Abstract
Mesenchymal stem/progenitor cells (MSCs) were reported to enhance the survival of cellular and organ transplants. However, their mode of action was not established. We here used a mouse model of corneal allotransplantation and demonstrated that peri-transplant intravenous (i.v.) infusion of human MSCs (hMSCs) decreased the early surgically induced inflammation and reduced the activation of antigen-presenting cells (APCs) in the cornea and draining lymph nodes (DLNs). Subsequently, immune rejection was decreased, and allograft survival was prolonged. Quantitative assays for human GAPDH revealed that <10 hMSCs out of 1 × 106 injected cells were recovered in the cornea 10 hours to 28 days after i.v. infusion. Most of hMSCs were trapped in lungs where they were activated to increase expression of the gene for a multifunctional anti-inflammatory protein tumor necrosis factor-α stimulated gene/protein 6 (TSG-6). i.v. hMSCs with a knockdown of TSG-6 did not suppress the early inflammation and failed to prolong the allograft survival. Also, i.v. infusion of recombinant TSG-6 reproduced the effects of hMSCs. Results suggest that hMSCs improve the survival of corneal allografts without engraftment and primarily by secreting TSG-6 that acts by aborting early inflammatory responses. The same mechanism may explain previous reports that MSCs decrease rejection of other organ transplants.
Introduction
Organ transplantation is the final therapeutic option in a variety of devastating diseases. However, postoperative survival is most often limited by rejection of the transplants by the immune system. Moreover, prolonged administration of immunosuppressive agents to prevent rejection can produce renal or hepatic toxicity and increase the susceptibility to malignancies or infections. One recent strategy for improving the engraftment of transplants of cells and organs is the pre- or concurrent administration of mesenchymal stem/progenitor cells (MSCs). Systemic infusion of MSCs was reported to decrease graft rejection in animal models, and thus the results have prompted a number of clinical trials.1,2,3,4 Previous studies largely attributed the improved survival of transplants to the immunomodulatory effects of MSCs.3,4,5,6 Most of the data, however, were based on in vitro experiments that may or may not reflect actions of MSCs in vivo.
In order to define how MSCs improve the engraftment of organ transplants, we here adopted a mouse model of allogeneic corneal transplantation, a model in which the temporal sequence of events from the introduction of the alloantigen to immune rejection is distinct and well-established.7,8,9 We demonstrated that intravenous (i.v.) administration of human MSCs (hMSCs) at the time of grafting decreased the immune response and prolonged the survival of corneal allografts primarily by suppressing the surgery-induced inflammation in the early postoperative period. Suppression of inflammation subsequently inhibited the afferent loop of the alloimmune response by decreasing the activation of dendritic cells (DCs) in the cornea and draining lymph nodes (DLNs). Of special interest was the finding that the beneficial effects of hMSCs were observed without engraftment of the cells in the cornea, but rather were dependent on hMSCs being trapped in the lungs after i.v. infusion with subsequent activation to express the gene for tumor necrosis factor-α stimulated gene/protein 6 (TSG-6), a multifunctional protein10,11 that acts in part by aborting the early phase of inflammation partially through the modulation of nuclear factor (NF)-κB signaling in resident macrophages.12,13
Results
i.v. hMSCs prolonged the survival of corneal allografts
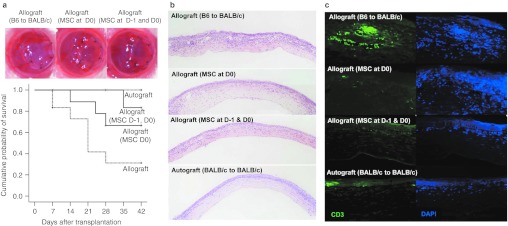
To determine whether i.v. hMSCs prolong the survival of corneal allografts, we performed orthotropic corneal allotransplantation using C57BL/6 mice (H-2b) as donors and BALB/c(H-2d) as recipients. Recipient mice received 1 × 106 hMSCs i.v. either once immediately after surgery (day 0) or twice at 1 day before surgery (day –1) and again immediately after surgery (day 0). Hank's balanced salt solution (HBSS) was injected i.v. as a vehicle control. Syngeneic corneal autografts (BALB/c-to-BALB/c) were performed to serve as negative controls. For the follow-up period of 42 days, 7 of 12 B6 corneal grafts in BALB/c mice (allografts) were rejected within 28 days with a mean survival time of 21.3 days, whereas all of the BALB/c corneal grafts in BALB/c mice (autografts, n = 12) survived (Figure 1a). i.v. hMSCs significantly prolonged the survival of corneal allografts. Eleven out of 12 allografts remained free of rejection in mice that received two injections of i.v. hMSCs (P = 0.006 versus allografts in the HBSS group), and 9 of 12 allografts survived in mice that received a single injection of hMSCs (P = 0.047 versus allografts in the HBSS group).
Figure 1.
Intravenous (i.v.) human mesenchymal stem cells (hMSCs) prolonged the survival and prevented the immune rejection of B6 corneal grafts in BALB/c mice. (a) Representative photographs of the cornea 14 days after transplantation and the Kaplan–Meier survival curve of corneal grafts. The graft survival was significantly prolonged by i.v. hMSCs. Seven out of 12 B6 corneal grafts in BALB/c mice (allografts) were rejected within 28 days (median survival time 23.1 days). In contrast, 11 out of 12 allografts survived in mice that received i.v. hMSCs both one day before surgery (day 1) and immediately after surgery (day 0), and 9 of 12 allografts survived in mice that received a single injection of hMSCs at day 0 (MSC at day 1 and 0 vs. Hank's balanced salt solution (HBSS), P = 0.006; MSC at day 0 vs. HBSS, P = 0.047; generalized Wilcoxon test). n = 12 in each group.(b) Hematoxylin–eosin staining of corneal grafts at day 28 showed heavy infiltration of inflammatory cells in the rejected allografts of control animals and much less inflammatory cell infiltration in the allografts that received hMSCs. (c) Immunohistochemical staining showed that many CD3+ T cells infiltrated the allografts of control animals, whereas there were rare T cells in the grafts that received hMSCs.
We performed histological studies on the grafts at day 28. In the rejected allografts of HBSS-injected animals, hematoxylin–eosin staining of sections showed extensive infiltration of inflammatory cells (Figure 1b). In contrast, inflammatory infiltrates were markedly decreased in the allografts from mice treated with hMSCs. Similar results were observed by immunostaining of the sections for CD3+ T cells (Figure 1c). There was extensive infiltration of CD3+ T cells in the rejected grafts from vehicle control animals and minimal infiltration of CD3+ T cells in the allografts from mice that received hMSCs.
Therefore, the data demonstrated that peri-transplant injection of i.v. hMSCs prolonged the survival of corneal allografts and prevented rejection. Two injections (day –1 and day 0) were more effective than a single injection (day 0).
i.v. hMSCs suppressed early inflammation and late rejection of corneal allografts
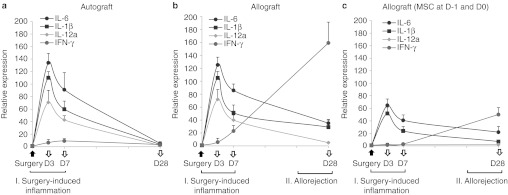
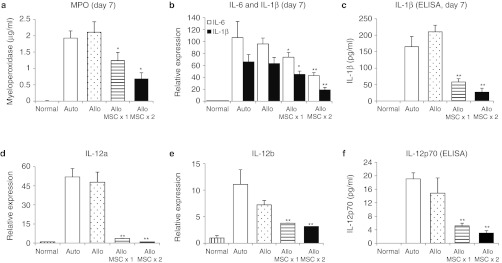
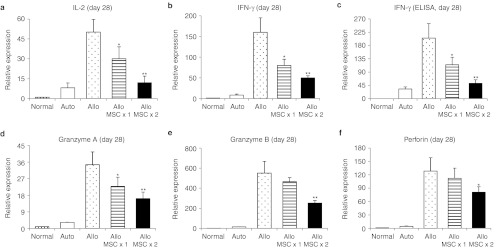
In order to examine the effects of hMSCs, we analyzed corneal grafts for the time course of expression of inflammation- and immune-related molecules. Since the rejection of corneal allografts occurred by day 28 and the surviving grafts remained free of rejection after day 28 (Figure 1a), we analyzed corneal grafts at day 28 for the immune rejection and the grafts at days 3 and 7 for the early surgery-induced inflammation. We found that both autografts and allografts demonstrated the same early increases at days 3 and 7 of the inflammatory cytokines interleukin (IL)-6, IL-1β, and IL-12 as well as myeloperoxidase as a semiquantitative measure of neutrophil infiltration(Figures 2a,b and 3).14 This finding indicates that the inflammation was induced by surgery and the similar amount of damage was applied to tissues by surgery between auto- and allografts. In contrast, there were marked differences between autografts and allografts in the late immune response. In allografts but not in autografts, there was a gradual increase up to day 28 in the transcript levels of T cell-derived cytokines (IL-2 and interferon (IFN)-γ) and effector molecules implicated in the allograft rejection (granzyme A, granzyme B, and perforin) (Figures 2a,b and 4).15 i.v. infusion of hMSCs decreased both early inflammatory phase and late immune response (Figure 2c). At days 3 and 7, the levels of inflammatory cytokines were markedly reduced in allografts from mice that received hMSCs. The levels of the transcripts for IL-6 and IL-1β were reduced by about half, and the transcript for IL-12a was reduced to baseline levels. Similar decreases were seen at day 7 in levels of myeloperoxidase and ELISA for IL-1β and IL-12 (Figure 3). The decrease in the inflammatory phase produced by hMSCs was accompanied by a decrease in the immune response. At 28 days, there was a marked decrease in the levels of transcripts for IL-2, IFN-γ, granzyme A, granzyme B, and perforin in the allografts from mice that received hMSCs as well as the protein level of IFN-γ (Figures 2c and 4). Also, flow cytometry demonstrated that the number of CD44+ CD69+ cells as markers for activated CD4 T cells was decreased in DLNs in mice treated with hMSCs (Supplementary Figure S1).
Figure 2.
Time course of gene expression levels in the cornea after transplantation surgery. Real-time reverse transcription (RT)-PCR showed that the levels of proinflammatory cytokines (interleukin (IL)-6, IL-1β, and IL-12a) were upregulated at similar levels in (a) autografts and (b) allografts at days 3 and 7 after transplantation, which defines the early phase of surgery-induced inflammation. The levels of T cell-derived cytokines (IFN-γ) were increased up to day 28 in allografts, but not in autografts, which indicates the late phase of the allogeneic immune rejection. In allografts that received intravenous (i.v.) human mesenchymal stem cells (hMSCs) (c), levels of IL-6, IL-1β, and IL-12a were significantly lower at days 3 and 7, and levels of IFN-γ were markedly decreased at day 28 compared to autografts or allografts that did not receive hMSCs. n = 5 at each time-point in all experimental groups.
Figure 3.
Intravenous (i.v.) human mesenchymal stem cells (hMSCs) decreased the early inflammatory response in corneal allografts. (a) The amount of myeloperoxidase as a measure of neutrophil infiltration was significantly decreased by hMSCs at day 7 after transplantation. (b–f) Also, the levels of proinflammatory cytokines, IL-6, IL-1β, and IL-12 were significantly decreased by i.v. hMSCs at day 7. Results indicate that inflammatory responses in the early postoperative period after transplantation surgery were suppressed by i.v. hMSCs. Auto: autografts, Allo: allografts, Allo MSCx1: allografts that received hMSCs once immediately after transplantation, Allo MSCx2:allografts that received hMSCs on the day before transplantation and immediately after transplantation. n = 5 in each group. *P < 0.05; **P < 0.01.
Figure 4.
Intravenous (i.v.) human mesenchymal stem cells (hMSCs) suppressed the late T cell-mediated immune response in corneal allografts. (a,b) The transcript levels of activated CD4 T-cell cytokines (IL-2 and IFN-γ) and protein level of (c) IFN-γ were significantly decreased in the allografts that received i.v. hMSCs at day 28 after surgery, compared to the grafts without i.v. hMSCs. (d–f) Also, the transcript levels of CD8 T-cell effector molecules (granzyme A, granzyme B, and perforin) were significantly decreased by i.v. hMSCs. Auto: autografts, Allo: allografts, Allo MSCx1: allografts that received hMSCs once immediately after transplantation, Allo MSCx2:allografts that received hMSCs on the day before transplantation and immediately after transplantation. n = 5 in each group. *P < 0.05; **P < 0.01.
Therefore, the results indicated that hMSCs suppressed the surgery-induced inflammation in the early postoperative period and, apparently as a result, decreased the subsequent immune rejection of corneal allografts.
i.v. hMSCs decreased activation of APCs
Previous studies demonstrated that the principal antigen-presenting cells (APCs) of the cornea, Langerhans cells, reside in basal epithelium in the limbal area of the cornea.7 Also, CD11b+CD11c+ cells having dendritic morphology are present in the anterior stroma, and a population of CD11b+CD11c– cells having macrophage morphology is present in the posterior stroma.16 In response to inflammatory insults including transplantation surgery, APCs undergo maturation by overexpressing major histocompatibility complex (MHC) class II.17,18Activated APCs, most of which are of host origin, take up graft-derived antigens in the cornea and migrate to DLNs, where they present antigens to host T cells causing the T cell-mediated immune rejection.7,19 Thus, we next examined whether the decrease in inflammation by i.v. hMSCs might lead to a reduction in the activation of APCs in the cornea and DLNs. First, we examined the whole-mounted epithelial sheets of the cornea for host-derived MHC class II (murine Iad) at one week after transplantation. We selected the 1-week time-point, because it is the time at which allosensitization takes place and allorejection is not yet initiated.7 Therefore, it allowed us to examine the afferent sensitization arm of alloimmunity. Immunostaining showed that the number of MHC class II+ cells in the cornea was markedly lower in the allografts from mice treated with hMSCs compared to the autografts or allografts without hMSCs (Figure 5). Next, we analyzed subsets of MHC class II+ cell population in DLNs (cervical LNs ipsilateral to the transplanted eye). Flow cytometry showed that the proportions of DCs, both MHC II+CD11b+CD11c+ cells and MHC II+CD11b–CD11c+ cells, were significantly decreased in mice treated with hMSCs (Figure 6). Two injections of hMSCs (day –1 and day 0) were more effective than a single injection (day 0). In addition to DCs, the proportion of MHC II+CD11b–CD11c+ cells representing macrophages was significantly decreased in the group treated with i.v. hMSCs. The findings of similar increases in APCs in the cornea and DLNs between auto- and allografts suggested that a transplant procedure and surgically induced inflammation contributed to activation of APCs, which is consistent with the data reported previously.18 Treatment with i.v. hMSCs was accompanied by reduced activation of APCs in the cornea and DLNs as well as reduced inflammation in the grafts. The data, therefore, indicated that the afferent limb of the alloimmune response was inhibited by hMSCs.
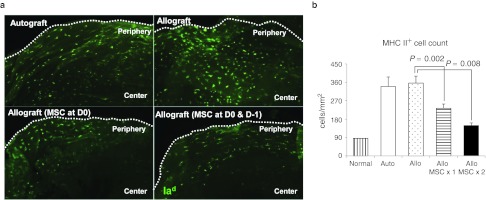
Figure 5.
Intravenous (i.v.) human mesenchymal stem cells (hMSCs) decreased the number of MHC class II+cells in the cornea 1 week following transplantation. Immunostaining for (a) murine Iad+ and enumeration of MHC class II+cells in the whole-mounted epithelial sheets of the cornea (b) showed that the number of MHC class II+cells indicating Langerhans cells was significantly increased both in autografts and allografts in response to transplantation surgery. However, the number of MHC class II+cells was significantly decreased in the allografts by i.v. hMSCs. n = 3 in each group. Auto: autografts, Allo: allografts, Allo MSCx1: allografts that received hMSCs once immediately after transplantation, Allo MSCx2:allografts that received hMSCs on the day before transplantation and immediately after transplantation.
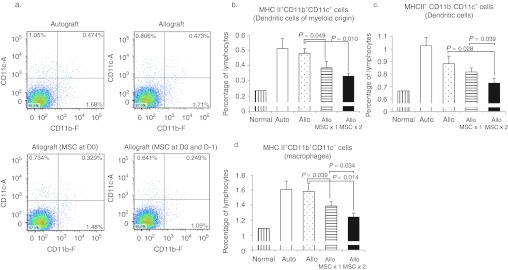
Figure 6.
Intravenous (i.v.) human mesenchymal stem cells (hMSCs) decreased the number of activated antigen-presenting cells in ipsilateral cervical lymph nodes 1 week following transplantation. (a) Flow cytometry showed that the proportions of dendritic cells (DCs), both MHC II+CD11b+CD11c+ cells (b) and MHC II+CD11b−CD11c+ cells (c),were significantly decreased in mice treated with hMSCs. In addition, the proportion of MHC II+CD11b+CD11c−cells representing macrophages was significantly decreased by i.v. (d) hMSCs. n = 4 in each group. Auto: autografts, Allo: allografts, Allo MSCx1: allografts that received hMSCs once immediately after transplantation, Allo MSCx2:allografts that received hMSCs on the day before transplantation and immediately after transplantation.
i.v. administered hMSCs did not engraft in the corneal allografts
Previous reports showed that the vast majority of hMSCs infused i.v. in mice were trapped in lungs and disappeared with a half-life of ~24 hours without long-term engraftment into injured tissues such as the cornea or heart.20,21 To determine whether hMSCs engrafted in the transplanted cornea after i.v. injection, we carried out quantitative reverse transcription (RT)-PCR assays for human-specific GAPDH in the corneas from mice that received two i.v. infusions of 1 × 106 hMSCs at day –1 and day 0 of corneal transplantation (Supplementary Figure S2 and Table S1). Results demonstrated that <10 hMSCs were present in corneas 10 hours to 28 days after the transplants. Therefore, the beneficial effects of the hMSCs were not explained by the i.v. administered cells engrafting in the cornea.
i.v. hMSCs trapped in lungs were activated to express the anti-inflammatory gene/protein TSG-6
Since the majority of i.v. hMSCs were trapped in lungs21 and did not engraft in the cornea (Supplementary Figure S2 and Table S1), we next examined the hypothesis that the effects of hMSCs on corneal grafts were mediated by trophic factors produced from the cells trapped in lungs. We used human-specific quantitative RT-PCR assays to screen the lungs 10 hours after corneal allotransplantation in mice that received i.v. hMSCs twice at day –1 and day 0. Data revealed that ~10% of injected cells were present in lungs (Supplementary Figure S3). We assayed for the expression of immunomodulatory and anti-inflammatory molecules that have been previously shown to be secreted by MSCs: COX2, NOS2, IDO, CCL2, TGF-β, TSG-6, STC-1, and PTX3.3,22 We found that the most highly upregulated human transcript was for the anti-inflammatory protein TSG-6 (113.8-fold) (Supplementary Figure S4).
i.v. hMSCs with TSG-6 siRNA knockdown did not either reduce the early inflammation or prolong allograft survival
To explore the role of TSG-6 in preventing graft rejection, we knocked down the expression of TSG-6 in hMSCs by transient transfection with siRNA and injected the cells into mice with corneal allografts twice at day –1 and day 0 of surgery. Three out of six allografts were rejected in mice that received a two-time injection of hMSCs with TSG-6 knockdown, whereas all (6/6) of the allografts remained free of rejection at 28 days in mice that received a two-time injection of hMSCs with scrambled siRNA control (P = 0.047; generalized Wilcoxon test) (Figure 7a). Additionally, hMSCs with TSG-6 knockdown were not effective in suppressing corneal inflammation at day 7 (Figure 7b). Also, levels of transcripts for activated T cell-derived cytokines and effector enzymes in corneal grafts were not suppressed by hMSCs with TSG-6 knockdown, whereas hMSCs with scrambled siRNA significantly decreased the levels of transcripts for T-cell cytokines and enzymes (Figure 7c-h).
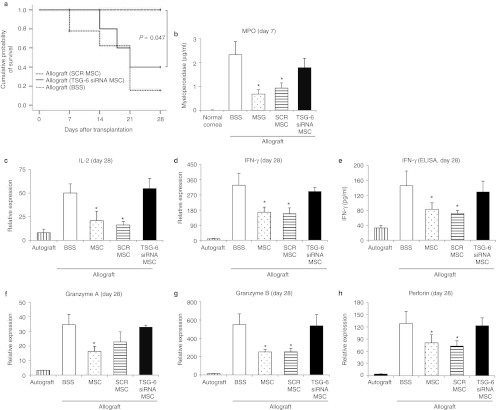
Figure 7.
intravenous (i.v.) human mesenchymal stem cells (hMSCs) with TSG-6 siRNA knockdown did not suppress the early surgery-induced inflammation and did not prolong the survival of corneal allografts. (a) The Kaplan–Meier survival curve of B6 corneal grafts in BALB/c mice. Three out of six allografts were rejected in mice that received a two-time injection of hMSCs with TSG-6 knockdown (TSG-6 siRNA MSC), whereas all allografts survived in mice that received a two-time injection of hMSCs with scrambled siRNA (SCR MSC). (b) The myeloperoxidase (MPO) amount as a measure of neutrophil infiltration was not significantly decreased by hMSCs with TSG-6 knockdown at day 7 after grafting. n = 3 in each group. The levels of activated T cell-derived cytokines (c–e) and effector enzymes (f–h) were not decreased in corneal grafts of mice treated with TSG-6 knockdown hMSCs, whereas hMSCs with scrambled siRNA significantly decreased the levels of T-cell cytokines and enzymes in corneal grafts. n = 3 in each group. Auto: autografts, Allo: allografts, Allo MSCx1: allografts that received hMSCs once immediately after transplantation, Allo MSCx2:allografts that received hMSCs on the day before transplantation and immediately after transplantation. n = 6 in each group. *P < 0.05; **P < 0.01.
i.v. injection of recombinant TSG-6 reduced early inflammation of the allografts and late rejection of corneal allografts
Next, we tested the hypothesis that systemically administered rhTSG-6 could reproduce the effects of i.v. hMSCs by reducing the surgery-induced inflammation in corneal allografts. We administered 35 µg of rhTSG-6 in 100 µl phosphate-buffered saline (PBS) by tail vein injection immediately after corneal allotransplantation. The survival of corneal allografts was significantly prolonged in mice treated with rhTSG-6, compared to PBS-injected controls (the mean survival time: 25.6 ± 1.5 days in the TSG-6 treated grafts and 18.6 ± 3.1 days in the PBS-treated grafts; P = 0.042; generalized Wilcoxon test) (Figure 8a). The expression of MPO and proinflammatory cytokines (IL-6, IL-1β, IL-12a, and IL-12b) in the cornea at day 7 was significantly decreased following rhTSG-6 injection (Figure 8b–f). Also, levels of transcripts for T cell-related cytokines, IL-2, IFN-γ, granzyme B, and perforin, were significantly decreased in the allografts from mice treated with TSG-6 at day 28 (Figure 8g–j).
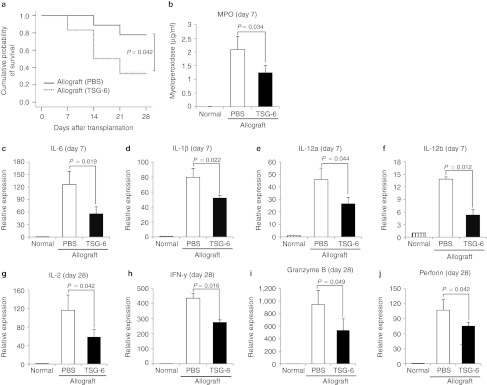
Figure 8.
Intravenous (i.v.) injection of recombinant TSG-6 suppressed the early surgery-induced inflammation and the late immune rejection of the corneal allografts. (a) The Kaplan–Meier survival curve of B6 corneal grafts in BALB/c mice. Six out of nine allografts survived in mice that received a single injection of rhTSG-6, whereas two out of nine allografts survived in mice that received phosphate-buffered saline (PBS). (b–f) The myeloperoxidase (MPO) amount and the levels of transcripts for proinflammatory cytokines were significantly decreased in the corneal allografts at day 7 by i.v. recombinant TSG-6 (35 µg) injected immediately after surgery. n = 3 in each group. (g–j) The levels of transcripts for T cell-related cytokines were also decreased by i.v. recombinant TSG-6 at day 28. n = 6 in each group.
Discussion
As summarized in Supplementary Figure S5, the results demonstrated that i.v. hMSCs increased the survival of the allografts without long-term engraftment by aborting the early inflammatory response and by decreasing activation of APCs in corneas after transplantation surgery. Similar results were obtained with i.v. recombinant TSG-6.
Our data are consistent with the current paradigm that hMSCs can produce therapeutic benefits without engraftment into injured tissues and primarily by upregulating the genes that modulate excessive inflammatory and immune reactions.23,24 In the present experiment, the multifunctional anti-inflammatory protein TSG-610,11 accounted for beneficial effects of the hMSCs that were infused i.v.. Since proinflammatory cytokines, including IL-1, play a critical role in recruitment, activation, and migration of APCs such as Langerhans cells,17,18 suppression of inflammation early after grafting by hMSCs might contribute to a prolonged survival of corneal allografts through inhibition of the afferent loop of the immune response.
In addition to suppressing inflammation, MSCs may also have other effects on the immune system. A large number of in vitro and in vivo data demonstrate that MSCs can be immunosuppressive through their interaction with a broad range of immune cells (T and B cells, regulatory T cells, NK cells, DCs, macrophages, and neutrophils) and by secreting a number of molecules such as IDO, PGE2, nitric oxide, CCL2, TGF-β, TSG-6, IL-10, or HLA-G.24,25,26,27,28 This considerable diversity and discrepancy in experimental findings for the immune-modulatory mechanisms of MSCs probably reflects their remarkable ability to respond to the microenvironments of injured tissues. In the present experiment, we injected hMSCs i.v. 1 day before and at the time of transplantation. Although the cells rapidly disappeared from the system after injection,21 the MSCs significantly decreased the early surgery-induced inflammation by upregulating the anti-inflammatory molecule TSG-6. Notably, in our present experimental setting, the expression of immunomodulatory and anti-inflammatory molecules that have been previously shown to be secreted by MSCs (COX2, NOS2, IDO, CCL2, TGF-β, STC-1, and PTX3) was not upregulated in hMSCs at the transcriptional level. However, we cannot rule out the possibility that consistent expression of molecules other than TSG-6 at protein levels might contribute to the action of hMSCs observed in the present study. Also, administration of hMSCs may have preconditioned the complex systems for inflammatory and immune responses so that the effects were apparent after most of hMSCs were no longer detected.
One of the critical observations here was that the hMSCs were more effective if they were infused i.v. both the day before surgery and immediately after the surgery than if they were infused only immediately after the surgery. The result may reflect the fact that hMSCs do not express therapeutic proteins such as TSG-6 in culture unless activated by proinflammatory cytokines such as tumor necrosis factor-α or IFN-γ, and they are not activated to express TSG-6 until about 10 hours after they are trapped in the lungs following i.v. infusion.21 Therefore, the preoperative administration of hMSCs might be more effective to reduce the surgery-induced inflammation as shown in this study.
We used a mouse model in the present study since corneal allotransplantation is the most well-studied in mice and thus the temporal sequence of events from the introduction of the alloantigen to immune rejection is well-established in this model.7,8,9 However, the surgical procedure of transplanting corneas in mouse eyes might have induced more severe tissue damage and surgically induced inflammation compared to transplantation in humans. Further studies in larger animal models will be beneficial.
The results may well explain the beneficial effects of MSCs observed in various animal models of organ transplantation such as heart, lung, skin, and islets.28,29,30,31,32 In addition, the data may extend upon previous studies that mostly focused on examining immune tolerance as a mechanism for MSCs to improve transplant survival. Early inflammatory responses may also be inviting targets for therapy. The results presented here add another rationale for using MSCs as a primary or secondary therapy to decrease the need for pharmacological immunosuppression in patients with transplants. Moreover, since i.v. administration of recombinant TSG-6 reproduced many of the beneficial effects of the hMSCs, the results raise the possibility that therapy with the protein may be more practical than therapy with the cells.
In summary, our results suggest that MSCs prolonged the survival of corneal allografts by suppressing the surgery-induced inflammation early after transplantation. The action of MSCs was exerted without significant engraftment of the cells in the cornea and primarily by secreting trophic factors including the anti-inflammatory molecule TSG-6. The observations may account for the favorable effects of MSCs seen previously in models of solid organ and cellular transplantation. Moreover, the data provide a basis for using either MSCs or TSG-6 to improve the survival of transplants of the cornea and possibly other organs.
Materials and Methods
Cell preparations. Vials of frozen passage one hMSCs were obtained from the Center for the Preparation and Distribution of Adult Stem Cells (http://medicine.tamhsc.edu/irm/msc-distribution.html) that supplies standardized preparations of MSCs enriched for early progenitor cells to over 300 laboratories under the auspices of an NIH/NCRR grant (P40 RR 17447-06). All of the experiments were performed with hMSCs from one donor. The cells consistently differentiated into three lineages in culture, were negative for hematopoietic markers (CD34, CD36, CD117, and CD45), and were positive for mesenchymal markers CD29 (95%), CD44 (>93%), CD49c (99%), CD49f (>70%), CD59 (>99%), CD90 (>99%), CD105 (>99%), and CD166 (>99%). Following culture at high density for 24 hours to recover viable cells, hMSCs were plated at low density (100 cells/cm2), incubated in complete culture medium with 16% fetal bovine serum for 8 days until 70% confluence was reached, and harvested with 0.25% trypsin/1 mmol/l EDTA at 37 °C for 2 minutes. The trypsin was thereafter inactivated by adding the complete culture medium to the cells, and the cells were washed with PBS by centrifugation at 1,200 r.p.m. for 5 minutes. The cells were frozen in α-MEM with 30% fetal bovine serum and 5% DMSO at a concentration of 1 × 106 cells/ml. Passage two cells were used for all experiments. Following lifting the cells before injection, a final wash was performed using HBSS (BioWhittaker, Walkersville, MD). After washing by centrifugation, the cells were suspended in HBSS at a concentration of 10,000 cells/µl for injection.
For siRNA experiments, hMSCs were transfected with siRNA for TSG-6 (sc-39819; Santa Cruz Biotechnology, Santa Cruz, CA) or scrambled siRNA (Stealth RNAi Negative Control; Invitrogen, Carlsbad, CA) with a commercial kit (Lipofectamine RNAiMAX reagent; Invitrogen). To confirm successful knockdown of TSG-6 expression, RNA was extracted from aliquots of the cells (RNeasy Mini kit; Qiagen, Valencia, CA) and assayed for TSG-6 by real-time RT-PCR. The knockdown efficiency of TSG-6 in hMSCs was 82–85% from 10 to 24 hours after the start of transfection (Supplementary Figure S6). The same cells used for assaying the knockdown efficiency were injected into mice for experiments. The cells were prepared for injection 10 hours after transfection with TSG-6-siRNA or scrambled siRNA.
Animal model of corneal transplantation. The experimental protocols were approved by the Institutional Animal Care and Use Committee of Texas A&M Health Science Center and Seoul National University Hospital Biomedical Research Institute. Eight-week-old female B6 mice (C57BL/6J, H-2b; Charles River Laboratories International, Wilmington, MA) were used as corneal donors and BALB/c mice (BALB/cAnNCrl, H-2d; Charles River Laboratories International) served as corneal transplant recipients.
Central 2-mm diameter corneal grafts were excised from donor corneas of B6 mice using a 2.0 mm trephine (Katena Products, Denville, NJ).The recipient corneal graft bed was prepared by removing a central 1.5-mm diameter button in recipient corneas of BALB/c host mice with a 1.5-mm trephine (Katena Products). The prepared donor corneal grafts were placed in the recipient bed and secured with eight interrupted 11–0 nylon sutures. Syngeneic autografts (BALB/c-to-BALB/c) served as experimental controls and were performed in the same fashion. The lids were closed with an 8-0 nylon tarsorrhaphy which was maintained (except for clinical evaluation) until graft rejection developed. All grafts were evaluated three times weekly for 6 weeks. Graft rejection was defined as a complete loss of graft transparency (i.e., the pupil margin and iris structure are not visible through the graft). Recipient mice received 1 × 106 hMSCs in 100 µl HBSS via tail vein either once (immediately after surgery) or twice (1 day before surgery and immediately after surgery). HBSS was injected i.v. in the control group. For i.v. TSG-6 experiments, each mouse received 35 µg of recombinant human (rh) TSG-6 (R&D Systems, Minneapolis, MN) in 100 µl PBS or PBS 100 µl via tail vein.
Histopathology. For tissue extraction, following sacrifice of the mice, the cornea was excised and fixed in 10% paraformaldehyde. The cornea was cut into 4 µm sections and stained with hematoxylin–eosin or subjected to immunohistochemistry. The formalin-fixed corneal sections were deparaffinized with ethanol and antigen was retrieved using a steamer in epitope retrieval solution (IHC WORLD, Woodstock, MD). Primary antibodies used were as follows: rabbit polyclonal antibody to CD3 (ab5690; Abcam, Cambridge, MA), rat monoclonal antibody to F4/80 (ab6640, Abcam), rabbit polyclonal antibody to iNOS (ab15323; Abcam), mouse monoclonal antibody to MRC (ab8918; Abcam), and mouse monoclonal antibody to MHC class II I Ad (ab64531; Abcam). A DAPI solution (VECTASHIELD Mounting Medium; Burlingame, CA) was used for counterstaining.
Real-time RT-PCR assays of cornea and lungs. For RNA extraction, the cornea or lung was minced into small pieces, lysed in RNA isolation reagent (RNA Bee; Tel-Test, Friendswood, TX), and homogenized using a motor-driven homogenizer. Total RNA was then extracted using RNeasy Mini kit (Qiagen) and used to synthesize double-stranded cDNA by reverse transcription (SuperScript III; Invitrogen). Real-time amplification was performed using TaqMan Universal PCR Master Mix (Applied Biosystems, Carlsbad, CA). An 18s rRNA probe (Taqman Gene Expression Assays ID, Hs03003631_g1) was used for normalization of gene expression. For all the PCR probe sets, Taqman Gene Expression Assay kits were purchased from Applied Biosystems. The assays were performed in triple technical replicates for each biological sample.
Real-time RT-PCR standard curve for hGAPDH. A standard curve was generated by adding serial dilutions of hMSCs to mouse tissue as previously described.20,21 Briefly, 10–100,000 hMSCs were added to a mouse cornea. Following RNA extraction (RNeasy Mini kit; Qiagen), cDNA was generated by reverse transcription (SuperScript III; Invitrogen) using 1 µg total RNA. A human-specific GAPDH (hGAPDH) primer and probe set (TaqMan Gene Expression Assays ID, GAPDH HS99999905_05) was used. The values were normalized to total eukaryotic 18s rRNA. The standard curve was made based on hGAPDH expression from a known number of hMSCs added to one mouse cornea.
ELISAs. For protein extraction, the cornea was minced into small pieces and lysed in tissue extraction reagent (Invitrogen) containing protease inhibitor coctail (Roche, Indianapolis, IN). The samples were sonicated on ice (Ultrasonic Processor; Cole Parmer Instruments, Vernon Hills, IL). After centrifugation at 12,000 r.p.m. at 4 °C for 20 minutes, the supernatant was collected and assayed by ELISA for IL-1β, IFN-γ, and IL-12p70 (Mouse Duoset kit; R&D Systems), CCR7 (USCN; Life Science, Missouri City, TX), and MPO (HyCult Biotech, Plymouth Meeting, PA).
Flow cytometry. DLNs were harvested from transplanted animals at days 7 and 28 after surgery. Each sample from an individual animal was separately prepared and anlayzed. No pooling of lymph node cells between animals was done. DLNs were placed and miced between the frosted ends of two glass slidesin RPMI media containing 10% fetal bovine serum and 1% penicillin–streptomycin. Cell suspensions were collected, and incubated for 30 minutes at 4 °C with fluorescein-conjugated anti-mouse antibodies. The primary antibodies used were as follows: I-Ad-PE, CD11b-FITC, CD11c-allophycocyanin, CD3-FITC, CD4-PE, and CD8-allophycocyanin (eBioscience, San Diego, CA). Three color phenotypic analyses were performed using a FACSCanto flow cytometer (BD BioSciences, Mountain View, CA). A total of 20,000 events from each sample were collected. The gate was set on either I-Ad+ or CD3+ cell population, and further analysis of surface markers was done within this gate. Data were analyzed using Flowjo program (Tree Star, Ashland, OR).
Statistical analysis. Survival analysis was performed using SPSS software (SPSS 12.0, Chicago, IL). The Kaplan–Meier method was used to evaluate the overall cumulative probability of graft survival, and the life-table method was used to estimate the median time to graft rejection. The graft survival between the groups was compared using Breslow (generalized Wilcoxon) test. Comparisons of parameters other than graft survival were made among the groups using one-way ANOVA using SPSS software. Differences were considered significant at P < 0.05.
SUPPLEMENTARY MATERIAL Figure S1. i.v. hMSCs decreased the number of activated T cells in ipsilateral cervical lymph nodes 4 week following transplantation. Figure S2. The quantitative assay for human mRNA for GAPDH in the cornea after i.v. administration of hMSCs. Figure S3. The quantitative assay for human mRNA for GAPDH in the lung after i.v. administration of hMSCs. Figure S4. Real-time RT-PCR analysis for human-specific transcripts of the anti-inflammatory and immunoregulatory molecules. Figure S5. Graphic summary of the effects of hMSCs on corneal allografts. Figure S6. The knockdown efficiency of TSG-6 in hMSCs was ~82% by real-time RT-PCR 10 hours after transfection with TSG-6-siRNA or scrambled siRNA which was the same time-point when TSG-6-siRNA MSCs were injected into mice. Table S1. The standard curve for the number of human mesenchymal stem cells (hMSCs) in one mouse cornea based on the expression of human-specific GAPDH (hGAPDH) relative to total eukaryotic 18s rRNA.
Acknowledgments
This study was supported in part by NIH grant R21EY020962 and in part by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A111609). We gratefully acknowledge support from Laura Quinlivan for assistance with animal experiments. D.J.P. is a member of the scientific advisory board of Temple Therapeutics LLC. The other authors declared no conflict of interest as described by the American Journal of Transplantation.
Supplementary Material
i.v. hMSCs decreased the number of activated T cells in ipsilateral cervical lymph nodes 4 week following transplantation.
. The quantitative assay for human mRNA for GAPDH in the cornea after i.v. administration of hMSCs.
The quantitative assay for human mRNA for GAPDH in the lung after i.v. administration of hMSCs.
Real-time RT-PCR analysis for human-specific transcripts of the anti-inflammatory and immunoregulatory molecules.
Graphic summary of the effects of hMSCs on corneal allografts.
The knockdown efficiency of TSG-6 in hMSCs was ~82% by real-time RT-PCR 10 hours after transfection with TSG-6-siRNA or scrambled siRNA which was the same time-point when TSG-6-siRNA MSCs were injected into mice.
The standard curve for the number of human mesenchymal stem cells (hMSCs) in one mouse cornea based on the expression of human-specific GAPDH (hGAPDH) relative to total eukaryotic 18s rRNA.
REFERENCES
- Hoogduijn MJ, Popp FC, Grohnert A, Crop MJ, van Rhijn M, Rowshani AT, MISOT Study Group et al. Advancement of mesenchymal stem cell therapy in solid organ transplantation (MISOT) Transplantation. 2010;90:124–126. doi: 10.1097/TP.0b013e3181ea4240. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Developmental Committee of the European Group for Blood and Marrow Transplantation et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- English K, French A., and, Wood KJ. Mesenchymal stromal cells: facilitators of successful transplantation. Cell Stem Cell. 2010;7:431–442. doi: 10.1016/j.stem.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Popp FC, Eggenhofer E, Renner P, Geissler EK, Piso P, Schlitt HJ.et al. (2009Mesenchymal stem cells can affect solid organ allograft survival Transplantation 879 SupplS57–S62. [DOI] [PubMed] [Google Scholar]
- Singer NG., and, Caplan AI. Mesenchymal stem cells: mechanisms of inflammation. Annu Rev Pathol. 2011;6:457–478. doi: 10.1146/annurev-pathol-011110-130230. [DOI] [PubMed] [Google Scholar]
- Uccelli A, Moretta L., and, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- Forrester JV, Xu H, Kuffová L, Dick AD., and, McMenamin PG. Dendritic cell physiology and function in the eye. Immunol Rev. 2010;234:282–304. doi: 10.1111/j.0105-2896.2009.00873.x. [DOI] [PubMed] [Google Scholar]
- Catron, DM, Rusch, LK, Hataye, J, Itano, AA., and, Jenkins, MK. CD4+ T cells that enter thedraining lymph nodes after antigen injection participate in the primary response andbecome central-memory cells. J Exp Med. 2006;203:1045–1054. doi: 10.1084/jem.20051954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffová L, Lumsden L, Veselá V, Taylor JA, Filipec M, Holán V.et al. (2001Kinetics of leukocyte and myeloid cell traffic in the murine corneal allograft response Transplantation 721292–1298. [DOI] [PubMed] [Google Scholar]
- Milner CM, Higman VA., and, Day AJ. TSG-6: a pluripotent inflammatory mediator. Biochem Soc Trans. 2006;34 Pt 3:446–450. doi: 10.1042/BST0340446. [DOI] [PubMed] [Google Scholar]
- Wisniewski HG., and, Vilcek J. Cytokine-induced gene expression at the crossroads of innate immunity, inflammation and fertility: TSG-6 and PTX3/TSG-14. Cytokine Growth Factor Rev. 2004;15:129–146. doi: 10.1016/j.cytogfr.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Choi H, Lee RH, Bazhanov N, Oh JY., and, Prockop DJ. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-?B signaling in resident macrophages. Blood. 2011;118:330–338. doi: 10.1182/blood-2010-12-327353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JY, Choi H, Lee RH, Roddy GW, Ylöstalo JH, Wawrousek E.et al. (2012Identification of the HSPB4/TLR2/NF-?B axis in macrophage as a therapeutic target for sterile inflammation of the cornea EMBO Mol Med 4435–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JY, Roddy GW, Choi H, Lee RH, Ylöstalo JH, Rosa RH., Jret al. (2010Anti-inflammatory protein TSG-6 reduces inflammatory damage to the cornea following chemical and mechanical injury Proc Natl Acad Sci USA 10716875–16880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy JC. Granzymes and perforin in solid organ transplant rejection. Cell Death Differ. 2010;17:567–576. doi: 10.1038/cdd.2009.161. [DOI] [PubMed] [Google Scholar]
- Hamrah P,, Huq SO, Zhang Q., and, Dana MR. Corneal immunity is mediated by heterogenous population of antigen-presenting cells. J Leukocyte Biol. 2003;74:172–178. doi: 10.1189/jlb.1102544. [DOI] [PubMed] [Google Scholar]
- Dana MR. Corneal antigen-presenting cells: diversity, plasticity, and disguise: the Cogan lecture. Invest Ophthalmol Vis Sci. 2004;45:722–7; 721. doi: 10.1167/iovs.03-0803. [DOI] [PubMed] [Google Scholar]
- Dana R. Comparison of topical interleukin-1 vs tumor necrosis factor-alpha blockade with corticosteroid therapy on murine corneal inflammation, neovascularization, and transplant survival (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2007;105:330–343. [PMC free article] [PubMed] [Google Scholar]
- Kuffová L, Netuková M, Duncan L, Porter A, Stockinger B., and, Forrester JV. Cross presentation of antigen on MHC class II via the draining lymph node after corneal transplantation in mice. J Immunol. 2008;180:1353–1361. doi: 10.4049/jimmunol.180.3.1353. [DOI] [PubMed] [Google Scholar]
- Roddy GW, Oh JY, Lee RH, Bartosh TJ, Ylostalo J, Coble K.et al. (2011Action at a distance: systemically administered adult stem/progenitor cells (MSCs) reduce inflammatory damage to the cornea without engraftment and primarily by secretion of TNF-a stimulated gene/protein 6 Stem Cells 291572–1579. [DOI] [PubMed] [Google Scholar]
- Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL.et al. (2009Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6 Cell Stem Cell 554–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RH, Oh JY, Choi H., and, Bazhanov N. Therapeutic factors secreted by mesenchymal stromal cells and tissue repair. J Cell Biochem. 2011;112:3073–3078. doi: 10.1002/jcb.23250. [DOI] [PubMed] [Google Scholar]
- Prockop DJ, Kota DJ, Bazhanov N., and, Reger RL. Evolving paradigms for repair of tissues by adult stem/progenitor cells (MSCs) J Cell Mol Med. 2010;14:2190–2199. doi: 10.1111/j.1582-4934.2010.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uccelli A., and, Prockop DJ. Why should mesenchymal stem cells (MSCs) cure autoimmune diseases. Curr Opin Immunol. 2010;22:768–774. doi: 10.1016/j.coi.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Siegel G, Schäfer R., and, Dazzi F. The immunosuppressive properties of mesenchymal stem cells. Transplantation. 2009;87 9 Suppl:S45–S49. doi: 10.1097/TP.0b013e3181a285b0. [DOI] [PubMed] [Google Scholar]
- Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI.et al. (2008Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide Cell Stem Cell 2141–150. [DOI] [PubMed] [Google Scholar]
- Rafei M, Campeau PM, Aguilar-Mahecha A, Buchanan M, Williams P, Birman E.et al. (2009Mesenchymal stromal cells ameliorate experimental autoimmune encephalomyelitis by inhibiting CD4 Th17 T cells in a CC chemokine ligand 2-dependent manner J Immunol 1825994–6002. [DOI] [PubMed] [Google Scholar]
- Crop M, Baan C, Weimar W., and, Hoogduijn M. Potential of mesenchymal stem cells as immune therapy in solid-organ transplantation. Transpl Int. 2009;22:365–376. doi: 10.1111/j.1432-2277.2008.00786.x. [DOI] [PubMed] [Google Scholar]
- Casiraghi F, Azzollini N, Cassis P, Imberti B, Morigi M, Cugini D.et al. (2008Pretransplant infusion of mesenchymal stem cells prolongs the survival of a semiallogeneic heart transplant through the generation of regulatory T cells J Immunol 1813933–3946. [DOI] [PubMed] [Google Scholar]
- Jarvinen L, Badri L, Wettlaufer S, Ohtsuka T, Standiford TJ, Toews GB.et al. (2008Lung resident mesenchymal stem cells isolated from human lung allografts inhibit T cell proliferation via a soluble mediator J Immunol 1814389–4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbano P, Cuccia A, Mazzanti B, Urbani S, Giusti B, Lapini I.et al. (2008Use of donor bone marrow mesenchymal stem cells for treatment of skin allograft rejection in a preclinical rat model Arch Dermatol Res 300115–124. [DOI] [PubMed] [Google Scholar]
- Ding Y, Bushell A., and, Wood KJ. Mesenchymal stem-cell immunosuppressive capabilities: therapeutic implications in islet transplantation. Transplantation. 2010;89:270–273. doi: 10.1097/TP.0b013e3181c6ffbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
i.v. hMSCs decreased the number of activated T cells in ipsilateral cervical lymph nodes 4 week following transplantation.
. The quantitative assay for human mRNA for GAPDH in the cornea after i.v. administration of hMSCs.
The quantitative assay for human mRNA for GAPDH in the lung after i.v. administration of hMSCs.
Real-time RT-PCR analysis for human-specific transcripts of the anti-inflammatory and immunoregulatory molecules.
Graphic summary of the effects of hMSCs on corneal allografts.
The knockdown efficiency of TSG-6 in hMSCs was ~82% by real-time RT-PCR 10 hours after transfection with TSG-6-siRNA or scrambled siRNA which was the same time-point when TSG-6-siRNA MSCs were injected into mice.
The standard curve for the number of human mesenchymal stem cells (hMSCs) in one mouse cornea based on the expression of human-specific GAPDH (hGAPDH) relative to total eukaryotic 18s rRNA.