Abstract
Background:
Entorhinal cortex (ERC), a multimodal sensory relay station for the hippocampus, is critically involved in learning, emotion, and novelty detection. One of the pathogenetic mechanistic bases in schizophrenia is proposed to involve aberrant information processing in the ERC. Several studies have looked at cytoarchitectural and morphometric changes in the ERC, but results have been inconsistent possibly due to the potential confounding effects of antipsychotic treatment.
Materials and Methods
In this study, we have examined the entorhinal cortex volume in antipsychotic-naïve schizophrenia patients (n=40; M:F=22:18) in comparison with age, sex, and handedness, matched (as a group) with healthy subjects (n=42; M:F=25:17) using a valid method. 3-Tesla MR images with 1-mm sections were used and the data was analyzed using the SPSS software.
Results:
Female schizophrenia patients (1.25±0.22 mL) showed significant volume deficit in the right ERC in comparison with female healthy controls (1.45±0.34 mL) (F=4.9; P=0.03), after controlling for the potential confounding effects of intracranial volume. However, male patients did not differ from male controls. The left ERC volume did not differ between patients and controls.
Conclusions:
Consistent with the findings of a few earlier studies we found a reduction in the right ERC volume in patients. However, this was limited to women. Contextually, our study finding supports the role for ERC deficit in schizophrenia pathogenesis — perhaps mediated through aberrant novelty detection. Sex-differential observation of ERC volume deficit in schizophrenia needs further studies.
Keywords: Entorhinal cortex, hippocampus, schizophrenia
INTRODUCTION
Schizophrenia is a disorder characterized by significant abnormalities in cognitive functioning and language, apart from the presence of delusions and hallucinations.[1] There is considerable evidence about the involvement of medial temporal lobe structures in this condition.[2,3] The entorhinal cortex (ERC) is an important brain area located in the medial temporal lobe and is involved in memory and learning.[4–6] Its position is almost in the rostral and ventral portion of the S-shaped infolding of the hippocampal formation corresponding to the Brodmann area 28 and 34.[7] This area is known to be an important gateway responsible for the integration of information between the hippocampus and neocortical regions[6,8]; information-processing deficits, perhaps secondary to aberrant integration, has been reported in schizophrenia. Hence, it is conceivable that ERC could be a substrate in the pathophysiology of schizophrenia. Indeed, there is significant evidence to suggest that ERC is an important brain region where neuronal migrational disturbances have been demonstrated to occur in schizophrenia, supporting the notion that developmental abnormalities are a core feature of this illness.[9] Post-mortem studies suggest an important role of this critical brain region in schizophrenia revealing cytoarchitectural changes like lesser neuronal size and neuronal displacement.[10,11]
The previous magnetic resonance imaging (MRI) studies examining the volume of ERC have provided conflicting results. To date, there have been eight studies aiming at the volumetric analysis of this structure using a region of interest (ROI) paradigm. Five studies,[12–15] including the most recent one,[16] have reported volume deficit, whereas, three studies[8,17,18] have found no volume change compared to the healthy controls. The interpretation of some of the earlier studies is limited because of the treatment of patients with psychotropic medications. In addition, the study that examined drug-naïve subjects had a limited sample size. In accordance with the background literature implicating ERC in schizophrenia (as reviewed earlier), especially due to its relevance in the neurodevelopmental model of the illness,[19] we hypothesized that antipsychotic naïve schizophrenia patients would have a deficit in ERC volume in comparison to normal healthy subjects. Hence, we have aimed to examine the volume of ERC in a large sample of antipsychotic naïve adult subjects with schizophrenia compared with matched healthy controls.
MATERIALS AND METHODS
Subjects
Forty patients, who fulfilled the DSM-IV criteria for schizophrenia, were recruited from the clinical services of the National Institute of Mental Health and Neurosciences (India), for the study. The Institute's ethics committee approved the study. The structured clinical interview for DSM-IV disorders[20] was initially administered for diagnosing schizophrenia. This diagnosis was confirmed following an independent clinical interview by an experienced psychiatrist. Illness duration and age-at-onset of the first psychotic episode, as defined by the report of psychotic symptoms, were assessed using the Interview for the Retrospective Assessment of the Onset of Schizophrenia.[21] These details related to the onset of illness and antipsychotic-naïve status were carefully ascertained by reliable information obtained from at least one first-degree relative. None of the patients were ever treated with any psychotropic medications, including antipsychotics. Also, none had received electroconvulsive therapy previously. Psychopathology was assessed using the Scale for the assessment of positive symptoms (SAPS) and Scale for the assessment of negative symptoms (SAPS).[22,23]
Age- and sex-matched (as a group) healthy comparison subjects (n=42) were recruited via ‘word of mouth’. Healthy controls (HC) were evaluated in detail to rule out history suggestive of psychiatric illness and were also screened using the General Health Questionnaire[24] and comprehensive mental status examination. None of the control subjects had a family history of psychosis in any of their first-degree relatives.
All subjects were right-handed as assessed by Annett's questionnaire.[25] None of the subjects had any contraindication to magnetic resonance imaging (MRI). None had any neurological / systemic illness, seizure disorder, history suggestive of delayed developmental milestones or history of significant head injury. Neither the patients nor the controls were in any clinically significant, nutritionally deprived state. The substance-use history was carefully ascertained from the subject with corroboration by at least one first-degree relative. Patients and controls did not have any current or past history of alcohol abuse or dependence. None of them used cannabis, opiates, stimulant or any other illicit drug of abuse. The female subjects were neither pregnant nor were within the postpartum period. None of the subjects had dyskinesia (as assessed using the Abnormal Involuntary Movements Scale[26]) or parkinsonism (as assessed using the Simpson and Angus Scale[27]). Clinical assessments and MRI acquisition were performed on the same day, before starting antipsychotics. After complete description of the study to the subjects (and caregivers of patients), a written informed consent was obtained.
Magnetic resonance imaging acquisition
Magnetic Resonance Imaging (MRI) was done using a Philips 3 Tesla scanner (Achieva, Best, The Netherlands). A T1-weighted, three-dimensional, structural MRI was performed (TR=8.1 msec, TE=3.7 msec, nutation angle=8°, FOV=256 mm, slice thickness 1 mm without inter-slice gap, NEX=1, matrix=256 × 256), yielding 165 sagittal slices. The images were stored with coded identification for blinded rating.
Anatomical guideline for entorhinal cortex
The anatomical localization guideline used has been adapted from Benasconi et al.[28] and is described as follows. The human ERC is located in the rostral half of the ventromedial temporal lobe and corresponds to Brodmann's areas 28 and 34. It covers the rostral parahippocampal gyrus, extending from approximately 2 to 3 mm behind the frontotemporal junction (temporal stem) to the rostral pole of the lateral geniculate nucleus. The major portion of the ERC is located within the region of the uncus, and approximately 50% of it lies ventromedial to the amygdale. The anterior border of the ERC begins 2 to 3 mm behind the frontotemporal junction (temporal stem). The temporal stem was defined as the junction between the white matter of the temporal lobe and the white matter underlying the insular cortex. Although the distance from the stem to the rostral pole may vary, the variation is approximately 2 mm. Hence, the anterior-most slice in which the temporal stem is visible was chosen. Medially, the ERC forms the surface of gyrus ambiens. The medial limit of the ERC is marked by the sulcus semiannularis rostrally and by the uncal cleft caudally. On the MR images, we have chosen the sulcus semiannularis and the uncal cleft as the superomedial boundaries. The sulcus semiannularis has been used as the boundary separating the amygdala above from the ERC below. The uncal cleft is used as the border between the hippocampal head above and the ERC below, when it is visible. The ERC is bordered laterally by the perirhinal cortex. The transition between the entorhinal and perirhinal cortices in humans is not defined by any gross morphological feature, and takes place at some point along the medial bank of the collateral sulcus. The lateral limit of the ERC is at the midpoint of the medial bank of the collateral sulcus. The posterior boundary is defined as being 1cm caudal to the beginning of the uncal cleft.
Volumetric method
All measurements were automatically calculated by the computer using the 3D Slicer software (http://www.slicer.org/).[29] The desired structure was outlined and measured by the rater (ES) using the computer mouse controlled pointer. The rater was blind to the subjects’ clinical details at the time of brain measurements on the coded MRI sections. The ERC contours were outlined in all brains by a single investigator (ES), blind to group status [Figure 1]. The intracranial volume was calculated automatically using the Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (FSL, http://www.fmrib.ox.ac.uk/fsl/).
Figure 1.
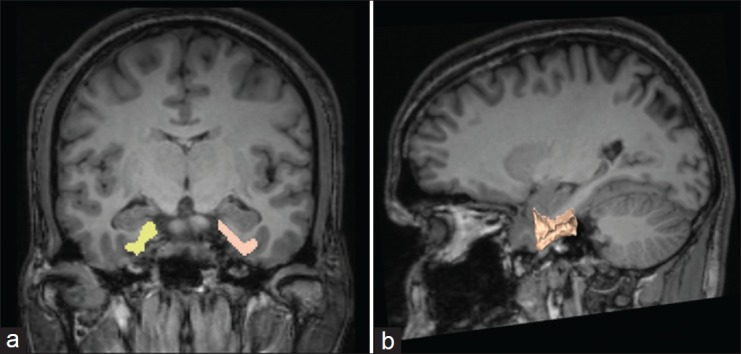
Representative tracings of ERC volume using 3D-slicer software
Statistical analysis
The statistical analyses were performed using the Statistical Package for Social Sciences (version-11) (SPSS Inc.,). The normality of the data was examined using the Shapiro–Wilk test, after satisfying which parametric statistical tests were applied for comparison. The sociodemographic data were analyzed using the independent samples t-test and the Chi-Square test. The Pearson's correlation analysis was employed to examine the relationship between the variables of age, duration of illness, the ERC volumes, and the clinical severity rating scores. In addition, the ERC volume differences between schizophrenia patients and HC were examined using the Analysis of Covariance (ANCOVA), with intracranial volume as a covariate.
RESULTS
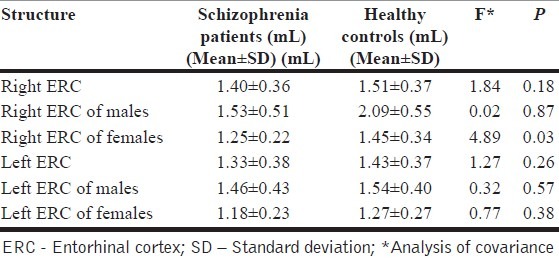
The gender distribution (M : F) in the schizophrenia group and HC group was 22 : 18 and 25 : 17, respectively. The groups did not differ based on the gender distribution (χ2=0.17, P=0.7) and age (mean±SD) (28.5±6.6 vs. 27.9±7.0, t=0.42, P=0.67). The mean duration of illness (same as duration of untreated psychosis) in the schizophrenia group was 30.2±35.2 months (median=15 months). The mean SANS and SAPS scores were 69.4±29.6 and 29.4±14.8, respectively. As shown in Table 1, there were no differences between the volumes of ERC on either side between the groups as a whole. However, the female schizophrenia patients showed a significant volume reduction in the right ERC in comparison with the female HC (F=4.9, P=0.03), after controlling for the potential confounding effects of intracranial volume. However, male patients did not differ from the male controls. The left ERC volume did not differ between patients and controls in both the sexes. Similarly, the asymmetry index (L-R) was also not different between the groups (F=0.01, P=0.95).
Table 1.
Comparison of entorhinal cortex volumes between schizophrenia patients and healthy controls
There were no significant correlations between the ERC volumes on either side with the clinical severity ratings using SANS and SAPS, duration of illness / duration of untreated psychosis (DUP), and age. In addition, there was no difference between male and female patients with schizophrenia in SAPS (26.0±12.1 vs. 33.5±17.0, t=1.6, P=0.1) and SANS (73.1±30.4 vs. 64.8±28.8, t=0.88, P=0.36) scores.
DISCUSSION
Although ERC has been proposed as an important brain region of aberration in schizophrenia, it has received less attention compared to other regions like the hippocampus. This study examines the volume of ERC in a relatively large sample of antipsychotic-naïve schizophrenia patients. The main study finding was that female schizophrenia patients demonstrated a significant deficit in the right ERC volume in comparison to female healthy controls.
As described earlier, the ERC has been proposed as a ‘gateway region’ between limbic structures and the neocortex. The ERC functions as a critical part of the elements that form the ‘novelty detection circuit,’ which include the prefrontal cortex, hippocampus, the parahippocampal region, and the cingulate.[4] ERC is postulated as a buffer that holds real sensory information, while the hippocampus compares it with the internal representation of stimuli, to detect novelty versus familiarity.[30,31] Thus, ERC plays a significant role in processing episodic and autobiographical memory, and in associative learning.[32,33] Aberration in the ERC is thus conceived as important in the pathophysiology of schizophrenia, as the aforesaid cognitive impairments are vital in schizophrenia.[30,34]
Neuropathological abnormalities in ERC point toward a neurodevelopmental etiology of an illness, as the changes involve decrease in neuron size, aberrant invagination of the surface, and disruption of cortical layers.[10,11] Microstructural changes in ERC have been documented in schizophrenia brains earlier in the study, which employed structural MRI as well as Diffusion Tensor Imaging (DTI).[8] According to this study, the observed deficits may lead to disturbances in the perforant path, which is essentially a large entorhinal efferent fiber tract to the limbic system, resulting in impairments of cognition. This data on change in the signal transduction has been further documented by the evidence of altered expression of specific microtubule proteins in the ERC of schizophrenia patients.[35] Chondroitin sulfate proteoglycan–related abnormalities that involve glial cells and the extracellular matrix have been detected in the amygdala and entorhinal cortex of subjects with schizophrenia.[36] This suggests impairments in mechanisms that regulate developmental and adult neural functions that are highly significant to the etiology of schizophrenia. The above data implies that there is significant pathology in the ERC, in schizophrenia, which provides information on its role in the developmental model of the illness and the associated cognitive changes seen in the illness.
The earlier attempts at examining the volume of the entorhinal cortex in schizophrenia brains compared to HC, using MRI, have yielded mixed results. Three studies have reported that there is no significant difference in the ERC between schizophrenia and HC.[8,17,18] However, there are five studies, to date, which have reported ERC volume deficits in schizophrenia. Joyal et al. detected bilateral ERC volume deficit in antipsychotic naïve subjects in a study with 18 subjects with schizophrenia.[12] A similar result was obtained in another study earlier, which found a specificity of this finding against bipolar disorder.[13] Bilateral ERC volume reduction was documented, even though there was no correlation with the olfactory threshold and memory function in schizophrenia, in another study.[14] In a study by Prasad et al., the patients with schizophrenia and related disorders and patients with non-schizophrenic psychotic disorders had smaller left entorhinal cortex volumes than healthy subjects.[15] In this study, in patients with schizophrenic disorders, the entorhinal cortex volume positively correlated with severity of delusions, suggesting that the ERC pathology could also be responsible for psychopathology of schizophrenia. More recently, a study with a larger sample of schizophrenia patients (n=70) reported a significant decrease in the right ERC volume compared to HC.[16] Our study with a good sample size, however, did not observe ERC volume deficit in schizophrenia subjects considered as a group compared to HC. The reason for this difference between our study and the recent study by Baiano et al. could be due to many factors. The mean age of the schizophrenia patients of our sample was 28.5±6.6 years as against the mean age of Baiano et al.'s sample of 39.9±12.1 years. The subjects in our sample were neuroleptic-naïve, whereas, the Baiano et al. sample had a long duration of antipsychotic treatment (12.6±10.6 years). In addition, the mean duration of illness in our sample was very short (median of 15 months), whereas, Baiano et al. had a sample with longer duration of illness (14.1±10.7 years). Thus, illness duration, antipsychotic exposure, and age of the subjects could have influenced the difference between the studies. However, what is intriguing is that the volume deficit of the right ERC in schizophrenia patients of the present study sample is restricted to females. The reason for this sex-specific volume alteration is unclear. There is evidence for sexual dimorphism in other limbic structures, like the amygdale, in schizophrenia.[37] The gender-specific lateralization in volume difference has been explained based on gender-specific lateralization of the limbic structures’ involvement in emotional processing.[37,38] Hence, the ERC volume difference based on sexual dimorphism in schizophrenia could possibly be explained on similar lines.
The present study has the advantage of a relatively large sample size of neuroleptic naïve schizophrenia patients and a very short duration of illness, thus avoiding the potential influences of important confounding variables. In conclusion, the ERC volume is found to be deficient in schizophrenia on the right side, and this deficit appears to display sexual dimorphism. The involvement of ERC as a substrate in schizophrenia underlines its role as a relay center between the limbic system and the cortex. This emphasizes its role in cognitive functions affected in schizophrenia, like the processing of emotionally salient information, memory processing, and associative learning; and thereby in the formation of psychopathology, like delusions. The sexual dimorphism in the structure could be related to the gender-specific lateralization of the limbic structures’ involvement in emotional processing. Future studies could examine this issue using larger samples, and both structural and functional imaging paradigms. In addition, given the importance of ERC in the developmental model of schizophrenia, it would be interesting to examine the volume of this structure in subjects at high risk to develop psychosis.
ACKNOWLEDGMENT
This study is supported by the Innovative Young Biotechnologist Award to GV by the Department of Biotechnology.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Van Os J, Kapur S. Schizophrenia. Lancet. 2009;374:635–45. doi: 10.1016/S0140-6736(09)60995-8. [DOI] [PubMed] [Google Scholar]
- 2.Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi T, Suzuki M, Zhou SY, Tanino R, Hagino H, Niu L, et al. Temporal lobe gray matter in schizophrenia spectrum: A volumetric MRI study of the fusiform gyrus, parahippocampal gyrus, and middle and inferior temporal gyri. Schizophr Res. 2006;87:116–26. doi: 10.1016/j.schres.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 4.Witter MP, Wouterlood FG, Naber PA, Van Haeften T. Anatomical organization of the parahippocampal-hippocampal network. Ann N Y Acad Sci. 2000;911:1–24. doi: 10.1111/j.1749-6632.2000.tb06716.x. [DOI] [PubMed] [Google Scholar]
- 5.Fransen E. Functional role of entorhinal cortex in working memory processing. Neural Netw. 2005;18:1141–9. doi: 10.1016/j.neunet.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Fyhn M, Molden S, Witter MP, Moser EI, Moser MB. Spatial representation in the entorhinal cortex. Science. 2004;305:1258–64. doi: 10.1126/science.1099901. [DOI] [PubMed] [Google Scholar]
- 7.Pruessner JC, Kohler S, Crane J, Pruessner M, Lord C, Byrne A, et al. Volumetry of temporopolar, perirhinal, entorhinal and parahippocampal cortex from high-resolution MR images: Considering the variability of the collateral sulcus. Cereb Cortex. 2002;12:1342–53. doi: 10.1093/cercor/12.12.1342. [DOI] [PubMed] [Google Scholar]
- 8.Kalus P, Slotboom J, Gallinat J, Federspiel A, Gralla J, Remonda L, et al. New evidence for involvement of the entorhinal region in schizophrenia: A combined MRI volumetric and DTI study. Neuroimage. 2005;24:1122–9. doi: 10.1016/j.neuroimage.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Kovalenko S, Bergmann A, Schneider-Axmann T, Ovary I, Majtenyi K, Havas L, et al. Regio entorhinalis in schizophrenia: More evidence for migrational disturbances and suggestions for a new biological hypothesis. Pharmacopsychiatry. 2003;36(Suppl. 3):S158–61. doi: 10.1055/s-2003-45124. [DOI] [PubMed] [Google Scholar]
- 10.Arnold SE, Hyman BT, Van Hoesen GW, Damasio AR. Some cytoarchitectural abnormalities of the entorhinal cortex in schizophrenia. Arch Gen Psychiatry. 1991;48:625–32. doi: 10.1001/archpsyc.1991.01810310043008. [DOI] [PubMed] [Google Scholar]
- 11.Jakob H, Beckmann H. Prenatal developmental disturbances in the limbic allocortex in schizophrenics. J Neural Transm. 1986;65:303–26. doi: 10.1007/BF01249090. [DOI] [PubMed] [Google Scholar]
- 12.Joyal CC, Laakso MP, Tiihonen J, Syvalahti E, Vilkman H, Laakso A, et al. A volumetric MRI study of the entorhinal cortex in first episode neuroleptic-naive schizophrenia. Biol Psychiatry. 2002;51:1005–7. doi: 10.1016/s0006-3223(01)01368-3. [DOI] [PubMed] [Google Scholar]
- 13.Pearlson GD, Barta PE, Powers RE, Menon RR, Richards SS, Aylward EH, et al. Ziskind-Somerfeld Research Award 1996. Medial and superior temporal gyral volumes and cerebral asymmetry in schizophrenia versus bipolar disorder. Biol Psychiatry. 1997;41:1–14. doi: 10.1016/s0006-3223(96)00373-3. [DOI] [PubMed] [Google Scholar]
- 14.Turetsky BI, Moberg PJ, Roalf DR, Arnold SE, Gur RE. Decrements in volume of anterior ventromedial temporal lobe and olfactory dysfunction in schizophrenia. Arch Gen Psychiatry. 2003;60:1193–200. doi: 10.1001/archpsyc.60.12.1193. [DOI] [PubMed] [Google Scholar]
- 15.Prasad KM, Patel AR, Muddasani S, Sweeney J, Keshavan MS. The entorhinal cortex in first-episode psychotic disorders: A structural magnetic resonance imaging study. Am J Psychiatry. 2004;161:1612–9. doi: 10.1176/appi.ajp.161.9.1612. [DOI] [PubMed] [Google Scholar]
- 16.Baiano M, Perlini C, Rambaldelli G, Cerini R, Dusi N, Bellani M, et al. Decreased entorhinal cortex volumes in schizophrenia. Schizophr Res. 2008;102:171–80. doi: 10.1016/j.schres.2007.11.035. [DOI] [PubMed] [Google Scholar]
- 17.Sim K, DeWitt I, Ditman T, Zalesak M, Greenhouse I, Goff D, et al. Hippocampal and parahippocampal volumes in schizophrenia: A structural MRI study. Schizophr Bull. 2006;32:332–40. doi: 10.1093/schbul/sbj030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nasrallah HA, Sharma S, Olson SC. The volume of the entorhinal cortex in schizophrenia: A controlled MRI study. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:1317–22. doi: 10.1016/s0278-5846(97)00166-8. [DOI] [PubMed] [Google Scholar]
- 19.Rapoport JL, Addington AM, Frangou S, Psych MR. The neurodevelopmental model of schizophrenia: Update 2005. Mol Psychiatry. 2005;10:434–49. doi: 10.1038/sj.mp.4001642. [DOI] [PubMed] [Google Scholar]
- 20.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders: User's Guide. New York: American Psychiatric Press; 1997. [Google Scholar]
- 21.Hafner H, Riecher-Rossler A, Hambrecht M, Maurer K, Meissner S, Schmidtke A, et al. IRAOS: An instrument for the assessment of onset and early course of schizophrenia. Schizophr Res. 1992;6:209–23. doi: 10.1016/0920-9964(92)90004-o. [DOI] [PubMed] [Google Scholar]
- 22.Andreasen NC. Scale for the assessment of positive symptoms (SAPS) lowa city: University of lowa; 1984. [Google Scholar]
- 23.Andreasen NC. Scale for the assessment of negative symptoms (SANS) lowa city: University of lowa; 1984. [PubMed] [Google Scholar]
- 24.Goldberg DP, Gater R, Sartorius N, Ustun TB, Piccinelli M, Gureje O, et al. The validity of two versions of the GHQ in the WHO study of mental illness in general health care. Psychol Med. 1997;27:191–7. doi: 10.1017/s0033291796004242. [DOI] [PubMed] [Google Scholar]
- 25.Annett M. The binomial distribution of right, mixed and left handedness. Q J Exp Psychol. 1967;19:327–33. doi: 10.1080/14640746708400109. [DOI] [PubMed] [Google Scholar]
- 26.Guy W. Abnormal Involuntary Movements Scale (AIMS) ECDEU Assessment Manual for Pharmacology: US Dept of Health, Education, and Welfare, Rockville, MD. 1976 [Google Scholar]
- 27.Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–9. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 28.Bernasconi N, Bernasconi A, Andermann F, Dubeau F, Feindel W, Reutens DC. Entorhinal cortex in temporal lobe epilepsy: A quantitative MRI study. Neurology. 1999;52:1870–6. doi: 10.1212/wnl.52.9.1870. [DOI] [PubMed] [Google Scholar]
- 29.Pieper S, Halle M, Kikinis R. 3D Slicer. IEEE International Symposium on Biomedical Imaging: From Nano to Macro. 2004:632–5. [Google Scholar]
- 30.Arnold OH. Schizophrenia - A disturbance of signal interaction between the entorhinal cortex and the dentate gyrus? The contribution of experimental dibenamine psychosis to the pathogenesis of schizophrenia: A hypothesis. Neuropsychobiology. 1999;40:21–32. doi: 10.1159/000026593. [DOI] [PubMed] [Google Scholar]
- 31.Lorincz A, Buzsaki G. Two-phase computational model training long-term memories in the entorhinal-hippocampal region. Ann N Y Acad Sci. 2000;911:83–111. doi: 10.1111/j.1749-6632.2000.tb06721.x. [DOI] [PubMed] [Google Scholar]
- 32.Hasselmo ME, Fransen E, Dickson C, Alonso AA. Computational modeling of entorhinal cortex. Ann N Y Acad Sci. 2000;911:418–46. doi: 10.1111/j.1749-6632.2000.tb06741.x. [DOI] [PubMed] [Google Scholar]
- 33.Fried I, MacDonald KA, Wilson CL. Single neuron activity in human hippocampus and amygdala during recognition of faces and objects. Neuron. 1997;18:753–65. doi: 10.1016/s0896-6273(00)80315-3. [DOI] [PubMed] [Google Scholar]
- 34.Lisman JE, Otmakhova NA. Storage, recall, and novelty detection of sequences by the hippocampus: Elaborating on the SOCRATIC model to account for normal and aberrant effects of dopamine. Hippocampus. 2001;11:551–68. doi: 10.1002/hipo.1071. [DOI] [PubMed] [Google Scholar]
- 35.Rioux L, Nissanov J, Lauber K, Bilker WB, Arnold SE. Distribution of microtubule-associated protein MAP2-immunoreactive interstitial neurons in the parahippocampal white matter in subjects with schizophrenia. Am J Psychiatry. 2003;160:149–55. doi: 10.1176/appi.ajp.160.1.149. [DOI] [PubMed] [Google Scholar]
- 36.Pantazopoulos H, Woo TU, Lim MP, Lange N, Berretta S. Extracellular matrix-glial abnormalities in the amygdala and entorhinal cortex of subjects diagnosed with schizophrenia. Arch Gen Psychiatry. 2010;67:155–66. doi: 10.1001/archgenpsychiatry.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gibbs AA, Dazzan P, Morgan KD, Naudts KH, Morgan C, Hutchinson G, et al. Sexually dimorphic changes in the amygdala in relation to delusional beliefs in first episode psychosis. J Psychiatr Res. 2008;42:913–9. doi: 10.1016/j.jpsychires.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 38.Cahill L, Uncapher M, Kilpatrick L, Alkire MT, Turner J. Sex-related hemispheric lateralization of amygdala function in emotionally influenced memory: An FMRI investigation. Learn Mem. 2004;11:261–6. doi: 10.1101/lm.70504. [DOI] [PMC free article] [PubMed] [Google Scholar]


