Abstract
The feasibility of allogeneic transplantation, without myeloablation or post-transplant immunosuppression, was tested using in vivo chemoselection of allogeneic hematopoietic stem cells (HSCs) after transduction with a novel tricistronic lentiviral vector (MGMTP140K-2A-GFP-IRES-TK (MAGIT)). This vector contains P140K-O6-methylguanine-methyltransferase (MGMTP140K), HSV-thymidine kinase (TKHSV), and enhanced green fluorescent protein (eGFP) enabling (i) in vivo chemoselection of HSC by conferring resistance to benzylguanine (BG), an inhibitor of endogenous MGMT, and to chloroethylating agents such as 1,3-bis(2-chloroethyl)nitrosourea (BCNU) and, (ii) depletion of proliferating cells such as malignant clones or transduced donor T cells mediating graft versus host disease (GVHD), by expression of the suicide gene TKHSV and Ganciclovir (GCV) administration. Non-myeloablative transplantation of transduced, syngeneic, lineage-depleted (Lin−) BM in neonates resulted in 0.67% GFP+ mononuclear cells in peripheral blood. BG/BCNU chemoselection, 4 and 8 weeks post-transplant, produced 50-fold donor cell enrichment. Transplantation and chemoselection of major histocompatibility complex (MHC)-mismatched MAGIT-transduced Lin− BM also produced similar expansion for >40 weeks. The efficacy of this allotransplant approach was validated in Hbbth3 heterozygous mice by correction of β-thalassemia intermedia, without toxicity or GVHD. Negative selection, by administration of GCV resulted in donor cell depletion without graft ablation, as re-expansion of donor cells was achieved with BG/BCNU treatment. These studies show promise for developing non-ablative allotransplant approaches using in vivo positive/negative selection.
Introduction
Sibling or matched unrelated allogeneic hematopoietic stem cell (HSC) transplantation remain the only curative therapy for many hereditary disorders.1 However, the toxicity of myeloablative preparative regimens, risks of graft versus host disease (GVHD), infectious complications of immunosuppression, and limited availability of suitable donors, restrict application of this approach. While early transplantation could reduce or abrogate the pathogenic consequences of many genetic disorders, myeloablative transplantation approaches, especially very early in life, have been associated not only with morbidity and mortality, but also with subsequent abnormal development and growth.2,3 Thus, approaches to diminish these risks have focused on reducing the intensity and toxicity of preparatory and immunosuppressive regimens, without compromising engraftment or increasing the incidence of GVHD.
One approach to addressing the risks of transplantation has been to genetically modify donor cell populations to enable positive selection and expansion of donor HSC, or negative selection of donor T cells causing GVHD. Treatment of GVHD caused by transduced donor T cells using HSV thymidine kinase suicide gene (TKHSV)/Ganciclovir (GCV)-mediated negative selection has been validated in a number of recent clinical studies.4 Introduction of a drug resistance gene in HSC followed by in vivo chemoselection has increased donor chimerism and may enable reduction of the intensity, and therefore toxicity, of conditioning regimens. In early studies, limitations of positive selection of HSC by in vivo chemotherapy included the requirement for ongoing drug administration with associated cumulative toxicity, and the unanticipated transformation and autonomous proliferation of selected cells.5,6,7
The observation that transfer of the O6-alkylguanine-DNA alkyltransferase gene into mammalian cells decreased sensitivity to 1,3-bis(2-chloroethyl)nitrosourea (BCNU), a well-established HSC toxin, suggested that alkyltransferases such as MGMT could be used for in vivo chemoselection.8,9 Identification of O6-Benzylguanine (BG)10 as an inhibitor of endogenous MGMT, and the derivation of BG-resistant forms of MGMT (P140K and G156A)11,12 provided dramatic improvements in the durability of this in vivo chemoselection strategy. The MGMTP140K mutant repair enzyme exhibits 1,000-fold resistance to alkylators and nitrosoureas compared with the wild-type MGMT enzyme following treatment with BG.13,14,15 When BG is administered to inhibit endogenous MGMT activity followed by delivery of the nitrosourea BCNU, or alkylating agents such as temozolomide, cells in the bone marrow not expressing MGMTP140K are eliminated. Unlike previous in vivo selection systems, genetic modification of HSC by transduction with the MGMTP140K gene results in in vivo chemoselection at the HSC level and stable donor chimerism, even after discontinuation of drug treatment.11,12,13,14,15,16,17,18,19,20
Using this approach, enrichment of MGMTP140K-expressing HSC has been successfully demonstrated in congenic, myeloablated adult mice,15 human nonobese diabetic/severe combined immunodeficiency repopulating cells,14,21 and with allogeneic transplantation requiring post-transplant immunosuppression in a canine model.18,19,20 Recently, this approach has also been evaluated in nonhuman primates.22,23 In these studies transplantation was predicated on myeloablation, the use of immuno-incompetent hosts, or on administration of standard immunosuppressive regimens, respectively.
Despite the promise of this in vivo HSC selection approach, concerns remain regarding the proliferative stress placed on small numbers of MGMTP140K modified repopulating HSC clones following successive cycles of chemoselection.19,20,24 In addition, there are well-established risks of genotoxicity associated with integrating gene transfer vectors and subsequent autonomous clonal proliferation.25,26,27 Thus, incorporation of a negative selection strategy to enable control or elimination of malignant clones, should they emerge, would be desirable. TKHSV-expressing cells metabolize GCVinto its active triphosphate form resulting in cell death.4 GCV is more toxic to proliferating cells28 than to quiescent populations such as HSC, suggesting that administration of GCV could preferentially eliminate autonomously replicating clones arising from insertional mutagenesis and also proliferative alloreactive T cells causing GVHD. In the clinical setting, infusion of donor T cells modified to express the TKHSV suicide gene to improve immune reconstitution and subsequent control of GVHD by GCV has been validated in several studies (reviewed in ref. 4).
To initially test the lentivirally based MGMTP140K/TKHSV “positive/negative ” selection strategy, a neonatal transplant model was established. Transplantation at days 1–2 of life, before maturation of alloreactive T cells, enables studies of engraftment and mechanisms of tolerance induction during immune ontogeny while reducing confounding variables of host-mediated immune responses and graft rejection.29,30
Donor HSC engraftment and in vivo chemoselection was assessed after HSC transduction with a tricistronic lentiviral vector (MGMTP140K-2A-GFP-IRES-TK (MAGIT)) that incorporates MGMTP140K for positive selection, enhanced green fluorescent protein (eGFP) for flow cytometric analysis, and the sr39TKHSV suicide gene for enhanced negative selection. Transduction and transplantation of either syngeneic or allogeneic HSC into neonates preconditioned with a busulfan (BU)-based non-toxic, non-ablative regimen, produced successful engraftment with up to 50-fold expansion of donor cells after in vivo chemoselection. Neither GVHD nor autonomous proliferation of transduced clones was observed. Importantly, negative selection by administration of GCV enabled depletion of committed progenitors and circulating cells, without ablation of engrafted, transduced HSC. The promise of this approach was validated by correction of anemia and RBC morphology after transplantation and chemoselection in a murine model of β-thalassemia intermedia.
Results
Efficient in vitro chemoselection of 293T cells and transduction of lineage-depleted BM with MAGIT lentivirus
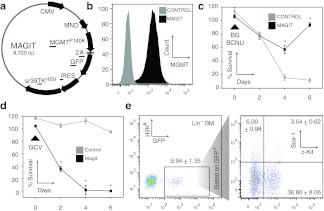
The expression efficiency of the tricistronic MAGIT lentivirus (Figure 1a) was first tested by transduction of 293T cells. After transduction and culture, >90% of 293T cells co-expressed eGFP and intracellular MGMTP140K (Figure 1b) and were viable, with similar efficiency to previously reported MGMTP140K-mediated selection,31 after one cycle of BG/BCNU treatment (Figure 1c). Control, untransduced cells were largely eliminated after BG/BCNU treatment (Figure 1c). Negative selection of 293T cells by co-culture with GCV confirmed the functionality of the sr39TKHSV cassette (Figure 1d). Efficient transduction of lineage-depleted primary bone marrow (Lin− BM) with MAGIT and expression of eGFP was also verified in c-Kit+Sca-1+ HSC (Figure 1e).
Figure 1.
In vitro validation of transduction, expression, and function of MGMTP140K-2A-GFP-IRES-TK (MAGIT) lentivirus. (a) Map of MAGIT tricistronic plasmid co-expressing MGMTP140K, enhanced green fluorescent protein (eGFP) linked by a 2A sequence, and followed by TKHSV separated by an internal ribosome entry site (IRES). (b) 293T cell line was transduced with MAGIT lentivirus and intracellular MGMTP140K and GFP expression analyzed by flow cytometry. The proportion of cells shown expressing intracellular MGMTP140K and GFP expression was >90% positive cells as compared with untransduced cells. In all histograms the y-axis represents cell counts. (c) The same cell population in b was exposed to BG followed by BCNU, separated by 1 hour. Survival as a percentage of untreated cells was determined in triplicates at 2-day intervals. (d) Simultaneous to c, MAGIT-transduced cells were exposed to Ganciclovir (GCV) and monitored similarly. (e) Lineage-depleted bone marrow from C57Bl/6 mice was transduced with MAGIT lentivirus and the levels of GFP, Sca-1, and c-Kit expression determined by flow cytometry 48 hours following transduction. The dot plot showing Sca-1 and c-Kit expression is gated to include only GFP+ cells. These data are representative of at least three independent experiments and any differences with P < 0.05 are indicated with an asterisk (*). Gray: control-untransduced cells; black: MAGIT-transduced cells. BCNU, 1,3-bis(2-chloroethyl)nitrosourea; BG, benzylguanine; CMV, cytomegalovirus.
Sustained multilineage hematopoietic repopulation by MAGIT-expressing cells after non-myeloablative syngeneic neonatal transplantation
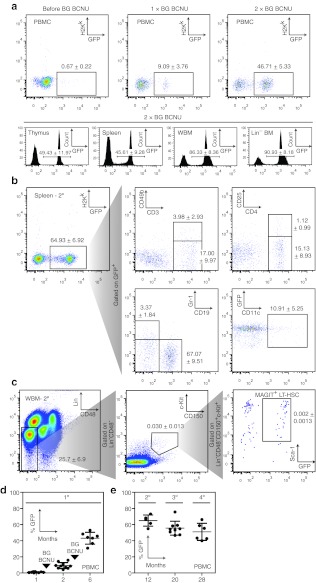
To test the feasibility of non-myeloablative transplantation and in vivo chemoselection in the absence of immune barriers to engraftment, transplantation of syngeneic MAGIT-transduced Lin− BM was performed at days 1–2 of life after a conditioning regimen that included BU and 200cGy radiation. Importantly, neonates treated with this regimen showed normal growth, and comparable survival to untreated, age-matched cohorts at 8 weeks, regardless of whether mice were subsequently transplanted or not (data not shown). Peripheral blood counts including total WBC (5.98 ± 0.63 × 104/µl), lymphocytes (2.37 ± 0.56 × 104/µl), and absolute neutrophil counts (3.16 ± 0.45 × 104/µl) in BU/200 treated neonates at day 2 of life, before transplantation, were not statistically different from those in untreated neonates (n = 3). After two cycles of BG/BCNU administered at 4 and 8 weeks of life, robust enrichment of transduced populations from low levels (0.67%) to ~50% of peripheral blood mononuclear cell (PBMC) was observed (Figure 2a, dot plots). The mean fluorescence of the GFP+ population did not differ significantly following each cycle of BG/BCNU suggesting that clones expressing higher levels of MGMTP140K were not being preferentially selected. The frequency of GFP expression in PBMC was proportional to the total number of transduced cells in the thymus and spleen, but under-represented the levels of transduced cells in BM and Lin− BM fractions from chemoselected animals (Figure 2a, histograms). Mice transplanted with MAGIT-transduced Lin− BM, without subsequent BG/BCNU treatment, had gradually decreasing numbers of GFP+ PBMC persisting for more than 10 months (data not shown). Initiation of chemoselection 10 months after transplantation of MAGIT-transduced cells was also successful in expanding transduced populations, suggesting targeting of long-lived HSC and persistence of MGMTP140K expression even in the absence of selection (data not shown). Co-expression of intracellular MGMTP140K and GFP was confirmed in these chemoselected populations by flow cytometry (data not shown).
Figure 2.
Non-myeloablative syngeneic neonatal transplantation with efficient chemoselection of MAGIT-expressing cells and multilineage hematopoietic repopulation. (a) Neonatal C57Bl/6 mice were transplanted with MAGIT-transduced C57Bl/6 Lin− BM and the percentage of peripheral blood cells expressing GFP and H-2Kk determined by FACS 1 month post-transplant, and after each successive cycle of chemoselection with BG/BCNU (n = 10). (b) Lin− BM from chemoselected mice (similar to a) were harvested and transplanted into lethally irradiated C57Bl/6 mice. One month post-transplantation, the repopulation of indicated lineages in secondary recipients was evaluated. The data shown is gated to include only GFP-positive cells (n = 6). (c) Bone marrow harvested from serially transplanted mice (similar to b) was assessed by flow cytometry for the presence of MAGIT-expressing GFP+ cells in long-term hematopoietic stem cells (LT-HSC). MAGIT+ LT-HSC were defined by coexpression of GFP, Sca-1, c-Kit, and CD150 on cells lacking lineage markers (lineage-specific antibodies to CD5, B220, CD11b, Anti-Gr-1, 7-4, and Ter-119) and CD48 were used in these studies (n = 5). These data are representative of at least two independent experiments. (d) Dot plot depicting the percent of GFP+ peripheral blood cells as determined by FACS at 1, 2, and 6 months after BG/BCNU administration, 1 and 2 months post-transplant as in a. (e) The percent GFP-expressing PBMC following serial transplantation of mice from e. Each dot represents a single mouse. The data from each panel are representative of at least two independent experiments. BCNU, 1,3-bis(2-chloroethyl)nitrosourea; BG, benzylguanine; FACS, fluorescence-activated cell sorting; GFP, green fluorescent protein; PBMC, peripheral blood mononuclear cell.
To further evaluate potential cumulative effects of successive cycles of chemoselection, a toxicity study was undertaken in which different doses of BG/BCNU were administered to adult mice, weekly four times, and total lymphocyte counts and survival monitored (Supplementary Figure S1). Cumulative lymphopenia was observed in all treated groups at day 28, after four doses of BG/BCNU (Supplementary Figure S1a). There was no impact on survival in groups receiving 10 mg/kg BG/5 mg/kg BCNU or 20 mg/kg BG/5 mg/kg BCNU (Supplementary Figure S1b). Decreased survival was observed in the groups receiving 30 mg/kg BG/5mg/kg BCNU or 10 mg/kg BG/10 mg/kg BCNU (Supplementary Figure S1b). Thereafter, recovery of counts was observed over the next 2 months in all treated mice (Supplementary Figure S1a).
Multilineage repopulation by MAGIT-transduced, chemoselected Lin− BM was assessed before and after secondary transplantation into lethally irradiated syngeneic hosts. Flow cytometric analysis after secondary transplantation of Lin− BM from mice receiving BG/BCNU in vivo chemoselection showed that GFP+ splenocytes contained lymphoid and myeloid progenitor populations including CD3+, CD3+CD49b+, CD4+, CD4+CD25+, CD19+, Gr-1+, and CD11c+ populations, respectively (Figure 2b). Similar frequencies of GFP-expressing cells, and multilineage reconstitution were observed in the primary and secondary recipients.
The presence, chemoselection, and persistence of long-term repopulating HSC was demonstrated by additional flow cytometric studies. A population of Lin−CD48−c-Kit+CD150+Sca-1+ cells32 expressing GFP were detected at comparable percentage to that of GFP+ cells within the bone marrow of serially transplanted recipients (Figure 2c). After discontinuing BG/BCNU in primary transplants at 2 months, the frequency of GFP+ PBMC populations persisted at 6 months (Figure 2d) and remained stable for 28 months in secondary, tertiary, and quaternary transplants (Figure 2e). These data are consistent with transduction and chemoselection of pluripotent HSC.
Sustained multilineage hematopoietic repopulation and chemoselection of MAGIT-expressing cells after non-myeloablative allogeneic neonatal transplantation
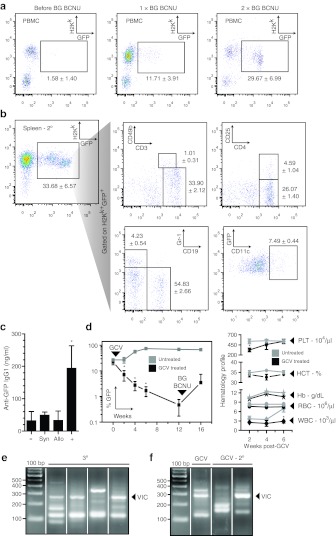
The efficacy of this non-myeloablative preparative regimen and post-transplant chemoselection protocol was then evaluated in a fully major histocompatibility complex (MHC) mismatched allogeneic setting by transplantation of MAGIT-transduced B10.Br (H-2k) Lin− BM into C57Bl/6 (H-2b) neonatal recipients. Engraftment of allogeneic Lin− BM and the presence of MAGIT-transduced GFP+ PBMC were detected before chemoselection (Figure 3a, left dot plot). Efficient expansion of transduced donor allogeneic GFP+ cells was also achieved with BG/BCNU treatment in this MHC-mismatched allogeneic transplant model (Figure 3a, middle and right dot plots). Recipients of MHC-mismatched cells had no signs of GVHD and showed comparable growth and appearance to naive littermates. Secondary transplantation of chemoselected B10.Br Lin− BM from primary C57Bl/6 recipients into lethally irradiated B10.Br adults produced multilineage reconstitution of lymphoid and myeloid progenitor compartments (Figure 3b). Humoral responses to GFP neoantigens described by others33 were not detected in neonatally transplanted mice (Figure 3c).
Figure 3.
Non-myeloablative allogeneic neonatal transplantation with chemoselection of MAGIT-expressing cells and multilineage hematopoietic repopulation. (a) Neonatal C57Bl/6 mice were transplanted with MAGIT-transduced B10.Br Lin− BM and the percentage of peripheral blood cells expressing GFP and H-2Kk determined by flow cytometry beginning 1 month after transplantation, and following two cycles of chemoselection with BG/BCNU at 8 and 12 weeks after transplant (n = 9 mice). (b) Lin− BM from mice after BG/BCNU treatment × 2 (similar to a) were harvested and secondarily transplanted into lethally irradiated B10.Br mice. One month after secondary transplantation, the repopulation of indicated lineages was re-evaluated by flow cytometry. The data shown is gated to include only GFP+ cells (n = 6 mice). These data are representative of at least two independent experiments. (c) The serum of mice after neonatal transplantation of syngeneic (Syn) or allogeneic (Allo) MAGIT-transduced Lin− BM and BG/BCNU chemoselection (n = 5 mice per group) was tested for the presence of antibodies specific to GFP. Positive control (+) samples were generated by injection of C57Bl/6 mice with alum/GFP. (d) Lin− BM from mice treated with BG/BCNU were harvested and transplanted into lethally irradiated C57Bl/6 mice (similar to Figure 2b). One month after secondary transplantation, Ganciclovir (GCV) was administered intraperitoneally. The frequency of GFP+ PBMC was monitored before administration of GCV and for 12 weeks after GCV administration at the indicated time points. One cycle of BG/BCNU was administered 12 weeks after GCV administration and the percent GFP-positive PBMC quantified (left graph). Hematocrit (HCT), hemoglobin (Hb), red blood cell (RBC), white blood cell (WBC), and platelet (PLT) levels were determined 2, 4, and 6 weeks post-GCV treatment (right graph). These data are representative of one experiment (n = 4 mice per group) and any differences with P < 0.05 are indicated with an asterisk (*). (e) Clonality as indicated by presence of 5′ lentiviral LTR adjacent genomic amplicons was imaged following LAM-PCR. Genomic DNA from PBMC following tertiary transplantation of Lin− BM as described in Figure 2b (n = 4 mice) was utilized. (f) Clonality of PBMC from mice treated with GCV as described in d and following secondary transplantation of Lin− BM from GCV-treated mice is shown. A 100 bp ladder and corresponding vector internal control (VIC) band are indicated. BCNU, 1,3-bis(2-chloroethyl)nitrosourea; BG, benzylguanine; GFP, green fluorescent protein; LAM-PCR, linear amplication-mediated PCR; LTR, long terminal repeat; PBMC, peripheral blood mononuclear cell.
Negative selection of MAGIT-expressing engrafted cells by GCV administration
Several potential risk factors were considered in developing this non-ablative allogeneic transplant model based on lentiviral HSC transduction. The first potential limitation was evolution of GVHD after allogeneic transplantation in the absence of any post-transplant immunosuppression. The second concern was the potential transformation/autonomous proliferation of lentivirally transduced cells due to insertional mutagenesis.25,26,27 We therefore incorporated a negative selection strategy into the MAGIT vector that would enable elimination of both alloreactive TKHSV-expressing T cell populations contributing to GVHD, and autonomously proliferating clonal populations. Since GVHD was not detected either by examining the appearance of animals, or by testing liver function, it was not feasible to directly evaluate the efficacy of TKHSV expression and GCV treatment in this regard. However, proof of concept for GCV-mediated negative selection in vivo was established by administration of GCV (50 mg/kg) to a cohort of lethally irradiated adult mice after secondary transplantation of MAGIT-transduced and -chemoselected, syngeneic Lin− BM. A decrease in the mean frequency of GFP+ donor cells to 0.47%, compared with 68.8% in untreated group, was demonstrated 12 weeks after a single cycle of GCV (Figure 3d, left graph). GCV-mediated negative selection in vivo was not associated with severe pancytopenia, as changes in blood counts were transient and returned to normal levels (Figure 3d, right graph). Re-expansion of transduced donor cells was achieved after re-administration of two cycles of BG/BCNU in animals previously treated with GCV (Figure 3d, left graph). Thus, although GCV mediated the depletion of committed progenitors and circulating transduced cells, these data suggest that quiescent HSC remained viable and that GCV may be administered in this setting without ablation of the graft.
Multiple studies have demonstrated that transplantation and in vivo chemoselection of MGMTP140K-expressing BM results in polyclonal engraftment and proliferation.18,19,20,24,34,35,36 To verify the multiclonality of engrafted, selected populations after positive and negative section with GCV and further re-expansion with BG/BCNU, linear amplification-mediated PCR (LAM-PCR) analysis of repopulating cells was performed (Figure 3e,f). Data from individual tertiary transplants demonstrate polyclonal engraftment (Figure 3e). After negative selection with GCV and re-expansion of transduced BM by re-administration of BG/BCNU multiple repopulating clones were also observed. Secondary transplants of Lin− BM from GCV-treated mice produced engraftment and persistence of multiple independent clones suggesting HSC remained viable (Figure 3f).
Correction of β-thalassemia with neonatal non-myeloablative allogeneic transplantation and in vivo chemoselection
Effective, non-myeloablative allogeneic transplantation protocols with less toxicity would enable broader application of this approach for treatment of hematopoietic and other genetic disorders. Mutations in the human globin loci causing thalassemia are among the most common genetic disorders worldwide. Several studies have demonstrated correction of mouse models of β-thalassemia or sickle cell disease by ex vivo lentiviral-mediated gene transfer of globin genes and transplantation.34,37,38,39,40,41 The feasibility of in vivo chemoselection of syngeneic BM transduced with globin/MGMT-expressing vectors has been demonstrated by transplantation of adult murine models of β-thalassemia.34,39 However, variability in persistence and levels of gene expression were observed.
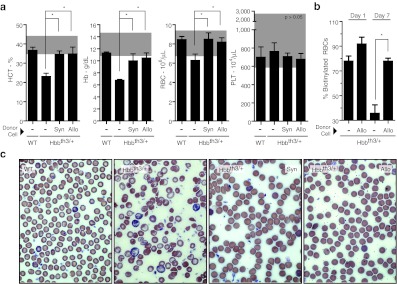
To assess the potential for correction of β-thalassemia with this non-ablative allogeneic neonatal transplantation approach, Hbbth-3 thalassemic mice carrying deletions of the adult murine β-minor and β-major globin genes42 were transplanted. Heterozygous Hbbth-3/+ mice have thalassemia intermedia with characteristic red cell changes on peripheral blood smear, and extramedullary hematopoiesis (Figure 4a,b).43 Homozygous Hbbth-3/th-3 knockout mice do not survive gestation due to severe anemia and hydrops fetalis. MAGIT transduction ex vivo and transplantation of either syngeneic normal C57Bl/6 Lin− BM or allogeneic B10.Br Lin− BM was performed at days 1–2 of life in Hbbth-3/+ mice. After two cycles of BG/BCNU at 4 and 8 weeks post-transplantation, normalization of hematocrit, hemoglobin, RBC counts (Figure 4a), and RBC half-life were achieved (Figure 4b). Representative blood smears from transplanted groups show reduced numbers of target cells and other red cell abnormalities, consistent with the correction of the disease phenotype (Figure 4c). The lack of toxicity of the preconditioning regimen was underscored by the comparable survival of BU-conditioned Hbbth-3/+ neonates that were not transplanted, to naive Hbbth-3/+ littermates. Also, adult Hbbth-3/+ naive controls tolerated at least two cycles of BG/BCNU without adverse effects on survival. Thus, both the BU/low-dose irradiation of this preparative regimen and in vivo chemoselection were well tolerated by the thalassemic Hbbth-3/+ mice.
Figure 4.
Correction of β-thalassemia following non-myeloablative neonatal transplantation and chemoselection of MAGIT. (a) Heterozygous Hbbth-3/+ neonatal mice underwent busulfan/200cGy preconditioning, and were transplanted with MAGIT-transduced syngeneic or allogeneic Lin− BM, and treated with two successive BG/BCNU chemoselection cycles. Peripheral blood counts were followed. Gray area indicates the normal range for mice. These data are representative of at least two independent experiments and any differences with P < 0.05 are indicated with an asterisk (*) (n = at least four mice per group). (b) RBC half-life, indicated by the decay of biotinylated RBCs in peripheral blood, was monitored by flow cytometry on days 1 and 7 following treatment with Biotin-NHS (n = 4 mice per group). (c) Representative Giemsa-stained blood smears of mice from each group from a are shown. BCNU, 1,3-bis(2-chloroethyl)nitrosourea; BG, benzylguanine; Hb, hemoglobin; HCT, hematocrit; PLT, platelet; RBC, red blood cell; WT, wild-type.
Discussion
Gene transfer approaches have shown great potential for the treatment of genetic disorders. In animal models, disease correction has required high-level transduction and ablative preconditioning. However, for many other genetic diseases, the corrective transgene cassette may not encompass all the regulatory elements required to reconstitute stable, tissue-specific, and physiologically appropriate levels of expression. Further, the design and validation of specific vectors for each disease constitutes a time-consuming and costly process.
Rather than seeking direct genetic correction of autologous cells, there may be significant advantages to employing gene transfer strategies to improve the safety and outcome of allogeneic transplantation, an established curative approach. However, application of allogeneic transplantation also has limitations due to the lack of suitably matched donors, the need for toxic recipient conditioning, risks of morbidity and mortality from GVHD, and infectious complications of immunosuppression and delayed immune reconstitution.
To address some of the current issues associated with allogeneic transplantation, a novel positive/negative selection strategy was pursued to provide both a selective advantage for donor HSC, and a mechanism for depletion of alloreactive T cells and potential autonomously proliferating clones after transplantation. The functionality of a tricistronic lentivirus including an eGFP reporter with linked MGMTP140K and TKHSV cassettes were demonstrated in both in vitro and in vivo studies. In these initial transplantation studies, positive/negative chemoselection was combined with transplantation in the neonatal period, when host-mediated immune responses are decreased.
The combination of a minimal, non-myeloablative conditioning regimen with in vivo BG/BCNU chemoselection was sufficient to enable engraftment and efficient in vivo expansion of lentivirally transduced HSC. Stable levels of donor chimerism were observed after cessation of drug therapy, and after secondary and tertiary transplantation. Significantly, this minimal preparative regimen also enabled stable engraftment and enrichment of lentivirally transduced allogeneic cells without post-transplant immunosuppression. No evidence of GVHD was detected after discontinuing in vivo chemoselection.
The efficacy of this approach was further validated by transplantation and chemoselection of lentivirally transduced allogeneic Lin− BM in a murine model of β-thalassemia. Neither the preparative regimen, nor the administration of successive cycles of BG/BCNU produced adverse effects or decreased survival in naive thalassemic controls. Secondary transplantation of lentivirally transduced, chemoselected Lin− BM resulted in full multilineage reconstitution in lethally irradiated recipients suggesting that self-renewing HSC were successfully transduced and expanded in thalassemic recipients.
Several transplantation studies in both murine and large animal models, using BM transduced with MGMTP140K or another variant, MGMTG156A, have shown that successive cycles of chemotherapy with BG/BCNU or temozolomide results in in vivo selection at the stem cell level.11,12,16,17,18,19,20,30,39 In the canine model, stable donor chimerism was observed for up to 6 years following discontinuation of the selective agent.18 Multiclonal, stable engraftment, and further enrichment of donor cells with re-administration of chemoselection more than a year after initial treatment was observed.18 LAM-PCR–based genomic studies of retrovirally transduced canine HSC-expressing MGMTP140K showed no evidence for increased transcriptional start sites in proximity to proto-oncogenes, or differences in retroviral integration site patterns after chemoselection with either BCNU or temozolomide.18 The successful transduction and in vivo selection of human CD34+ cells transduced with MGMTP140K in immunodeficient models14,21 further supports the promise of clinical application of MGMTP140K-mediated in vivo expansion of modified HSC.
Importantly, to date there are no reports of malignant transformation of BM with viral transduction and in vivo expansion of MGMT-expressing donor cells. The incorporation of the HSVTK suicide gene described here facilitates depletion of autonomous clones that might arise from insertional mutagenesis. The goal of the GCV-mediated negative selection experiment after BG/BCNU chemoselection was to test the hypothesis that proliferating clones in the periphery or in the bone marrow would have greater sensitivity to GCV than more quiescent HSC in the bone marrow. Hence, a single dose of GCV was administered to assess the degree of peripheral depletion and to demonstrate that multilineage re-expansion of selected donor HSC was feasible after GCV. Since autonomous clones could persist after single dose GCV further negative selection might be required to eliminate these clones.
In contrast to the successful in vivo chemoselection and stable chimerism reported in canine models, recent studies in a rhesus macaque model, showed only transient expansion of autologous cells transduced with MGMTP140K, and toxicity with administration of a more dose-intensive BG/BCNU regimen.22 Use of the MND promoter used in our and other successful chemoselection studies may have advantages in directing higher levels of gene expression and decreased silencing effects.44
Although genetic modification of donor cells to enable engraftment and in vivo amplification may make further reduction of the intensity and toxicity of preparative regimens feasible, risks of GVHD and the infectious consequences of required immunosuppression remain.4 T-cell depletion of donor cells reduces the incidence and severity of GVHD, but has been associated with protracted immunodeficiency and increased mortality.4 The TKHSV-based negative selection strategy incorporated in our studies has already been validated in clinical trials where recipients developing GVHD after donor TKHSV transduced T-cell lymphocyte infusion post-transplant were uniformly responsive to administration GCV.4
In the present study, efficient depletion of transduced donor cell populations was also observed with GCV administration after in vivo chemoselection of MAGIT-transduced BM. Importantly, the graft was not ablated after GCV administration enabling re-expansion with further chemoselection. The use of BM transduced with the bifunctional MAGIT lentiviral construct may enable modulation of the donor graft to limit risks associated with transplantation and gene delivery. This model system also enables studies of mechanisms underlying tolerance induction to neoantigens, and allogeneic stem cells during immune ontogeny.
Materials and Methods
Mice. Wild-type C57Bl/6 (H-2b), and B10.Br (H-2k) mice were obtained from Charles River (Wilmington, MA), and the Jackson Laboratory (Bar Harbor, ME), respectively. Hbbth-3 mice carrying a deletion of the murine β-major and β-minor globin genes (gift of Dr Oliver Smithies, University of North Carolina, Chapel Hill, NC)43 were backcrossed at least 20 generations and maintained on a C57Bl/6 background. The mouse β-globin deletion was detected by PCR screening for a 385 bp product using sense primer 5′-AACAAGAGCAAACTAAGTAAGATGC-3′ and antisense primer 5′-AATTCGCCAATGACAAGACG-3′. PCR results were confirmed by analysis of hematocrit and hemoglobin using a Hemavet 850FS system (Drew Scientific, Waterbury, CT). All strains were housed under specific pathogen-free conditions at the University of California, San Francisco, CA. Experiments were conducted under the supervision of the University of California, San Francisco Institutional Animal Care and Use Committee according to an approved protocol.
Fluorescence-activated cell sorting. Antibodies to H-2Kk (36-7-5), MGMT (MT5.1), CD3 (2C11), CD4 (RM4-5), CD25 (PC61), CD49b (HMa2), Ly-6G and Ly-6C (Gr-1), CD19 (1D3), CD11c (HL3), c-Kit (2B8), Sca-1 (D7), and Fc Block (2.4G2) were purchased from BD Biosciences (San Jose, CA). Antibodies to CD150 (TC15-12F12.2) and CD48 (HM48-1) were purchased from BioLegend (San Diego, CA). Flow cytometry was performed on a LSR II and data analyzed on FlowJo software (Treestar, Ashland, OR) using indicated antibodies and Fc Block, with shown gates excluding at least 99.5% of control cells. Intracellular staining specific for human MGMT was performed after fixation with 4% paraformaldhyde, permeabilization with 0.5% saponin, and detection of bound anti-MGMT with a Goat anti-mouse IgG1 PE as described (Invitrogen, Carlsbad, CA).45
Construction of MAGIT plasmid. The tricistronic MAGIT expression plasmid (pMAGIT) coexpressing MGMTP140K, eGFP linked by a 2A sequence, and followed by TKHSV separated by an internal ribosome entry site (IRES) was constructed by inserting an 2,873 bp NdeI/SpeI fragment containing the MGMTP140K and GFP sequences from MAG plasmid31 into NdeI/SpeI digested MGMTP140K-IRES-TK (a bicistronic plasmid lacking the GFP gene that was generated in our laboratory). This approach reintroduced MGMTP140K and added a GFP cassette to create a tricistronic plasmid under the control of the MND promoter for stable long-term expression.44 The plasmid containing the sr39TKHSV was a gift from Dr Noriyuki Kasahara (University of California Los Angeles, Los Angeles, CA). This sr39 mutant of TKHSV has enhanced ability to convert acyclovir/GCV into cytotoxic agents and greater in vivo positron emission tomography imaging sensitivity.46 The IRES-TKHSV cassette was cloned from plasmid pACE-TK, which encodes a replication-competent amphotropic MLV vector derived from pACE-GFP47 but with the GFP gene replaced by the TKHSV gene amplified from plasmid pFRA-TK48 by PCR using Pfu polymerase (Stratagene, La Jolla, CA). Plasmid DNA was prepared with PureLink HiPure Plasmid DNA Megaprep and Gigaprep Kits according to the manufacturer's instructions (Invitrogen).
Production and titering of lentivirus vectors. MAGIT lentivirus was produced by overnight transfection of 293T cells grown to 80% confluence on poly-lysine coated (Sigma-Aldrich, St Louis, MO) 10 cm dishes with three plasmids (pCMVδR8.91,49 pMD.G,49 and pMAGIT) and Lipofectamine 2000 (Invitrogen). Supernatants were collected 24 and 48 hours after removal of transfection media, and pooled before concentration on 70% ethanol washed Centricon-80 columns (Millipore, Billerica, MA). Aliquots of virus were frozen at −80 °C. The titer of concentrated virus was determined by serial dilution onto 293T cells in the presence of 8 µg/ml polybrene. Using this procedure, we observed titers at 1.2 ± 0.12 × 109 IU/ml (data from six viral lots).
In vitro BG/BCNU chemoselection and GCV killing curves. MAGIT-transduced 293T cells (>90% GFP expression by fluorescence-activated cell sorting) were either treated with 10 µmol/l BG (Gift from Dr Gary Pauly, National Cancer Institute, Frederick, MD) followed 1 hour later by 200 µmol/l BCNU (Bristol-Myers Squibb, Princeton, NJ) or with 1 µg/ml GCV (InvivoGen, San Diego, California). Cell survival was assessed every other day by evaluating conversion of MTS (Promega, Madison, WI) into the aqueous soluble formazan product. The quantity of formazan product as measured by the amount of 490 nm absorbance is directly proportional to the number of living cells in culture.
Lentiviral transduction of murine Lin− BM cells. Bone marrow was harvested from femurs and tibias of C57Bl/6 or B10.Br mice. Lineage positive populations were depleted using the Lineage Cell Depletion Kit (Miltenyi Biotec, Auburn, CA) according to the manufacturer's instructions. The resulting Lin− BM was >98% depleted of lineage populations with undetectable CD3+ cells (data not shown). Lin− BM cells were transduced with 100 multiplicity of infection of MAGIT lentivirus at a density of 106 cells/ml in DMEM (Invitrogen) containing 10% fetal calf serum supplemented with 100 ng/ml rmFlt-3L, 100 ng/ml rmSCF (R&D Systems, Minneapolis, MN), and 8 µg/ml polybrene (Sigma-Aldrich). Following overnight incubation, the transduced cells were harvested, washed, and 106 cells injected via facial vein in 50 µl of phosphate-buffered saline. GFP expression, an indicator of transduction efficiency, was evaluated by flow cytometric analysis after culture for an additional 48 hours. This transduction protocol consistently produced GFP expression in 9–12% of Lin− BM.
Preconditioning and transplantation of neonates. C57Bl/6 females were timed-mated with either wild-type C57Bl/6 or Hbbth-3/+ heterozygous C57Bl/6 male mice. On day 18 of gestation (E18), 15 mg/kg of Busulfan (Aldrich, Milwaukee, WI) was delivered intraperitoneally into pregnant dams.3 Neonates from BU-treated dams were exposed to 2 Gy (200 rad) on day 1 or 2 of life and injected with the indicated cell numbers on the same day.
In vivo positive and negative selection procedures for MAGIT-transduced populations. At 1 month of life and before chemoselection, mice were bled via submandibular vein. The fractions of GFP- and H-2Kk-expressing PBMC were determined via flow cytometry. In vivo chemoselection was performed by intraperitoneal administration of BG at 30 mg/kg and then BCNU at 7.5 mg/kg, 1 hour later. The level of donor cell enrichment was evaluated 1 month after each cycle of chemoselection before drug administration was repeated. Negative selection by in vivo depletion of MAGIT-expressing cells was performed by a single intraperitoneal administration of GCV at 50 mg/kg.50
Secondary transplantation of MAGIT-transduced cells. Bone marrow, spleen, and thymus were harvested after neonatal transplantation and chemoselection. Lineage repopulation of each of these tissues with MAGIT-transduced cells was evaluated via flow cytometric analysis after staining with the panel of indicated antibodies. The remainder of the harvested bone marrow was depleted of lineage positive populations, as described above, and injected intravenously into lethally irradiated (10 GY) syngeneic adult recipients. The frequency of GFP-expressing cells and repopulation of different lineages were again evaluated after secondary transplantation.
Anti-GFP ELISA. Plates were coated with eGFP (Promega) overnight. After blocking, the plate was incubated with serum for 1 hour followed by incubation with anti-mouse IgG HRP (Calbiochem, Gibbstown, NJ) for an additional hour separated by a wash step. The amount of anti-GFP antibody was quantified with reference to a known control anti-mouse GFP IgG (Abcam, Cambridge, MA). Positive control serum was produced by repeated injection of C57Bl/6 mice with a suspension of Alum and eGFP.
RBC biotinylation decay assay. Red blood cells were pulse biotinylated with N-hydroxysuccinimide biotin (Biotin-NHS; Calbiochem, San Diego, CA) dissolved in N,-N,-dimethylacetamide at 50 mg/ml. Before tail vein injection, the dissolved Biotin-NHS was diluted in phosphate-buffered saline to 5 mg/ml and delivered at 50 mg/kg per mouse. The percentage of biotinylated RBCs in peripheral blood was quantified by flow cytometry at indicated time points after incubation with strepavidin-PE (BD Biosciences, San Jose, California).
Lentivirus insertion site analysis by LAM-PCR. LAM-PCR analyses to identify 5′ long terminal repeat lentiviral vector adjacent genomic sequences was performed in collaboration with the National Gene Vector Biorepository (NGVB) and Dr. Kenneth Cornetta (Indiana University, Indianapolis, IN) as described previously.24 PBMC from indicated mice were collected and 200 ng of DNA used as template for two rounds of 50-cycle linear PCR with biotinylated lentiviral long terminal repeat-specific primer. Following magnetic separation of PCR products, the linear product was exposed to hexanucleotide random priming, restriction digestion with Mse1, ligation with a linker, and exponential PCR. Resulting amplicons adjacent to the 5′ long terminal repeat were imaged following a second round of magnetic separation and nested PCR as described.24
Statistics. All experiments were repeated at least two times unless indicated otherwise. Results are reported as the mean ± SD of independent experiments. The significance of differences was determined using Student's paired t-test.
SUPPLEMENTARY MATERIAL Figure S1. Effects of different weekly BG/BCNU regimens on total lymphocyte counts and survival.
Acknowledgments
This work was supported by NIH National Heart, Lung, and Blood Institute (NHLBI) grant R01HL082665, R21DK069430, and by a University of California, San Francisco, School of Medicine, Bridge Fund Award. We greatly appreciate the constructive suggestions of William J Murphy (University of California, Davis, CA), Kenneth Cornetta (Indiana University, Indianapolis, IN), Troy B. Hawkins (Indiana University, Indianapolis, IN), Noriyuki Kasahara (University of California, Los Angeles, CA), Damien Reynaud (University of California, San Francisco, CA), Emmanuelle Passegue (University of California, San Francisco, CA), and Joan E Etzell (University of California, San Francisco, CA). The authors declared no conflict of interest.
Supplementary Material
Effects of different weekly BG/BCNU regimens on total lymphocyte counts and survival.
REFERENCES
- Lucarelli G., and, Gaziev J. Advances in the allogeneic transplantation for thalassemia. Blood Rev. 2008;22:53–63. doi: 10.1016/j.blre.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Sands MS, Barker JE, Vogler C, Levy B, Gwynn B, Galvin N.et al. (1993Treatment of murine mucopolysaccharidosis type VII by syngeneic bone marrow transplantation in neonates Lab Invest 68676–686. [PubMed] [Google Scholar]
- Yoder MC, Cumming JG, Hiatt K, Mukherjee P., and, Williams DA. A novel method of myeloablation to enhance engraftment of adult bone marrow cells in newborn mice. Biol Blood Marrow Transplant. 1996;2:59–67. [PubMed] [Google Scholar]
- Lupo-Stanghellini MT, Provasi E, Bondanza A, Ciceri F, Bordignon C., and, Bonini C. Clinical impact of suicide gene therapy in allogeneic hematopoietic stem cell transplantation. Hum Gene Ther. 2010;21:241–250. doi: 10.1089/hum.2010.014. [DOI] [PubMed] [Google Scholar]
- Abonour R, Williams DA, Einhorn L, Hall KM, Chen J, Coffman J.et al. (2000Efficient retrovirus-mediated transfer of the multidrug resistance 1 gene into autologous human long-term repopulating hematopoietic stem cells Nat Med 6652–658. [DOI] [PubMed] [Google Scholar]
- Hesdorffer C, Ayello J, Ward M, Kaubisch A, Vahdat L, Balmaceda C.et al. (1998Phase I trial of retroviral-mediated transfer of the human MDR1 gene as marrow chemoprotection in patients undergoing high-dose chemotherapy and autologous stem-cell transplantation J Clin Oncol 16165–172. [DOI] [PubMed] [Google Scholar]
- Williams DA, Hsieh K, DeSilva A., and, Mulligan RC. Protection of bone marrow transplant recipients from lethal doses of methotrexate by the generation of methotrexate-resistant bone marrow. J Exp Med. 1987;166:210–218. doi: 10.1084/jem.166.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumenco LL, Warman B, Hatzoglou M, Lim IK, Abboud SL., and, Gerson SL. Increase in nitrosourea resistance in mammalian cells by retrovirally mediated gene transfer of bacterial O6-alkylguanine-DNA alkyltransferase. Cancer Res. 1989;49:6044–6051. [PubMed] [Google Scholar]
- Allay JA, Dumenco LL, Koc ON, Liu L., and, Gerson SL. Retroviral transduction and expression of the human alkyltransferase cDNA provides nitrosourea resistance to hematopoietic cells. Blood. 1995;85:3342–3351. [PubMed] [Google Scholar]
- Gerson SL, Zborowska E, Norton K, Gordon NH., and, Willson JK. Synergistic efficacy of O6-benzylguanine and 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) in a human colon cancer xenograft completely resistant to BCNU alone. Biochem Pharmacol. 1993;45:483–491. doi: 10.1016/0006-2952(93)90086-c. [DOI] [PubMed] [Google Scholar]
- Davis BM, Roth JC, Liu L, Xu-Welliver M, Pegg AE., and, Gerson SL. Characterization of the P140K, PVP(138-140)MLK, and G156A O6-methylguanine-DNA methyltransferase mutants: implications for drug resistance gene therapy. Hum Gene Ther. 1999;10:2769–2778. doi: 10.1089/10430349950016500. [DOI] [PubMed] [Google Scholar]
- Christians FC, Dawson BJ, Coates MM., and, Loeb LA. Creation of human alkyltransferases resistant to O6-benzylguanine. Cancer Res. 1997;57:2007–2012. [PubMed] [Google Scholar]
- Sawai N, Zhou S, Vanin EF, Houghton P, Brent TP., and, Sorrentino BP. Protection and in vivo selection of hematopoietic stem cells using temozolomide, O6-benzylguanine, and an alkyltransferase-expressing retroviral vector. Mol Ther. 2001;3:78–87. doi: 10.1006/mthe.2000.0223. [DOI] [PubMed] [Google Scholar]
- Zielske SP, Reese JS, Lingas KT, Donze JR., and, Gerson SL. In vivo selection of MGMT(P140K) lentivirus-transduced human NOD/SCID repopulating cells without pretransplant irradiation conditioning. J Clin Invest. 2003;112:1561–1570. doi: 10.1172/JCI17922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman JE, Reese JS, Lingas KT., and, Gerson SL. Myeloablation is not required to select and maintain expression of the drug-resistance gene, mutant MGMT, in primary and secondary recipients. Mol Ther. 2003;8:42–50. doi: 10.1016/s1525-0016(03)00141-2. [DOI] [PubMed] [Google Scholar]
- Reese JS, Koç ON, Lee KM, Liu L, Allay JA, Phillips WP., Jret al. (1996Retroviral transduction of a mutant methylguanine DNA methyltransferase gene into human CD34 cells confers resistance to O6-benzylguanine plus 1,3-bis(2-chloroethyl)-1-nitrosourea Proc Natl Acad Sci USA 9314088–14093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christians FC., and, Loeb LA. Novel human DNA alkyltransferases obtained by random substitution and genetic selection in bacteria. Proc Natl Acad Sci USA. 1996;93:6124–6128. doi: 10.1073/pnas.93.12.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard BC, Sud R, Keyser KA, Ironside C, Neff T, Gerull S.et al. (2009Long-term polyclonal and multilineage engraftment of methylguanine methyltransferase P140K gene-modified dog hematopoietic cells in primary and secondary recipients Blood 1135094–5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff T, Beard BC, Peterson LJ, Anandakumar P, Thompson J., and, Kiem HP. Polyclonal chemoprotection against temozolomide in a large-animal model of drug resistance gene therapy. Blood. 2005;105:997–1002. doi: 10.1182/blood-2004-08-3169. [DOI] [PubMed] [Google Scholar]
- Neff T, Horn PA, Peterson LJ, Thomasson BM, Thompson J, Williams DA.et al. (2003Methylguanine methyltransferase-mediated in vivo selection and chemoprotection of allogeneic stem cells in a large-animal model J Clin Invest 1121581–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollok KE, Hartwell JR, Braber A, Cooper RJ, Jansen M, Ragg S.et al. (2003In vivo selection of human hematopoietic cells in a xenograft model using combined pharmacologic and genetic manipulations Hum Gene Ther 141703–1714. [DOI] [PubMed] [Google Scholar]
- Larochelle A, Choi U, Shou Y, Naumann N, Loktionova NA, Clevenger JR.et al. (2009In vivo selection of hematopoietic progenitor cells and temozolomide dose intensification in rhesus macaques through lentiviral transduction with a drug resistance gene J Clin Invest 1191952–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trobridge GD, Wu RA, Beard BC, Chiu SY, Muñoz NM, von Laer D.et al. (2009Protection of stem cell-derived lymphocytes in a primate AIDS gene therapy model after in vivo selection PLoS ONE 4e7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball CR, Pilz IH, Schmidt M, Fessler S, Williams DA, von Kalle C.et al. (2007Stable differentiation and clonality of murine long-term hematopoiesis after extended reduced-intensity selection for MGMT P140K transgene expression Blood 1101779–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le Deist F, Wulffraat N, McIntyre E.et al. (2003A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency N Engl J Med 348255–256. [DOI] [PubMed] [Google Scholar]
- Ott MG, Schmidt M, Schwarzwaelder K, Stein S, Siler U, Koehl U.et al. (2006Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1 Nat Med 12401–409. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P.et al. (2003LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1 Science 302415–419. [DOI] [PubMed] [Google Scholar]
- Engelmann C, Panis Y, Bolard J, Diquet B, Fabre M, Nagy H.et al. (1999Liposomal encapsulation of ganciclovir enhances the efficacy of herpes simplex virus type 1 thymidine kinase suicide gene therapy against hepatic tumors in rats Hum Gene Ther 101545–1551. [DOI] [PubMed] [Google Scholar]
- Soper BW, Lessard MD, Jude CD, Schuldt AJ, Bunte RM., and, Barker JE. Successful allogeneic neonatal bone marrow transplantation devoid of myeloablation requires costimulatory blockade. J Immunol. 2003;171:3270–3277. doi: 10.4049/jimmunol.171.6.3270. [DOI] [PubMed] [Google Scholar]
- Hacke K, Falahati R, Flebbe-Rehwaldt L, Kasahara N., and, Gaensler KM. Suppression of HLA expression by lentivirus-mediated gene transfer of siRNA cassettes and in vivo chemoselection to enhance hematopoietic stem cell transplantation. Immunol Res. 2009;44:112–126. doi: 10.1007/s12026-008-8088-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielske SP., and, Gerson SL. Lentiviral transduction of P140K MGMT into human CD34(+) hematopoietic progenitors at low multiplicity of infection confers significant resistance to BG/BCNU and allows selection in vitro. Mol Ther. 2002;5:381–387. doi: 10.1006/mthe.2002.0571. [DOI] [PubMed] [Google Scholar]
- Reynaud D, Pietras E, Barry-Holson K, Mir A, Binnewies M, Jeanne M.et al. (2011IL-6 controls leukemic multipotent progenitor cell fate and contributes to chronic myelogenous leukemia development Cancer Cell 20661–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stripecke R, Carmen Villacres M, Skelton D, Satake N, Halene S., and, Kohn D. Immune response to green fluorescent protein: implications for gene therapy. Gene Ther. 1999;6:1305–1312. doi: 10.1038/sj.gt.3300951. [DOI] [PubMed] [Google Scholar]
- Zhao H, Pestina TI, Nasimuzzaman M, Mehta P, Hargrove PW., and, Persons DA. Amelioration of murine beta-thalassemia through drug selection of hematopoietic stem cells transduced with a lentiviral vector encoding both gamma-globin and the MGMT drug-resistance gene. Blood. 2009;113:5747–5756. doi: 10.1182/blood-2008-10-186684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Cheung P, Roth JC, Wilson DL., and, Gerson SL. Imaging stem cell-derived persistent foci after in vivo selection of lentiviral MGMT-P140K transduced murine bone marrow cells. Mol Ther. 2011;19:1342–1352. doi: 10.1038/mt.2010.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano FA, Sorg UR, Appelt JU, Lachmann N, Bleier S, Roeder I.et al. (2011Clonal inventory screens uncover monoclonality following serial transplantation of MGMT P140K-transduced stem cells and dose-intense chemotherapy Hum Gene Ther 22697–710. [DOI] [PubMed] [Google Scholar]
- May C, Rivella S, Callegari J, Heller G, Gaensler KM, Luzzatto L.et al. (2000Therapeutic haemoglobin synthesis in beta-thalassaemic mice expressing lentivirus-encoded human beta-globin Nature 40682–86. [DOI] [PubMed] [Google Scholar]
- Pawliuk R, Westerman KA, Fabry ME, Payen E, Tighe R, Bouhassira EE.et al. (2001Correction of sickle cell disease in transgenic mouse models by gene therapy Science 2942368–2371. [DOI] [PubMed] [Google Scholar]
- Persons DA, Allay ER, Sawai N, Hargrove PW, Brent TP, Hanawa H.et al. (2003Successful treatment of murine beta-thalassemia using in vivo selection of genetically modified, drug-resistant hematopoietic stem cells Blood 102506–513. [DOI] [PubMed] [Google Scholar]
- Imren S, Payen E, Westerman KA, Pawliuk R, Fabry ME, Eaves CJ.et al. (2002Permanent and panerythroid correction of murine beta thalassemia by multiple lentiviral integration in hematopoietic stem cells Proc Natl Acad Sci USA 9914380–14385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persons DA, Hargrove PW, Allay ER, Hanawa H., and, Nienhuis AW. The degree of phenotypic correction of murine beta -thalassemia intermedia following lentiviral-mediated transfer of a human gamma-globin gene is influenced by chromosomal position effects and vector copy number. Blood. 2003;101:2175–2183. doi: 10.1182/blood-2002-07-2211. [DOI] [PubMed] [Google Scholar]
- Detloff PJ, Lewis J, John SW, Shehee WR, Langenbach R, Maeda N.et al. (1994Deletion and replacement of the mouse adult beta-globin genes by a “plug and socket ” repeated targeting strategy Mol Cell Biol 146936–6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Kirby S, Lewis J, Detloff PJ, Maeda N., and, Smithies O. A mouse model for beta 0-thalassemia. Proc Natl Acad Sci USA. 1995;92:11608–11612. doi: 10.1073/pnas.92.25.11608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halene S, Wang L, Cooper RM, Bockstoce DC, Robbins PB., and, Kohn DB. Improved expression in hematopoietic and lymphoid cells in mice after transplantation of bone marrow transduced with a modified retroviral vector. Blood. 1999;94:3349–3357. [PMC free article] [PubMed] [Google Scholar]
- Zielske SP, Lingas KT, Li Y., and, Gerson SL. Limited lentiviral transgene expression with increasing copy number in an MGMT selection model: lack of copy number selection by drug treatment. Mol Ther. 2004;9:923–931. doi: 10.1016/j.ymthe.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Gambhir SS, Bauer E, Black ME, Liang Q, Kokoris MS, Barrio JR.et al. (2000A mutant herpes simplex virus type 1 thymidine kinase reporter gene shows improved sensitivity for imaging reporter gene expression with positron emission tomography Proc Natl Acad Sci USA 972785–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai CK, Wang WJ, Chen TC., and, Kasahara N. Single-shot, multicycle suicide gene therapy by replication-competent retrovirus vectors achieves long-term survival benefit in experimental glioma. Mol Ther. 2005;12:842–851. doi: 10.1016/j.ymthe.2005.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Zhong C, Chang Z., and, Roy-Burman P. A potential therapeutic strategy to combat leukemia virus infection. Cancer Biol Ther. 2003;2:92–99. doi: 10.4161/cbt.236. [DOI] [PubMed] [Google Scholar]
- Tarantal AF, O'Rourke JP, Case SS, Newbound GC, Li J, Lee CI.et al. (2001Rhesus monkey model for fetal gene transfer: studies with retroviral- based vector systems Mol Ther 3128–138. [DOI] [PubMed] [Google Scholar]
- Drobyski WR, Morse HC., 3rd, , Burns WH, Casper JT., and, Sandford G. Protection from lethal murine graft-versus-host disease without compromise of alloengraftment using transgenic donor T cells expressing a thymidine kinase suicide gene. Blood. 2001;97:2506–2513. doi: 10.1182/blood.v97.8.2506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effects of different weekly BG/BCNU regimens on total lymphocyte counts and survival.