors: A novel mechanism for receptor-independent antipsychotic medications. Neuroscience. 2004;129:101–7. doi: 10.1016/j.neuroscience.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 17.Campbell E, Edwards T. Zaprinast consolidates long-term memory when administered to neonate chicks trained using a weakly reinforced single trial passive avoidance task. Behav Brain Res. 2006;169:181–5. doi: 10.1016/j.bbr.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Itoh T, Tokumura M, Abe K. Effects of rolipram, a phosphodiesterase 4 inhibitor, in combination with imipramine on depressive behavior, CRE-binding activity and BDNF level in learned helplessness rats. Eur J Pharmacol. 2004;498:135–42. doi: 10.1016/j.ejphar.2004.07.084. [DOI] [PubMed] [Google Scholar]
- 19.Menniti FS, Chappie TA, Humphrey JM, Schmidt CJ. Phosphodiesterase 10A inhibitors: a novel approach to the treatment of the symptoms of schizophrenia. Curr Opin Investig Drugs. 2007;8:54–9. [PubMed] [Google Scholar]
- 20.Nishimura K, Ishigooka J, Imamura Y, Ihara S. Cilostazol, a cAMP Phosphodiesterase 3 Inhibitor in the Treatment of Poststroke Depression. J Neuropsychiatry Clin Neurosci. 2007;19:471–2. doi: 10.1176/jnp.2007.19.4.471. [DOI] [PubMed] [Google Scholar]
- 21.Kalra BS, Tayal V, Chawla S. Antidepressant like activity of tramadol in mice. Indian J Psychiatry. 2008;50:51–3. doi: 10.4103/0019-5545.39760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology. 1985;85:367–70. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 23.Santosh P, Venugopal R, Nilakash AS, Kunjbihari S, Mangala L. Antidepressant activity of methanolic extract of passiflora foetida leaves in mice. Int J Pharm Pharm Sci. 2011;3:112–5. [Google Scholar]
- 24.Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–2. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 25.Rahman H, Muralidharan P, Sivaraman D, Kartika B, Saha D. Evaluation of anxiolytic activity of ethanolic extracts from the leaves of Trichosanthes cucumerina L. in mice. Der Pharmacia Sinica. 2010;1:86–94. [Google Scholar]
- 26.Njunge K, Handley SL. Evaluation of Marble-Burying Behavior as a Model of Anxiety, Pharmacology Biochemistry and Behavior. 1991;38:63–7. doi: 10.1016/0091-3057(91)90590-x. [DOI] [PubMed] [Google Scholar]
- 27.Patient information leaflet. [Assessed on 2010 Dec 15]. Available from: http://www.cipladoc.com/therapeutic/pdf_cipla/pletoz.pdf , updated on May 2010 .
- 28.Badhe SR, Badhe RV, Ghaisas MM, Chopade VV, Deshpande AD. Evaluations of antidepressant activity of Anacyclus pyrethrum root extract. Int J Green Pharm. 2010;4:79–82. [Google Scholar]
- 29.Bhatt PA, Patel DS, Anand IS, Shah UG, Patel SB. Antidepressant Activity of Phosphodiesterase 3 Inhibitor: Cilostazol. Int J Pharm Clin Res. 2011;3:52–4. [Google Scholar]
- 30.Roach SS. 7th ed. NY: Lippincott Williams and Wilkins; 2007. Introduction to clinical pharmacology and Drug Guide. (Visible Productions LLC) © 2004 ISBN: 0-7817-3696-X, © 2003 ISBN: 0-7817-1751-5. [Google Scholar]
- 31.Homma C, Yamada K. Physical Properties of Bedding Materials Determine the Marble Burying Behavior of Mice (C57BL/6J) Open Behav Sci J. 2009;3:34–9. [Google Scholar]
- 32.Duman CH, Schlesinger L, Terwilliger R, Russell DS, Newton SS, Duman RS. Peripheral insulin-like growth factor-I produces antidepressant-like behavior and contributes to the effect of exercise. Behav Brain Res. 2009;198:366–71. doi: 10.1016/j.bbr.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]


