Abstract
Globoid cell leukodystrophy (GLD) or Krabbe disease is a neurodegenerative disorder caused by the deficiency of the lysosomal enzyme galactocerebrosidase (GALC). This deficiency results in accumulation of certain galactolipids including psychosine which is cytotoxic for myelin-producing cells. Treatment of human patients at this time is limited to hematopoietic stem cell transplantation (HSCT) that appears to slow the progression of the disease when performed in presymptomatic patients. In this study, adeno-associated virus (AAV) serotype rh10-(AAVrh10) expressing mouse GALC was used in treating twitcher (twi) mice, the mouse model of GLD. The combination of intracerebroventricular, intracerebellar, and intravenous (iv) injection of viral particles in neonate twi mice resulted in high GALC activity in brain and cerebellum and moderate to high GALC activity in spinal cord, sciatic nerve, and some peripheral organs. Successfully treated mice maintained their weight with no or very little twitching, living up to 8 months. The physical activities of the long-lived treated mice were comparable to wild type for most of their lives. Treated mice showed normal abilities to mate, to deliver pups, to nurse and to care for the newborns. This strategy alone or in combination with other therapeutic options may be applicable to treatment of human patients.
Introduction
Globoid cell leukodystrophy (GLD) or Krabbe disease is a progressive central nervous system (CNS) and peripheral nervous system (PNS) disease caused by the deficiency of lysosomal galactocerebrosidase (GALC). GALC is responsible for the degradation of specific galactolipids, including several that are important in the production of myelin (reviewed in refs. 1,2). In the absence of GALC, accumulation of psychosine (galactosylsphingosine) impairs the function of the myelin-producing cells.3,4 While most patients have the infantile form of GLD, older individuals are also diagnosed. At this time the only treatment is hematopoietic stem cell transplantation (HSCT) that has been successful in patients who do not yet have severe neurological disease.5,6 The HSCT seems to slow the disease progression. This disease is unique in having several well characterized animal models available for study.7,8 Twitcher (twi) mice, the authentic murine model of GLD, appear normal at birth but develop tremor by ~21 days of age. This is followed by steady weight loss, muscle weakness, and hind-leg paralysis. They become hunchbacked by 30 days and die usually around 40 days of age.7 Treatment trials in twi mice include bone marrow transplantation (BMT),9,10 stem cell transplantation,11,12,13,14 in vivo and ex vivo gene therapies,15,16,17,18,19,20,21 substrate reduction therapy,22 enzyme replacement therapy,23 and small molecule therapy.24,25,26 BMT has been so far the most effective therapeutic strategy in the treatment of twi mice. If performed early, BMT prolongs the life of the affected mice from ~40 days to an average of 80 days.
One approach needing additional study is gene therapy for treating the CNS and PNS. Adeno-associated viruses (AAV) are currently among the most frequently used viral vectors for gene therapy. Their unique life cycle and their ability to infect both dividing and nondividing cells have made them an attractive vector to carry a variety of transgenes. Our previous studies on intracerebroventricular injection of AAV serotypes 1 and 5 into twi mice demonstrated many pathological improvements with modest increase in life span of the twi mice.17,18 However, for diseases that affect large areas of the CNS, local injection of gene delivery vectors is less than optimal, since it provides transgene expression only to limited regions in the CNS. An ideal approach to address CNS disorders that affect large areas of the brain and spinal cord is the vector delivery through the vasculature system. However, the blood–brain barrier (BBB) is the major obstacle to this approach. Significant amount of studies have focused in finding an efficient and safe vector to deliver the transgene to CNS through systemic administration.27,28,29,30,31 Recently, Foust et al. demonstrated that intravenous (iv) administration of AAV9 resulted in extensive transduction of neuronal cells in neonates and astrocytes in adult mice.32
More recently, Hu et al. demonstrated that AAVrh10 serotype provides high expression and greater stability of expression compared to the other serotypes studied when iv administrated into neonatal mice.33 This was followed by a comparative study of 10 different AAV serotypes by Zhang et al.34 They concluded that the ability of AAVrh10 in crossing the BBB is comparable to that of AAV9. In the present study, AAV2 genome construct expressing mouse GALC was packaged in AAVrh10 capsid. Three delivery strategies alone or in combination were applied to neonatal twi mice: combined intracerebroventricular and intracerebellar injections, iv injection alone, and a combination of all three injections. We have shown that the successfully treated mice are active and symptom-free up to 8 months of age. When symptoms such as hind legs paralysis start, near the end of their life, the progression of these symptoms is much slower compared to the rapid downhill course seen in untreated mice. In their active period of life, these mice are capable of mating, delivering and caring for the pups. No other previous treatments have resulted in fertile mice.
Results
GALC activity
When neonate twi mice received only iv injection, the increase in GALC activities of brain and cerebellum were modest. The GALC activity measured in whole brain reached up to 0.2 nmol/h/mg protein which is ~10-fold increase above the activity measured in untreated twi mice. However, icv-ic and icv-ic-iv injections of neonate twi mice with AAVrh10-mGALC, resulted in high levels of GALC activity throughout the brain. With both treatment methods GALC activity in whole brain homogenate was around 13–15 nmol/h/mg protein. Figure 1 shows the brain regions analyzed and sites of injections of one representative mouse subjected to icv-ic on PND2 and sacrificed on PND35. The GALC activities are shown in the different brain regions. These numbers are compared to 0.02 nmol/h/mg protein, the mean GALC activity in untreated twi mice and ~3.0 nmol/h/mg protein in wild-type mice. The high expression of GALC could be demonstrated throughout their lifespan. For example, the GALC activity measured in midbrain of three icv-ic-iv-treated mice that lived between 72 and 140 days was between 40 and 44 nmol/h/mg protein, similar to what was measured in the 35-day-old icv-ic-treated mouse shown in Figure 1. The mean GALC activity measured in the olfactory bulb area of two icv-ic-iv-iv-treated mice, analyzed 8 months after injection, was 25.8 nmol/h/mg protein. These values measured at death of long-lived treated mice are still well above normal. While high GALC activity could be demonstrated in peripheral organs such as liver, heart and lung following iv and icv-ic-iv injections, no GALC activity was found in the gonads of these mice. PCR to amplify the viral vector genome in gonads were also negative.
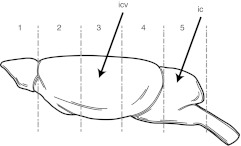
Figure 1.
Galactocerebrosidase (GALC) activity in central nervous system (CNS) of an icv-ic-treated mouse injected on PND2 and sacrificed at PND35. The diagram identifies the different CNS sections analyzed for GALC activity. The arrows point to the injection sites of injections. GALC activities expressed as nmol/h/mg protein are as follows: Section 1 (olfactory bulbs), 12.8; section 2 (forebrain), 9.7; section 3 (midbrain), 42.9; section 4 (hindbrain), 13.3; section 5 (cerebellum), 1.3; and section 6 (spinal cord), 0.4.
While GALC activity in CNS and PNS of iv-treated mice was low but detectable using biochemical measurement, it could be readily visualized by X-gal staining of the brain sections (Figure 2a, panel B). Strong blue staining could also be seen in some peripheral organs, such as liver, heart, and muscle tissues of iv-treated mice (data not shown).
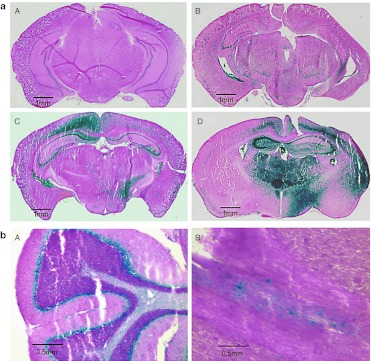
Figure 2.
Histochemical detection of galactocerebrosidase (GALC) activity using modified X-gal staining. The blue color represents GALC. (a) Coronal brain sections from a 65-day-old uninjected wild-type mouse (A), a 60-day-old mouse that received iv injection only (B), a 60-day-old icv-ic-treated mouse (C), and a 72-day-old icv-ic-iv-treated mouse (D). (b) Sections of cerebellum (A) and spinal cord (B) of a 105-day-old icv-ic- iv-treated mouse.
Robust GALC activity could be easily visualized in brain in icv-ic and icv-ic-iv-treated mice (Figure 2a,b). Areas of the brain stained with X-gal included the choroid plexus in the lateral and dorsal 3rd ventricles, CA3 field of hippocampus, fimbria of hippocampus, stria terminalis, corpus callosum, and cortex (Figure 2a). Using this method, cerebellum from icv-ic or icv-ic-iv-treated mice demonstrated strong GALC activity in Purkinje cell layer (Figure 2b, panel A).
Growth monitoring and life span
Body weights of the AAVrh10-mGALC-treated mice were monitored as an indicator of general health. The untreated twi and wild-type mice as well as all AAVrh10-treated mice had similar weight gain up to ~3 weeks of age. By week 6 or 7 all untreated mice had died following steady weight loss, while successfully treated mice continued to gain weight for a longer period (Figure 3a). Mice receiving an extra iv injection on PND7 (icv-ic-iv-iv) achieved the highest weights, very similar to wild-type mice. Although the lifespan was not the same for the different treatment strategies, the mice maintained their body weight until ~30 days before they died. Gradual and slow weight loss starts 15 to 30 days before their expiration. While mice are alert and quite active, weaknesses and sometime paralysis in the hind legs will limit their mobility. Death may occur at any time during this stage. Post-mortem examination of many mice did not reveal any significant organ damage to explain their death. However, occasional full urinary bladder and urinary retention were observed.
Figure 3.
Impact of AAVrh10-mGALC administration on growth, lifespan, and grip strength. (a) Weight maintenance of icv-ic-iv (n = 14) and icv-ic-iv-iv-treated affected mice (n = 10) are compared to untreated affected mice (n = 7) and wild-type mice (n = 5). (b) Survival rates of iv, ivc-ic, and icv-ic-iv-treated mice are compared to untreated affected mice. While iv-injected mice had modest increase in lifespan, the icv-ic and icv-ic-iv-injected mice had dramatic increases in lifespan. (c) Effect of an extra iv administration of AAVrh10-mGALC is compared to icv-ic-iv treatment alone. Note that some of the mice receiving the second iv injection lived shorter than icv-ic-iv-treated mice while some others had much extended lifespan. (d) The average lifespan of the mice treated with different viral administration strategies are compared to average lifespan of the untreated twitcher (twi) mice (untreated: n = 9, iv-treated: n = 10, icv-ic-treated: n = 10, icv-ic-iv-treated: n = 15, icv-ic-iv-iv-treated: n = 7). All treatment strategies resulted in a significant increase in life span of the treated mice compared to untreated twi mice (iv: P < 0.0001, icv-ic: P < 0.001, icv-ic-iv: P < 0.0001, icv-ic-iv-iv: P < 0.05). GALC, galactocerebrosidase.
The median lifespan of untreated twi mice is ~40 days. They generally start twitching at PND21, showing hind leg paralyses at the terminal stage and hunched-back starting at PND30. We have shown an increase in lifespan of all AAVrh10-mGALC-treated twi mice (Figure 3b–d) with an improvement in all functions for most of their lives. While both iv and icv-ic treatment strategies resulted in a significant increase in lifespan, the combination of these two approaches had a synergic effect in extending their active lifetime. As shown in Figure 3d the average lifespan for iv-treated mice was 55 days with the maximum of 63 days; these values for icv-ic-treated mice were 81 and 140 days. Combination of these two strategies (icv-ic-iv treatment) resulted in average lifespan of 104 days with several mice living up to 150 days. While average life span of mice receiving icv-ic-iv-iv injections was 120 days (Figure 3d), two lived near 8 months (Figure 3c) before being sacrificed for pathological studies.
Behavioral monitoring
Behavioral performance of wild type, untreated, and treated mice was assessed by evaluation of their grip strength, monitoring their open field activities, analyzing their walking pattern and observing their daily tasks performance. Grip strength test was used to determine neuromuscular strength in the treated and untreated mice. As shown in Figure 4a, the hanging time measured at different time points in icv-ic-iv-treated mice was comparable to the age-matched wild-type mice at PND120.
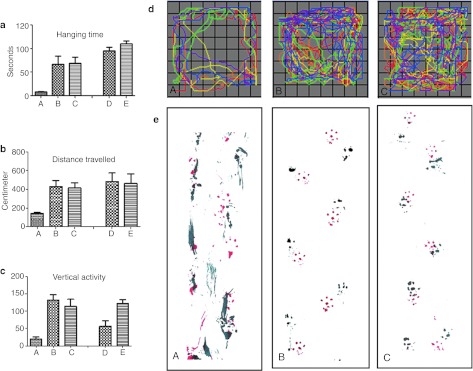
Figure 4.
Muscle strength and behavioral monitoring of the AAVrh10-mGALC-treated mice in an open field setting and gait analysis. (a) Grip strength of the treated and untreated mice is evaluated by the length of their hanging time (seconds) from a metal rod. Columns (A), (B), and (C) are the mean of three sets of experiments carried out between PND25 and 35. Column (A) represents the average of experiments for untreated mice (n = 3), column (B) represents icv-ic-iv-treated mice (n = 3) and column (C) stands for age-matched wild-type mice (n = 3). Column (D) represents the average hanging time for two (n = 2) 120-day-old icv-ic-iv-treated mice and column (E) represents the average of four (n = 4) age-matched wild-type mice. While the hanging times recorded at period of PND25–35 and PND110–120 were significantly higher in the treated mice compared to untreated twitcher (twi) mice (P < 0.01 for the measurements recorded at PND25–35 and P < 0.0001 for PND110–120), it was not statistically different when compared to the age-matched wild-type mice (P < 1.0 for PND25–35 and P < 0.5 for PND110–120). Figures (b) through (d) are the results from an open field studies. For each testing groups, two sets of three repetitions in three consecutive days were conducted. First testing group (A–C) is comprised of: (A) untreated affected mice (n = 3), (B) icv-ic-iv-treated affected mice (n = 3) and (C) wild-type mice (n = 3), all tested between PND25 and 35. Second group comprised of: (D) icv-ic-iv-treated affected mice (n = 2), and (E) untreated wild-type mice (n = 4), all tested between PND110 and 120. (b) Total distance traveled (cm). A significant difference was recorded between untreated (column A) and treated mice (column B): P < 0.002 between columns (A) and (B), P < 0.003; between columns (A) and (D). There was no a significant difference between treated mice and wild-type mice. (c) Vertical activity. While the vertical activities recorded for icv-ic-iv-treated mice at PND25–35 were comparable to the activity recorded for wild-type mice (P < 0.50) they were significantly higher than untreated affected mice (P < 0.0001). However, the vertical activities of the icv-ic-iv-treated mice measured at PND110–120 were significantly lower than wild-type mice (P < 0.007). (d) Movements recorded by one untreated (A), one icv-ic-iv-treated (B) and one wild-type mouse (C). Each color represents 50-second movement segment for a total of 5 minutes. (e) Gait analysis. The foot prints of mice were recorded as described in Method section. Foot prints from a 42-day-old untreated affected mouse (A), a 150-day-old icv-ic-iv-iv-treated mouse (B) and an 80-day-old wild-type mouse (C). GALC, galactocerebrosidase; PND, postnatal day.
The motor function and coordination ability were monitored using an activity monitor (VersaMax; AccuScan Instruments, Columbus, OH). Total distances travelled as well as the vertical activities in a novel environment were evaluated for 5 minutes (Figure 4b,c). The icv-ic-iv-treated mice activities at 25–35-PND (Figure 4b, column C and Figure 4c, column C) were comparable to the patterns recorded for wild type (Figure 4b, column B and Figure 4c, column B). A marked decrease in the total distance traveled (Figure 4b, column A), and vertical activity (Figure 4c, column A) was observed in untreated affected mice. For icv-ic-iv-treated mice tested between PND 110 and 120, treated mice showed decreased vertical activities (Figure 4c, column D) while the total distance travelled was comparable to the age-matched wild-type mice (Figure 4b, column D).
A representative movement pattern from 25 to 35-day-old mice is shown in Figure 4d. Wild type and treated mice would explore the entire field including the center part while the untreated affected mice would spend most of the time in the periphery.
Gait analysis of the treated, untreated, and wild-type mice was conducted at different ages. In untreated twi mice, the gait was disordered with poor coordination between the forepaw and the hind paw along with a dragging foot patterns (Figure 4e, panel A). In wild-type mice, the footprints for the hind paws were mainly overlaid on top of the footprints for the front paws and there was no limb dragging (Figure 4e, panel C). The walking pattern of the icv-ic-iv-iv-treated mice, during the active period of their lives, was comparable with the one from wild-type mice with no limb dragging. The walking pattern of a 150-day-old icv-ic-iv-iv-treated mouse is shown in Figure 4e, panel B.
Among the other normal behavioral indicators observed in the icv-ic-iv or icv-ic-iv-iv-treated affected mice were the absence of tremor and twitching for most of their lives, many successful matings, full-term pregnancy, normal nest making, and postnatal care of the newborns. Many successful mating and pregnancies between treated affected males and females with carriers, and between treated affected males and treated affected females were recorded. One long-lived-treated female who received a second iv injection of AAVrh10-mGALC mated twice with similarly treated male, and delivered average size litters of all genetically affected pups. None of these untreated affected mice produced from treated affected parents lived beyond the life expectancy for untreated twi mice. This may be taken as an additional indicator that there was no viral transduction in germ lines of treated male and female. Negative PCR amplification of the gonads for viral construct supports this hypothesis (data not shown).
Immunohistochemistry
Immunostaining for GALC antigen of the brains sections from mice that were only iv-injected did not result in detectable signals in cerebral tissue. It is also difficult to detect GALC antigen in wild-type mice by immunostaining. However, intense staining was observed in the choroids plexus of the cerebral ventricles of iv-treated mice (data not shown).
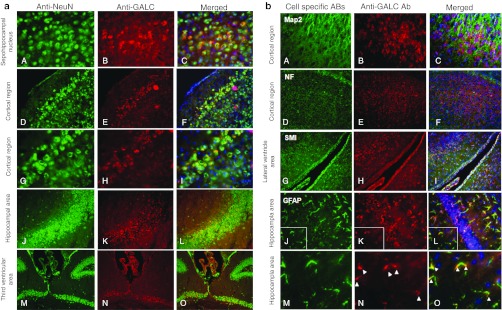
In contrast to the mice that received only iv injection, brain sections from the mice receiving icv-ic or icv-ic-iv injections demonstrated widespread cytoplasmic staining with anti-GALC antibody (Figure 5a,b). Strong GALC expression was seen in all neural areas, choroids plexus, and ependymal cells and periventricular areas (Figure 5a). Strong tropism of AAVrh10 toward neuronal cells could be demonstrated by colocalization of GALC antigen and neuron-specific antibodies such as anti-NeuN antibody (Figure 5a, panels A through O) and anti-Map2 and anti-neurofilament antibodies (Figure 5b, panels A through F). While there was a slight overlap between myelin-specific markers such as SMI-91 and SMI-94, strong colocalization of anti-GALC and anti-GFAP in the vicinity of the strongly GALC-positive neural cells was noted (Figure 5b, panels J through O). The GALC antigen detected in astrocytes could be the result of the viral transduction or uptake from transduced neuronal cells.
Figure 5.
Immunohistochemistry. (a) Different cerebral regions of a 221-day-old icv-ic-iv-iv-treated mouse, immunostained with NeuN neuronal marker and affinity-purified anti-GALC antibody. [Original magnification for images (A–I): ×400 and for (M–O): ×200]. (b) Brain section from 114-day-old icv-ic-iv-treated mouse stained with different cell type-specific antibodies and anti-GALC antibody. Images (A) and (D) are stained with neuronal-specific antibodies (anti-Map2 and anti-neurofilament respectively), image (G) is stained with oligodendrocyte antibodies (anti-myelin CNPase: SMI91 + SMI94), and image (I) is stained with astrocyte antibody (anti-GFAP). Images (B), (E), (H), (K) and (N) are stained against GALC antibody and images (C), (F), (I), (L) and (O) are the merged pictures from cell specific and GALC antibodies with DAPI staining. Images (M) through (O) enlarge the boxed areas of images (I) through (L). [Original magnification for images (A–I): ×400. Approximate magnification for images (M–O): ×1,000]. GALC, galactocerebrosidase.
Pathological studies
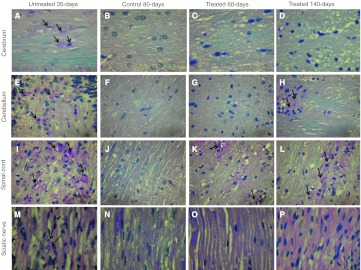
The pathological studies of AAVrh10-mGALC-treated mice were carried out by LFB/PAS staining of formalin fixed, paraffin embedded sections from different areas in CNS and PNS (Figure 6), immunohistochemical of frozen sections from cerebral tissues (Figure 7a) and electron microscopy of spinal cord (Figure 7b). LFB/PAS staining of multiple sites in the CNS and the sciatic nerve from a 60-day-old icv-ic-iv-treated twi mouse that was active with no apparent symptoms and a similarly treated 140-day-old mouse at its terminal state were compared to a 35-day-old untreated affected mouse and to an 80-day-old wild-type mouse. The untreated affected mouse showed focal dense infiltration of foamy macrophages containing PAS-positive myelin debris consistent with myelin breakdown in different regions of the CNS and PNS including the cerebral hemispheres, the cerebellum, the spinal cord and sciatic nerve (Figure 6, panels A, E, I, and M). In contrast to the untreated mouse, the cerebral white matter from a 60-day-old and 140-day-old icv-ic-iv-treated twi mice, had normal-appearing white matter with no macrophage infiltration and no areas of demyelination (Figure 6, panels C and D). The cerebellum of the 60-day-old treated mouse (Figure 6, panel G) also showed no macrophage infiltration, however the cerebellum of the 140-day-old treated mouse did show scattered foci of PAS-positive foamy macrophages and demyelination (Figure 6, panel H). In addition the brainstem of the 60-day-old-treated mouse had completely normal appearance while the brainstem of the 140-day-old treated mouse showed dispersed foci of PAS-positive foamy macrophages and demyelination (data not shown). In spite of improved ultrastructure detected by electron microscopy in the spinal cord of a 140-day-old icv-ic-iv-treated mouse (Figure 7b, panel C), the LFB/PAS stained sections of spinal cord white matter tracts of both 60-day-old and 140-day-old-treated mice showed scattered foci of PAS-positive foamy macrophages with myelin loss (Figure 6, panels K and L). Sciatic nerves from the 60-day-old mouse contains normal looking myelinated axons with no macrophage infiltration (Figure 6o). The sciatic nerve from the 140-day-old-treated mouse showed normal-appearing myelinated axons (Figure 6, panel p). However, a very few infiltrating macrophages were noted (data not shown).
Figure 6.
Pathological studies of central nervous system (CNS) and peripheral nervous system (PNS) in untreated twitcher (twi), wild type, and icv-ic-iv-treated twi mice. All images are from paraffin sections stained with LFB/PAS. Images (A) through (D) are from cerebral hemisphere white matter (original magnification ×640); images (E) through (H) are from cerebellum (original magnification ×400); images (I) through (K) are from spinal cord white matter (original magnification ×400); and images (M) through (P) are longitudinal sections from sciatic nerve (original magnification ×640). Arrows point to infiltrating foamy macrophages.
Figure 7.
Reduction of astrogliosis and improved myelination in icv-ic-iv-treated mice. (a) Anti-GFAP-stained brain sections from a (A) 35-day-old untreated affected, (B) 114-day-old wild type, and (C) 114-day-old treated mouse. (Original magnification: ×400). (b) Electron micrographs of the spinal cord from a (A) 35-day-old, untreated affected, (B) wild type, and (C) 140-day-old icv-ic-iv-treated mice. Demyelinated axons are shown by arrow heads and the tubular profiles, characteristic of globoid cell leukodystrophy (GLD), are shown by arrows (see B panel A). Scales are shown on the pictures.
In another set of experiments, we examined the frozen brain sections from a 35-day-old untreated twi mouse, a 114-day-old wild-type mouse and a 114-day-old icv-ic-iv-treated mouse to evaluate the appearance of astrocytes (Figure 7a). The anti-GFAP antibody staining in brain of a 35-day-old untreated twi mouse demonstrated an increased number of hypertrophied, reactive appearing astrocytes (Figure 7a, panel A). In contrast to the untreated affected mouse, the wild-type mouse showed an orderly arrangement of normal-appearing astrocytes (Figure 7a panel B). The astrocytic density in the icv-ic-iv-treated mouse was comparable to the wild-type mouse; however, very mild reactive astrocytic changes were present (Figure 7a, panel C).
Electron micrograph from spinal cord of an untreated twi mouse showed numerous abnormalities including marked reduction in the number of myelinated axons and scattered tubular profiles characteristic of Krabbe disease (Figure 7b, panel A). In contrast to the untreated twi mouse, the spinal cord from a wild-type mouse showed normal-appearing myelin profiles with numerous myelinated axons (Figure 7b, panel B). Electron micrograph of spinal cord from a 140-day-old icv-ic-iv-treated mouse showed many normal-appearing axons (Figure 7b, panel C). While the ultrastructure of the myelin in the spinal cord of the treated mouse did not appear significantly different from the myelin ultrastructure of the wild-type specimen the number of myelinated axons in 140-day-old icv-ic-iv-treated mouse was reduced by ~15% when compared to the wild-type mouse (data not shown).
Discussion
GLD or Krabbe disease is caused by the lack of GALC enzyme, a lysosomal hydrolase responsible for the degradation of certain galactolipids, including the cytotoxic sphingolipid called psychosine. The classic form of this disease, in human and in animal models, starts at an early age. Therefore, any methodology aiming to treat this disease should be carried out as early as possible to protect or rescue the myelin-producing cells. While there is no cure for GLD at this time, early HSCT has been proven to be beneficial in slowing the progression of the disease in infants treated before symptoms become obvious and in patients with the late-onset forms of this disease.5,6 BMT in animal models of GLD have resulted in positive outcomes.10 Several other treatment strategies such as stem cell transplantation, in vivo and ex vivo gene therapies,15,16,17,18,20,21 substrate reduction therapy,22 enzyme replacement therapy,23 and small molecule administration24,25,26 have also been applied to animal models of GLD but none of them resulted in satisfactory outcomes.
In the present study, we have demonstrated successful AAV-mediated delivery of GALC enzyme throughout the CNS and PNS that resulted in dramatic improvements in the general health and lifespan in the twi mouse. Such improvements are unprecedented with the use of any gene or stem cell therapies in mouse model of GLD. This was achieved by using the newly developed AAVrh10 serotype and combination of gene delivery directly to the CNS and indirectly to the CNS and PNS through the blood circulation. The choice of AAVrh10 was based on its ability to provide widespread distribution in the CNS.35 When we started to use this vector, there were no studies showing that the AAVrh10 could cross the BBB. However, our findings are in full agreement with the recent publications reporting that certain AAV serotypes especially serotypes 9 and rh10 can efficiently cross the BBB and transduce primarily neurons and to some extent astrocytes.33,34
Our methodology in gene delivery using AAVrh10-mGALC, was composed of two strategies of direct CNS injection (icv-ic) and systemic injection alone (iv) or in combination. All methodologies resulted in clinical, pathological, and behavioral improvements with no or minimal twitching until very late in the disease course. The extent of the improvements was different depending on the methodology used. The iv administration of AAVrh10-mGALC alone resulted in modest but global viral delivery in CNS as detected by X-gal staining (Figure 2a, panel B) and significant delivery to several peripheral organs. Some of the affected mice that received an extra iv injection of AAVrh10-mGALC lived up to 8 months which is over five times the average lifespan of untreated twi mice. Nevertheless, some other similarly treated mice (icv-ic-iv-iv) were terminal by 2 months of age. One explanation for the early deaths may be insufficient delivery of viral vector. Although care has been taken to ensure the success of the tail vein and CNS injections at PND2, variations in the injected volume and site of injection is plausible. An alternative explanation for early death in the mice with the second iv injection, a week after the first injection, might be the immunologic response from the mouse that has already been exposed to AAVrh10. AAV vectors have been favored for in vivo gene therapy applications not only because of their high efficiency in transducing different cell types, but also because of their low immunogenicity and strong safety record in animal models. However, injecting animal models that are pre-exposed to AAV capsid protein may stimulate their immunologic responses. Development of such T-cell response to AAV capsid protein has been reported in human patients and nonhuman primate models.36,37,38
The successfully icv-ic-iv-treated mice could not be distinguished from normal or heterozygote siblings during their active life. The improvements included the lack of twitching, a key feature of the twi mice that is even present in BMT-treated twi mice and maintenance of body weight. They were also capable in carrying out their everyday activities such as mating, litter delivery, nest making, taking care of newborn pups and other behavioral acts. While such improvement has not been reported in twi mice, reversal of infertility has been demonstrated in another mouse model of a lysosomal storage disease39. Activity patterns and exploratory behavior of our treated mice were comparable to age-matched wild-type mice (Figure 4a–d). Unlike the untreated twi mice that demonstrate irregularly scattered footprints, the footprints produced by icv-ic-iv and icv-ic-iv-iv-treated mice are coordinated during their active life (Figure 4e). Approximately 2 or 3 weeks before death, the gradual weight loss and hind leg weakness would start. Even when dragging the hind legs they could move around quite quickly and would continue their routine activities. The common arched spinal cord symptom known as “hunched-back” appearance in twi mice was very mild in the treated long-living mice.
Consistent with the increase in lifespan, a significant reversal of pathology in CNS and PNS was observed. Pathological studies of the brain and cerebellum from a 60-day-old icv-ic-iv-treated twi mice showed completely normal-appearing morphology with no macrophage infiltration (Figure 6, panels C and G). Similar studies carried out on a 140-day-old icv-ic-iv-treated mouse at its terminal stage, showed completely normal brain structure but moderate infiltration of PAS-positive foamy macrophages in the cerebellum (Figure 6, panels D and H).
Although spinal cord from both 60-day-old and 140-day-old-treated mice had much improved morphology (Figure 6, panel K, L and Figure 7b) compared to the spinal cord from untreated affected mouse, this was still the least improved area of the CNS, containing scattered foci of foamy macrophages.
The widespread transduction and high GALC activity pattern achieved by AAVrh10-mGALC was maintained for many months until the death of the mice. The mean GALC activity measured in the olfactory bulb area of two icv-ic-iv-iv-treated mice, analyzed 8 months after injection, was 25.8 nmol/h/mg protein. The mean activity of the cerebellum from the same mice was 7.0 nmol/h/mg protein. These values are compared to ~3 nmol/h/mg protein in uninjected wild-type mice. In every brain structure analyzed, strong GALC-positive signals were co-localized with the neuron-specific marker NeuN (Figure 5a). Using both anti-GALC and anti-GFAP antibodies, we could also demonstrate GALC-positive staining in some astrocytic processes (Figure 5b). The ability of AAVrh10 to transduce astrocytes of adult mice was reported by Zhang et al.34 It is also possible that some GALC antigen seen in astrocytes has been internalized by these cells as a part of the cross-correction event between high-GALC-expressing neurons and GALC-deficient astrocytes. Although we could not demonstrate GALC-positive oligodendrocytes, some GALC activity must have reached these cells since normal-appearing myelin was present where the macrophage infiltration was greatly diminished. It appears that only a very low level of GALC activity is needed for proper functioning of oligodendrocytes. This low level antigen might not be detected using antibody due to the lack of sensitivity of the method. Much stronger GALC activity could be detected using X-gal staining in brain, cerebellum and spinal cord. Strong X-gal staining of the Purkinje cell layer and granular layer (Figure 2b, panel A) indicates AAVrh10's potential effectiveness in treatment of disorders where there is Purkinje cell involvement.
With high GALC activity measured in the CNS, dramatic extension of lifespan and normal behavioral performance observed in successfully treated mice, the pending question is why these long-lived mice are dying after several months of active and normal life. As the mice treated with BMT and other treatment methodologies have never been symptom-free, the perception was that there could be early and irreversible damage to the nervous system of twi mice. With nearly complete disappearance of the symptoms and emergence of a normal life for quite an extended period of time, it is hard to believe that any irreversible pathological event had occurred in CNS or PNS at an early age. The effectiveness of the AAVrh10-mGALC treatment on the PNS is also highlighted by the improved grip test, which is an indicator of peripheral nerve and muscle function. However, reemerging symptoms such as weakness of hind limbs and slight twitching at the terminal stage of the AAVrh-10-treated mice may have resulted from impaired peripheral nerve function or a demyelinating event resulting from insufficient GALC activity in the PNS at this stage. Therefore, in spite of high GALC activity seen in CNS and PNS of the icv-ic-iv-treated mice throughout their life, some critical regions may have sufficient GALC activity for an extended period of time but not enough to cope with animal's development. In fact as AAV vectors are not integrating in the genome, the gradual loss of the episomal form in adult mice40 might be the reason for gradual reduction of transgene expression in some critical areas. In one perspective, these long-lived mice may mimic the late-onset GLD in human patients where most of them seem perfectly normal until some event or a secondary insult initiates the neurological deterioration.
An alternative explanation for the unexpected downhill after a period of normal life of the successfully treated mice could be resulted from over-production of GALC protein causing unfolded protein response.41 The unfolded protein response is a cellular stress response related to the endoplasmic reticulum. The imbalance between the protein-folding load and the capacity of the endoplasmic reticulum, resulting in an accumulation of unfolded or misfolded proteins in the endoplasmic reticulum lumen, is referred to as endoplasmic reticulum stress which is capable in initiating an inflammatory response.42 If this is the case for premature death in AAVrh10-treated mice a regulatable or adjustable GALC expression should be considered.
In conclusion, using AAVrh10 as delivery vehicle for GALC, we were successful in eradicate the disease symptoms in twi mice for an extended period of time. Intracerebroventricular and iv administration of AAVrh10-mGALC produced global GALC delivery to the CNS and PNS with robust expression profiles within the brain and moderate expression in spinal cord and PNS. Although some peripheral organs were found highly transduced by iv injection of AAVrh10, the male and female gonads seem to be untransduced and germ lines are not altered. Previous studies have shown sperm abnormalities in twi mice.43 Although there is no evidence for transduction of gonads, these mice can reproduce normally. Further studies are underway to investigate the health of gonads of male and female treated mice. The lower than sufficient GALC expression in PNS at terminal stage or higher than manageable amount of GALC production in CNS may account for the ultimate death of the treated mice. Therefore, we believe that both the CNS and PNS will need to be effectively targeted to achieve completely successful therapy. This could be achieved by generating viral vector with controllable GALC expression. Generating viral serotypes that are able to cross BBB and not get trapped in peripheral organs may also result in increased PNS delivery and improved outcomes.
Our findings hold considerable clinical significance for gene therapy of Krabbe disease and other CNS-related disorders. Our next goal is to evaluate the potential therapeutic effect of AAVrh10 expressing canine GALC in the dog model of GLD. Meanwhile, we are working to understand the mechanism behind the premature death of the treated mice and potential strategies to bypass this concern. If studies in canine models prove this approach safe and effective, plans will be made for human trial.
Materials and Methods
Generation of AAV2/rh10-mGALC vector. Mouse GALC cDNA was cloned into pCB7plasmid, an improved version of AAV2 genome vector, which was received from the Institute for Human Gene Therapy at the University of Pennsylvania. The recombinant genome contained AAV2 inverted terminal repeats that flanked the human cytomegalovirus-enhancer/chicken β-actin promoter, mouse GALC cDNA, and rabbit globin poly(A) sequences, totaling ~7.2 kb. The plasmid was then sequenced throughout the expression cassette, and integrity of the ITRs was confirmed by restriction analysis with SmaI and NcoI. The functionality of the construct was verified by in vitro cell transfection and measurement of GALC enzyme activity. STBL2 competent cells were used for transformation of the construct followed by carbenicillin (Invitrogen, Carlsbad, CA) selection. Large-scale plasmid preparation was achieved using the Plasmid Maxi kit from Qiagen (Valencia, CA). The recombinant genome was cross-packaged into AAVrh10 capsid by utilizing a chimeric AAV2-Rep/AAV1-Cap and helper plasmids during a triple-transfection procedure.44,45 Viral packaging and purification were accomplished by the Institute for Human Gene Therapy at the University of Pennsylvania and the vector was called AAVrh10-mGALC. Viral titer, determined by PCR of the simian virus 40 poly(A) sequence44,46 was 7.6 × 1011 genomic equivalents/ml.
Animal model. The original twi mice with W339X mutation in the C57BL background were used in this study. Affected mice obtained from heterozygous mating were genotyped on postnatal day 1 (PND1). Genotypes of pups were determined by PCR and restriction enzyme analysis of DNA extracted from toe clips. Toe clipping also served as animal numbering and identification. All injections were done on PND2 unless stated otherwise. Three times per week, mice were observed and, if deemed moribund, (inactive with severe twitching and weight loss), were killed by CO2 euthanasia and the age of death was recorded. All treatments of mice were approved and carried out according to the guidelines of the Institutional Animal Care and Use Committee.
Viral delivery routes. Three treatment strategies were designed to evaluate their potential treatment efficacies: (i) combination of intracerebroventricular in both hemispheres and a single intracerebellar injection (icv-ic) treatment, (ii) iv injection using the tail vein, (iii) combination of icv-ic and iv injections (icv-ic-iv). All injections were carried out on PND2 unless mentioned otherwise. Depending on the nature of the experiment, some mice received a second iv injection on PND7 (icv-ic-iv-iv). Trypan blue was added to the viral stock at a final concentration of 0.05% (wt/vol) to facilitate visual assessment of the injections. The animals were cryo-anesthetized on ice before the injections and warmed up by hand before returning to their mothers. Both icv and ic injections were accomplished using a finely drawn (1.0 mm OD) glass micropipette (FHC, Bowdoinham, ME) attached to a 25-µl Hamilton syringe.44,47 Injections were carried out on a light box to facilitate visualizing the whole cerebral and cerebellar regions. A total of 1.5 × 109 viral particles in 2 µl were administered into each site of injection. Intraventricular injections were performed bilaterally at a 45° angle 1 mm from both the bregma line and the sagittal suture on the parietal bone. The success of injection could be determined by the appearance of blue color in the ventricles. Cerebellar administrations of the viral stock were done by perpendicular injections 2-mm deep in cerebellum using the same finely drawn glass micropipette and Hamilton syringe. The iv injections also were carried out on a light box to visualize the tail vein. Ten microliters of vector solution (7.6 × 109 viral particles) was drawn into a 30-gauge insulin syringe. The needle was inserted into the vein, and the solution containing the virus was injected manually. A correct injection was verified by noting blanching of the vein. After the injection, pups were warmed up and returned to their cage. Only the successfully injected pups were considered for further analysis. Prematurely dead mice or mice with little sign of improvement (<10% of the cases) were not included in this study.
Tissue preparation. The treated and untreated mice were anesthetized with pentobarbital sodium (100 g/g body wt) and perfused initially with 40 ml of ice-cold 1× phosphate-buffered saline (PBS) by the transcardiac route, followed by perfusion with 40-ml ice-cold 4% paraformaldehyde in PBS (PFA/PBS). The entire brain and cerebellum were removed from the skull, postfixed in fresh 4% PFA/PBS for an additional 4 hours at room temperature, and cryoprotected by soaking in 25% glucose (wt/vol) for 18–24 hours at 4 °C. Once the tissues sank to the bottom of the sucrose solution, they were embedded in Tissue-Tek OCT compound (Sakura Finetek, Torrance, CA) and frozen in a liquid nitrogen-chilled isopentane (Sigma-Aldrich, St Louis, MO) bath. The tissue blocks were stored at −80 °C until sectioning. Six-micrometer-thick coronal sections were prepared using an HM-505N Microm cryostat (Richard-Allan, Kalamazoo, MI). Recovered sections on the glass slides were processed directly for immunohistochemical staining or stored at –80 °C. Alternatively tissues from the PFA/PBS perfused mice were post fixed in 10% formalin (3.7% formaldehyde) and processed for paraffin embedding and sectioning. Two-micrometer-thick paraffin sections were prepared and stained with Luxol-fast blue/periodic acid Schiff using standard procedures and examined by light microscopy. For electron microscopy, tissue was fixed in 2% of glutaraldehyde (vol/vol) in PBS and examined at 100 kV with an H-7000 electron microscope (Hitachi, Tokyo, Japan).
Biochemical analysis. Animals were sacrificed by CO2 euthanasia at different time points after injection. The whole brain or the desired sections were homogenized in distilled water using a Polytron apparatus (Brinkmann Instruments, Westbury, NY), and the protein concentration was determined according to the method of Lowry et al.48 GALC activity was measured using [3H]galactosylceramide, according to our published method.49
Immunohistochemistry. Frozen brain, cerebellum and spinal cord sections were thawed and fixed for an extra 15 minutes with freshly made 4% PFA/PBS at room temperature, permeabilized with ethanol/acetic acid (95/5) for 10 minutes at –20 °C, and treated for 1 hour with blocking reagent from Vector Laboratories (Burlingame, CA). Affinity-purified anti-GALC antibody (prepared by Proteintech, Chicago, IL) was used to detect GALC antigen followed by visualization with a secondary anti-rabbit antibody (Alexa 594 from Molecular Probes, Eugene, OR). Cell-type-specific antibodies such as NeuN, Map2, and NF (neuronal specific), SMI91 and AMI94 (oligodendrocyte specific), and anti-glial fibrillary acidic protein (GFAP, astrocyte specific) antibodies (Chemicon, Temecula, CA) were also utilized and visualized by Alexa 488 (Molecular Probes). Tissues were incubated for 2 hours at 25 °C in the appropriate secondary antibodies. Immunostained slides were mounted with Vectashield mounting medium containing DAPI as a nuclear marker (Vector Laboratories). Although coronal sections from the entire brain were monitored, most sections studied were from the area between bregma and interaural lines.
Histochemical staining for GALC enzyme activity. Frozen sections were thawed, equilibrated with citrate/phosphate buffer (pH 4.5) for 15 minutes, then incubated in 5 mg/ml taurodeoxycholic acid and 5 mg/ml oleic acid in C/P buffer and stained with X-gal according the method described by Dolcetta et al.50 Samples were dehydrated in serial alcohols, cleared in Histoclear (Fisher Scientific, Pittsburgh, PA) and cover-slipped with Permount (Fisher Scientific) and examined under light microscopy.
PCR analysis of different organs for GALC cDNA. DNA was isolated from different tissues using the DNeasy tissue kit from Qiagen. A 283-bp fragment of mouse cDNA was amplified using a sense primer (5′-CCAAGCATTATCATGACCTG-3′) starting at cDNA location 463 (counting from “A” of the initiation codon) and an antisense primer (5′-TCTTTGCATTCCACACTGTG-3′) ending at cDNA position 745. The genomic DNA is interrupted by an intron at cDNA location 534 and would not be amplified. PCR were carried out at 95 °C denaturing for 30 seconds, 58 °C annealing, for 1 minute, and 72 °C extension for 1 minute in 30 cycles, and the PCR products were analyzed on a 2% Metaphor gel (Invitrogen).
Microscopy and analysis of micrographs. The fluorescent stains were visualized using an Olympus BX51 microscope with FITC, TRITC, and UV filters (Chroma Technology, Brattleboro, VT). The digital images were captured with a SPOT-RT camera (Diagnostic Instruments, Sterling Heights, MI). The compiled images were processed with Adobe Photoshop CS/8.0 software (Adobe Systems, San Jose, CA).
Behavioral assessment. In all behavioral testing an age-matched wild-type mouse was used as normal control and whenever, possible, an age-matched or terminal untreated affected mouse was tested for comparison. The repeated tests were performed at the same time each week and under similar conditions.
Gait analysis. A blind tunnel was made from polyvinyl chloride pipe 34 cm long, 4 cm wide, and 3 cm high. The front paws of the mice were dipped in nontoxic red food color, and back paws in blue food color. The mouse was placed in the front of the tunnel on the paper so that it could walk down the tunnel towards the light source. The piece of paper with the colored paw prints was retained for comparative analysis.
Grip test. A 25-cm long horizontal metal beam of 1 cm in diameter blocked at both ends with Styrofoam panels covered with smooth aluminum sheets (to prevent mouse grasp out of the metal beam) was affixed at a height of 20 cm on a metal cage. The cage base was covered with a 2-cm thick sponge cushion bedding to provide a soft landing for any mouse that fell off. The mouse suspended by its tail, was slowly lowered from above and was placed in the center of the metal beam. It was allowed to move along the beam and the time until a fall was recorded. After 2 minutes, any mouse that had not fallen was returned to its cage, and the time was recorded as 2 minutes. The test was repeated three times once a week. The mean of all values was used for analysis. Data points from three independent experimental series were combined for statistical analysis.
Activity monitoring. To evaluate motor balance and coordination ability in wild type, untreated, and treated mice a digital activity monitor (VersaMax; AccuScan Instruments) was used. Mice were placed in a novel open field, 20 cm × 20 cm × 45 cm chambers made of clear Plexiglas, and covered with a Plexiglas lid with holes for ventilation. Their activities were monitored for 5 minutes and data were analyzed using VersaMax software.
Statistical analysis. Statistical significance was determined using GraphPad Prism software. All data are depicted by the magnitudes of means and their related SE. Comparisons between two groups were performed using two-tailed Student t-test. A survival rate between two groups was assessed using Kaplan–Meier test.
Acknowledgments
The authors thank Dr Diane E. Merry, Associate Professor, Department of Biochemistry and Molecular Biology, Thomas Jefferson University for her scientific assistance and allowing us to use the digital activity monitor (VersaMax, AccuScan Instruments) and Dr Mohammadreza Hojat Professor, Department of Psychiatry & Human Behavior for his assistance in statistical analysis.
REFERENCES
- Wenger DA.1997. Krabbe disease (globoid cell leukodystrophy). Rosenberg, RNP, DiMauro S., and, Barchi RL.eds). The Molecular and Genetic Basis of Neurological Disease2nd ed. Butterworth–Heinemann: Boston,421–431. [Google Scholar]
- Wenger DA, Suzuki K, Suzuki Y., and, Suzuki K.2001Galactosylceramide lipidosis: Globoid cell leukodystrophy (Krabbe disease) Scriver, CR, Sly WS,, Valle, D, Childs B, Kinzler, KW., and, Vogelstein B.ed). The Metabolic and Molecular Bases of Inherited Disease8th ed. McGraw–Hill: New York,3669–3687. [Google Scholar]
- Suzuki K. Twenty five years of the “psychosine hypothesis”: a personal perspective of its history and present status. Neurochem Res. 1998;23:251–259. doi: 10.1023/a:1022436928925. [DOI] [PubMed] [Google Scholar]
- Zaka M., and, Wenger DA. Psychosine-induced apoptosis in a mouse oligodendrocyte progenitor cell line is mediated by caspase activation. Neurosci Lett. 2004;358:205–209. doi: 10.1016/j.neulet.2003.12.126. [DOI] [PubMed] [Google Scholar]
- Escolar ML, Poe MD, Provenzale JM, Richards KC, Allison J, Wood S.et al. (2005Transplantation of umbilical-cord blood in babies with infantile Krabbe's disease N Engl J Med 3522069–2081. [DOI] [PubMed] [Google Scholar]
- Shapiro EG, Lockman LA, Balthazor M., and, Krivit W. Neuropsychological outcomes of several storage diseases with and without bone marrow transplantation. J Inherit Metab Dis. 1995;18:413–429. doi: 10.1007/BF00710053. [DOI] [PubMed] [Google Scholar]
- Suzuki K., and, Suzuki K. The twitcher mouse: a model for Krabbe disease and for experimental therapies. Brain Pathol. 1995;5:249–258. doi: 10.1111/j.1750-3639.1995.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Wenger DA. Murine, canine and non-human primate models of Krabbe disease. Mol Med Today. 2000;6:449–451. doi: 10.1016/s1357-4310(00)01800-1. [DOI] [PubMed] [Google Scholar]
- Hoogerbrugge PM, Suzuki K, Suzuki K, Poorthuis BJ, Kobayashi T, Wagemaker G.et al. (1988Donor-derived cells in the central nervous system of twitcher mice after bone marrow transplantation Science 2391035–1038. [DOI] [PubMed] [Google Scholar]
- Luzi P, Rafi MA, Zaka M, Rao HZ, Curtis M, Vanier MT.et al. (2005Biochemical and pathological evaluation of long-lived mice with globoid cell leukodystrophy after bone marrow transplantation Mol Genet Metab 86150–159. [DOI] [PubMed] [Google Scholar]
- Neri M, Ricca A, di Girolamo I, Alcala'-Franco B, Cavazzin C, Orlacchio A.et al. (2011Neural stem cell gene therapy ameliorates pathology and function in a mouse model of globoid cell leukodystrophy Stem Cells 291559–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripoll CB, Flaat M, Klopf-Eiermann J, Fisher-Perkins JM, Trygg CB, Scruggs BA.et al. (2011Mesenchymal lineage stem cells have pronounced anti-inflammatory effects in the twitcher mouse model of Krabbe's disease Stem Cells 2967–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RM, Lee JP, Palacino JJ, Bower KA, Li J, Vanier MT.et al. (2006Intrinsic resistance of neural stem cells to toxic metabolites may make them well suited for cell non-autonomous disorders: evidence from a mouse model of Krabbe leukodystrophy J Neurochem 971585–1599. [DOI] [PubMed] [Google Scholar]
- Taylor RM., and, Snyder EY. Widespread engraftment of neural progenitor and stem-like cells throughout the mouse brain. Transplant Proc. 1997;29:845–847. doi: 10.1016/s0041-1345(96)00163-7. [DOI] [PubMed] [Google Scholar]
- Galbiati F, Givogri MI, Cantuti L, Rosas AL, Cao H, van Breemen R.et al. (2009Combined hematopoietic and lentiviral gene-transfer therapies in newborn Twitcher mice reveal contemporaneous neurodegeneration and demyelination in Krabbe disease J Neurosci Res 871748–1759. [DOI] [PubMed] [Google Scholar]
- Lin D, Donsante A, Macauley S, Levy B, Vogler C., and, Sands MS. Central nervous system-directed AAV2/5-mediated gene therapy synergizes with bone marrow transplantation in the murine model of globoid-cell leukodystrophy. Mol Ther. 2007;15:44–52. doi: 10.1038/sj.mt.6300026. [DOI] [PubMed] [Google Scholar]
- Lin D, Fantz CR, Levy B, Rafi MA, Vogler C, Wenger DA.et al. (2005AAV2/5 vector expressing galactocerebrosidase ameliorates CNS disease in the murine model of globoid-cell leukodystrophy more efficiently than AAV2 Mol Ther 12422–430. [DOI] [PubMed] [Google Scholar]
- Rafi MA, Zhi Rao H, Passini MA, Curtis M, Vanier MT, Zaka M.et al. (2005AAV-mediated expression of galactocerebrosidase in brain results in attenuated symptoms and extended life span in murine models of globoid cell leukodystrophy Mol Ther 11734–744. [DOI] [PubMed] [Google Scholar]
- Shen JS, Meng XL, Yokoo T, Sakurai K, Watabe K, Ohashi T.et al. (2005Widespread and highly persistent gene transfer to the CNS by retrovirus vector in utero: implication for gene therapy to Krabbe disease J Gene Med 7540–551. [DOI] [PubMed] [Google Scholar]
- Biswas S., and, LeVine SM. Substrate-reduction therapy enhances the benefits of bone marrow transplantation in young mice with globoid cell leukodystrophy. Pediatr Res. 2002;51:40–47. doi: 10.1203/00006450-200201000-00009. [DOI] [PubMed] [Google Scholar]
- Strazza M, Luddi A, Carbone M, Rafi MA, Costantino-Ceccarini E., and, Wenger DA. Significant correction of pathology in brains of twitcher mice following injection of genetically modified mouse neural progenitor cells. Mol Genet Metab. 2009;97:27–34. doi: 10.1016/j.ymgme.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Biswas S., and, LeVine SM. Substrate-reduction therapy enhances the benefits of bone marrow transplantation in young mice with globoid cell leukodystrophy. Pediatr Res. 2002;51:40–47. doi: 10.1203/00006450-200201000-00009. [DOI] [PubMed] [Google Scholar]
- Lee WC, Courtenay A, Troendle FJ, Stallings-Mann ML, Dickey CA, DeLucia MW.et al. (2005Enzyme replacement therapy results in substantial improvements in early clinical phenotype in a mouse model of globoid cell leukodystrophy FASEB J 191549–1551. [DOI] [PubMed] [Google Scholar]
- Kagitani-Shimono K, Mohri I, Fujitani Y, Suzuki K, Ozono K, Urade Y.et al. (2005Anti-inflammatory therapy by ibudilast, a phosphodiesterase inhibitor, in demyelination of twitcher, a genetic demyelination model J Neuroinflammation 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WC, Kang D, Causevic E, Herdt AR, Eckman EA., and, Eckman CB. Molecular characterization of mutations that cause globoid cell leukodystrophy and pharmacological rescue using small molecule chemical chaperones. J Neurosci. 2010;30:5489–5497. doi: 10.1523/JNEUROSCI.6383-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzi P, Abraham RM, Rafi MA, Curtis M, Hooper DC., and, Wenger DA. Effects of treatments on inflammatory and apoptotic markers in the CNS of mice with globoid cell leukodystrophy. Brain Res. 2009;1300:146–158. doi: 10.1016/j.brainres.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque S, Joussemet B, Riviere C, Marais T, Dubreil L, Douar AM.et al. (2009Intravenous administration of self-complementary AAV9 enables transgene delivery to adult motor neurons Mol Ther 171187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foust KD, Wang X, McGovern VL, Braun L, Bevan AK, Haidet AM.et al. (2010Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN Nat Biotechnol 28271–274. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gray SJ, Blake BL, Criswell HE, Nicolson SC, Samulski RJ, McCown TJ.et al. (2010Directed evolution of a novel adeno-associated virus (AAV) vector that crosses the seizure-compromised blood-brain barrier (BBB) Mol Ther 18570–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester ME, Foust KD, Kaspar RW., and, Kaspar BK. AAV as a gene transfer vector for the treatment of neurological disorders: novel treatment thoughts for ALS. Curr Gene Ther. 2009;9:428–433. doi: 10.2174/156652309789753383. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Preparation of Trojan horse liposomes (THLs) for gene transfer across the blood-brain barrier. Cold Spring Harb Protoc. 2010;2010:pdb.prot5407. doi: 10.1101/pdb.prot5407. [DOI] [PubMed] [Google Scholar]
- Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM., and, Kaspar BK. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol. 2009;27:59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C, Busuttil RW., and, Lipshutz GS. RH10 provides superior transgene expression in mice when compared with natural AAV serotypes for neonatal gene therapy. J Gene Med. 2010;12:766–778. doi: 10.1002/jgm.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Yang B, Mu X, Ahmed SS, Su Q, He R.et al. (2011Several rAAV vectors efficiently cross the blood-brain barrier and transduce neurons and astrocytes in the neonatal mouse central nervous system Mol Ther 191440–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondhi D, Hackett NR, Peterson DA, Stratton J, Baad M, Travis KM.et al. (2007Enhanced survival of the LINCL mouse following CLN2 gene transfer using the rh.10 rhesus macaque-derived adeno-associated virus vector Mol Ther 15481–491. [DOI] [PubMed] [Google Scholar]
- Hurlbut GD, Ziegler RJ, Nietupski JB, Foley JW, Woodworth LA, Meyers E.et al. (2010Preexisting immunity and low expression in primates highlight translational challenges for liver-directed AAV8-mediated gene therapy Mol Ther 181983–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Lasaro MO, Jia B, Lin SW, Haut LH, High KA.et al. (2011Capsid-specific T-cell responses to natural infections with adeno-associated viruses in humans differ from those of nonhuman primates Mol Ther 192021–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nietupski JB, Hurlbut GD, Ziegler RJ, Chu Q, Hodges BL, Ashe KM.et al. (2011Systemic administration of AAV8-a-galactosidase A induces humoral tolerance in nonhuman primates despite low hepatic expression Mol Ther 191999–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Gomero E, Bonten E, Gray JT, Allay J, Wu Y.et al. (2012Preclinical dose-finding study with a liver-tropic, recombinant AAV-2/8 vector in the mouse model of galactosialidosis Mol Ther 20267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Bell P, Lin J, Calcedo R, Tarantal AF., and, Wilson JM. AAV8-mediated hepatic gene transfer in infant rhesus monkeys (Macaca mulatta) Mol Ther. 2011;19:2012–2020. doi: 10.1038/mt.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidrauski C, Chapman R., and, Walter P. The unfolded protein response: an intracellular signalling pathway with many surprising features. Trends Cell Biol. 1998;8:245–249. doi: 10.1016/s0962-8924(98)01267-7. [DOI] [PubMed] [Google Scholar]
- Zhang K., and, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luddi A, Strazza M, Carbone M, Moretti E., and, Costantino-Ceccarini E. Galactosylceramidase deficiency causes sperm abnormalities in the mouse model of globoid cell leukodystrophy. Exp Cell Res. 2005;304:59–68. doi: 10.1016/j.yexcr.2004.10.034. [DOI] [PubMed] [Google Scholar]
- Hildinger M, Auricchio A, Gao G, Wang L, Chirmule N., and, Wilson JM. Hybrid vectors based on adeno-associated virus serotypes 2 and 5 for muscle-directed gene transfer. J Virol. 2001;75:6199–6203. doi: 10.1128/JVI.75.13.6199-6203.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W, Chirmule N, Berta SC, McCullough B, Gao G., and, Wilson JM. Gene therapy vectors based on adeno-associated virus type 1. J Virol. 1999;73:3994–4003. doi: 10.1128/jvi.73.5.3994-4003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passini MA., and, Wolfe JH. Widespread gene delivery and structure-specific patterns of expression in the brain after intraventricular injections of neonatal mice with an adeno-associated virus vector. J Virol. 2001;75:12382–12392. doi: 10.1128/JVI.75.24.12382-12392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EY, Taylor RM., and, Wolfe JH. Neural progenitor cell engraftment corrects lysosomal storage throughout the MPS VII mouse brain. Nature. 1995;374:367–370. doi: 10.1038/374367a0. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL., and, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Wenger DA., and, Williams CA.1991Hommes, FA.ed). Techniques in Diagnostic Human Biochemical Genetics Wiley–Liss: New York; 587–617. [Google Scholar]
- Dolcetta D, Perani L, Givogri MI, Galbiati F, Orlacchio A, Martino S.et al. (2004Analysis of galactocerebrosidase activity in the mouse brain by a new histological staining method J Neurosci Res 77462–464. [DOI] [PubMed] [Google Scholar]