In a study reported in the 27 June 2012 issue of Science Translational Medicine, Tedesco and collaborators1 put into practice methodologies for generating, differentiating, and transplanting pluripotent cells derived from human patients with limb-girdle muscular dystrophy type 2D (LGMD2D, also called α-sarcoglycanopathy). Starting from patient fibroblasts or myoblasts, the authors first established and validated human induced pluripotent stem (iPS) cell lines, which were then coaxed toward differentiation into mesoangioblast-like cells termed “human iPS-derived mesoangioblast-like stem/progenitor cells” (HIDEMs). These cells were then supplemented with complementary DNAs encoding human α-sarcoglycan (Sgca) and a tamoxifen-inducible myogenic transcription factor (MyoD). These cells participated in muscle regeneration after intramuscular administration into the Sgca-null mouse, a model of the human disease. Moreover, when injected by the arterial route, the cells were able to colonize the downstream muscles and to improve their phenotype and their functional capacities. The findings suggest that cells prepared from simple biopsy specimens from human patients can be committed through a multistep procedure into corrected cells able to cross the endothelial barriers, allowing systemic distribution over large muscle volumes and widespread correction of myopathic features.
Muscular dystrophies are a heterogeneous family of orphan genetic diseases for which no cure is yet available. LGMD2D is a rare disorder (prevalence between 1 and 9 per million worldwide) caused by mutations in the small gene encoding Sgca, a member of the sarcoglycan complex located at the muscle membrane and involved in linking the cytoskeleton to the extracellular matrix. LGMD2D is characterized by a symmetrical involvement of trunk and limb muscles, calf hypertrophy, proximal weakness, high levels of serum creatine kinase, but absence of cardiac dysfunction.2 Muscular dystrophies are being targeted by innovative therapeutic strategies.3 Gene supplementation approaches using viral vectors4 and post-transcriptional “gene surgery” targeting messenger RNAs are being investigated to restore the expression of functional proteins, but these approaches do not supply a pool of regenerative cells to the degenerating muscle tissues of the patients.
Cell therapy approaches take advantage of the natural fusion between syncytial fibers and individual progenitors during muscle regeneration. The transplanted cells not only constitute a myogenic supply but also are able to carry new genetic material into the hybrid muscle fibers.5 Initial cell transplantation trials for muscular dystrophies involved myoblasts, which are committed myogenic progenitors expanded in culture from satellite cells, and produced mitigated results underlining their limitations. Although localized tissue repair could be observed, the myoblasts were susceptible to high mortality and could not be delivered through systemic routes. Several new myogenic cell types have been identified, and some among the family of perivascular cells might be amenable to systemic delivery.6,7,8,9,10 Mesoangioblasts (MABs) in particular are a type of pericyte of muscle origin that can cross endothelial barriers and are under clinical testing in an allogeneic context in children affected by Duchenne muscular dystrophy.1,6
The initial goal of the authors of the new study was to produce and use MABs extracted from muscles of LGMD2D patients to correct the pathology in Sgca-null mice. Unexpectedly, the choice of this model disease underlined a potential involvement of pericytes in the physiopathological process of LGMD2D. It was indeed impossible to expand MABs from biopsy samples of adult patients or of adult Sgca-null mice, whereas MABs had been prepared from juvenile Sgca-null mice in a previous study.7 Therefore, the exhaustion of these cells might constitute a hallmark of LGMD2D and appears comparable to the exhaustion of satellite cells associated with the ongoing degeneration in Duchenne muscular dystrophy.11 To obtain the desired MABs, the authors then decided to convert somatic cells first into pluripotent cells, and then into MAB-like cells.
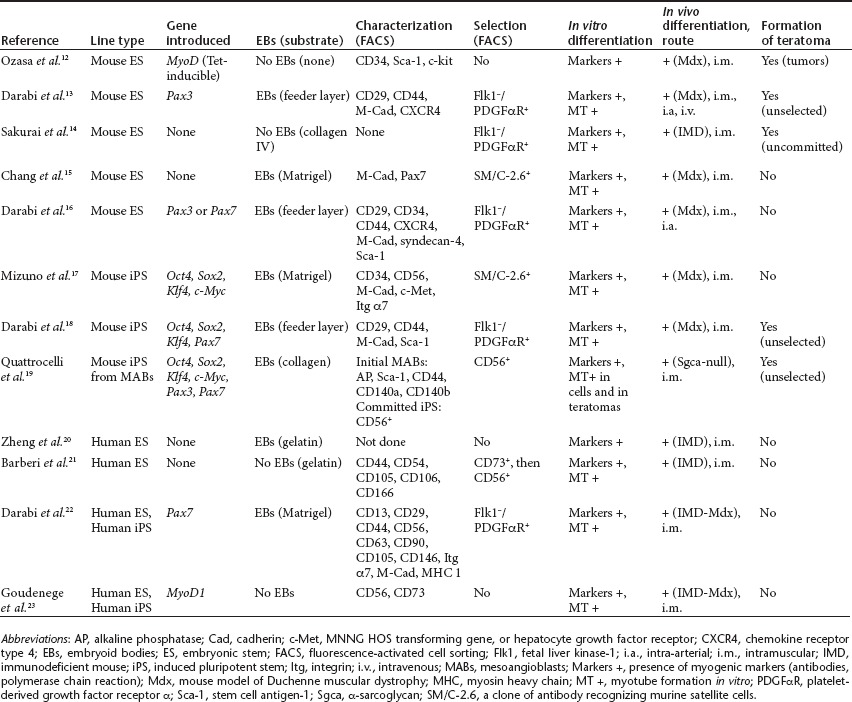
The use of embryonic stem (ES) cells for the treatment of myopathies was conceptualized long ago but was hampered by the low efficiency of myogenic commitment. Recently several groups have developed multistep procedures to produce myogenic cells (Table 1).12,13,14,15,16,17,18,19,20,21,22,23 These are generally based on combinations of mesodermal conversion of ES or iPS cells using specific media; the induction of early progenitors using commitment factors such as Pax3, Pax7, and MyoD; their nesting within embryoid bodies; and, finally, the sorting of differentiated progenitors using flow cytometry or immunomagnetic beads.24 The originality of the approach by Tedesco and collaborators lies in the commitment toward MAB-like cells (HIDEMs) instead of satellite- or myoblast-like cells, so as to benefit from the ability of MABs to cross endothelial barriers. A few teams have attempted to produce vasculogenic or pericyte-like cells from healthy ES or iPS cells but did not evaluate their capacities in the context of myopathies or through systemic distribution, or both.19,25 These HIDEMs harbored some characteristics of pericytes such as membrane markers (CD13, CD44, CD49b, and CD146), the expression of molecular markers, the proliferative capacity, and karyotype stability; moreover, they were not tumorigenic in vivo. At variance with natural pericytes,8 HIDEMs required cultivation in the presence of myogenic (i.e., C2C12) cells or transduction with a vector encoding MyoD, a strong and polyvalent muscle transcription factor, to exhibit myogenic differentiation in vitro. The HIDEM cells were then engineered to express the therapeutic gene (the complementary DNA encoding Sgca) under control of a muscle-specific promoter so as to avoid ubiquitous expression in other cell types, such as professional immunological presenting cells. To improve their myogenicity, the cells were further transduced with a vector encoding a tamoxifen-inducible MyoD transgene, which exerts a potent myogenic commitment in a self-activating manner.26
Table 1. Recent protocols developed to commit pluripotent cells toward myogenic lineages.
HIDEMs could exit the vascular system upon intra-arterial injection and home into diseased muscle. Some HIDEMs participated in muscle regeneration through fusion with muscle fibers, while others remained within the interstitium in a pericyte-like position, and even replenished this niche in the pathological Sgca-null mouse model. This capacity underlines their angiogenic potential and is reminiscent of the capacity of satellite cells and some myogenic progenitors to home to satellite cell niches when the latter are depleted. In the future, if HIDEMs are to become of widespread use, their efficacy to replenish niches should be assessed in models of muscular dystrophies showing no spontaneous defect in pericyte biology. Their efficacy may also be compared with that of other mesodermal-oriented ES or iPS cell types presenting a homing capacity upon intra-arterial injection.13,16,18
Tedesco and collaborators also compared the efficacy of human and murine cells in the Sgca-null model.1 They observed a fourfold increase in histological integration and improved muscular capacities when murine MAB-like cells (termed MIDEMs) were used in a mouse-to-mouse transplantation context instead of HIDEMs in a human-to-mouse context. This discrepancy might be explained by the difference in size between murine and human cells resulting in divergences in the spreading or clotting into vessels. Also, slight incompatibilities between human and murine molecules or physiological systems may cause defective recognition of cell surface antigens involved in migration, extravasation, or fusion. Finally, the combination of the exogenous human α-sarcoglycan with the murine sarcoglycans β, γ, δ leads to the formation of a tetrameric but chimeric complex of unknown functionality.
An ultimate goal of cell therapy approaches is to generate biological products amenable to clinical trials, which impose several restrictions regarding production, characterization, quality control, and delivery to prevent the worsening of the pathologies by embolisms, immune reactions, unexpected differentiations, or fibroses. With this in mind the authors have tested the robustness of their protocols using iPS cell lines certified to be free of viral integrations, and the cells proposed by Tedesco and collaborators may constitute the first generation of a new category of progenitors that, despite not being completely equivalent to their natural in situ counterparts, may share on demand some angiogenic and/or myogenic capacities. Further work will be necessary to (i) assess their safety and stability, (ii) document the colonization of specific muscles affected in several myopathies (diaphragm, intercostal muscles, heart), (iii) compare HIDEMs derived from various initial cell types, and (iv) assess their potential immunogenicity, even in an autologous context.27 The production of HIDEMS may benefit from ongoing progress in our understanding of the biology of pluripotent stem cells, which are emerging as important weapons in the armamentarium of cell, gene, and molecular therapy.
REFERENCES
- Tedesco FS, Gerli MF, Perani L, Benedetti S, Ungaro F, Cassano M.et al. (2012Transplantation of genetically corrected Human iPSC-derived progenitors in mice with limb-girdle muscular dystrophy Sci Transl Med 4140ra89. [DOI] [PubMed] [Google Scholar]
- Eymard B, Romero NB, Leturcq F, Piccolo F, Carrié A, Jeanpierre M.et al. (1997Primary adhalinopathy (alpha-sarcoglycanopathy): clinical, pathologic, and genetic correlation in 20 patients with autosomal recessive muscular dystrophy Neurology 481227–1234. [DOI] [PubMed] [Google Scholar]
- Pichavant C, Aartsma-Rus A, Clemens PR, Davies KE, Dickson G, Takeda S.et al. (2011Current status of pharmaceutical and genetic therapeutic approaches to treat DMD Mol Ther 19830–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell JR, Rodino-Klapac LR, Rosales XQ, Coley BD, Galloway G, Lewis S.et al. (2010Sustained alpha-sarcoglycan gene expression after gene transfer in limb-girdle muscular dystrophy, type 2D Ann Neurol 68629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuk D., and, Tremblay JP. Intramuscular cell transplantation as a potential treatment of myopathies: clinical and preclinical relevant data. Expert Opin Biol Ther. 2011;11:359–374. doi: 10.1517/14712598.2011.548800. [DOI] [PubMed] [Google Scholar]
- Tedesco FS, Dellavalle A, Diaz-Manera J, Messina G., and, Cossu G. Repairing skeletal muscle: regenerative potential of skeletal muscle stem cells. J Clin Invest. 2010;120:11–19. doi: 10.1172/JCI40373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaolesi M, Torrente Y, Innocenzi A, Tonlorenzi R, D'Antona G, Pellegrino MA.et al. (2003Cell therapy of alpha-sarcoglycan null dystrophic mice through intra-arterial delivery of mesoangioblasts Science 301487–492. [DOI] [PubMed] [Google Scholar]
- Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L.et al. (2007Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells Nat Cell Biol 9255–267. [DOI] [PubMed] [Google Scholar]
- Dellavalle A, Maroli G, Covarello D, Azzoni E, Innocenzi A, Perani L.et al. (2011Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells Nat Commun 2499. [DOI] [PubMed] [Google Scholar]
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS.et al. (2008A perivascular origin for mesenchymal stem cells in multiple human organs Cell Stem Cell 3301–313. [DOI] [PubMed] [Google Scholar]
- Morgan JE., and, Zammit PS. Direct effects of the pathogenic mutation on satellite cell function in muscular dystrophy. Exp Cell Res. 2010;316:3100–3108. doi: 10.1016/j.yexcr.2010.05.014. [DOI] [PubMed] [Google Scholar]
- Ozasa S, Kimura S, Ito K, Ueno H, Ikezawa M, Matsukura M.et al. (2007Efficient conversion of ES cells into myogenic lineage using the gene-inducible system Biochem Biophys Res Commun 357957–963. [DOI] [PubMed] [Google Scholar]
- Darabi R, Gehlbach K, Bachoo RM, Kamath S, Osawa M, Kamm KE.et al. (2008Functional skeletal muscle regeneration from differentiating embryonic stem cells Nat Med 14134–143. [DOI] [PubMed] [Google Scholar]
- Sakurai H, Okawa Y, Inami Y, Nishio N., and, Isobe K. Paraxial mesodermal progenitors derived from mouse embryonic stem cells contribute to muscle regeneration via differentiation into muscle satellite cells. Stem Cells. 2008;26:1865–1873. doi: 10.1634/stemcells.2008-0173. [DOI] [PubMed] [Google Scholar]
- Chang H, Yoshimoto M, Umeda K, Iwasa T, Mizuno Y, Fukada S.et al. (2009Generation of transplantable, functional satellite-like cells from mouse embryonic stem cells FASEB J 231907–1919. [DOI] [PubMed] [Google Scholar]
- Darabi R, Santos FN, Filareto A, Pan W, Koene R, Rudnicki MA.et al. (2011Assessment of the myogenic stem cell compartment following transplantation of Pax3/Pax7-induced embryonic stem cell–derived progenitors Stem Cells 29777–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno Y, Chang H, Umeda K, Niwa A, Iwasa T, Awaya T.et al. (2010Generation of skeletal muscle stem/progenitor cells from murine induced pluripotent stem cells FASEB J 242245–2253. [DOI] [PubMed] [Google Scholar]
- Darabi R, Pan W, Bosnakovski D, Baik J, Kyba M., and, Perlingeiro RC. Functional myogenic engraftment from mouse iPS cells. Stem Cell Rev. 2011;7:948–957. doi: 10.1007/s12015-011-9258-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quattrocelli M, Palazzolo G, Floris G, Schöffski P, Anastasia L, Orlacchio A.et al. (2011Intrinsic cell memory reinforces myogenic commitment of pericyte-derived iPSCs J Pathol 225593–603. [DOI] [PubMed] [Google Scholar]
- Zheng JK, Wang Y, Karandikar A, Wang Q, Gai H, Liu AL.et al. (2006Skeletal myogenesis by human embryonic stem cells Cell Res 16713–722. [DOI] [PubMed] [Google Scholar]
- Barberi T, Bradbury M, Dincer Z, Panagiotakos G, Socci ND., and, Studer L. Derivation of engraftable skeletal myoblasts from human embryonic stem cells. Nat Med. 2007;13:642–648. doi: 10.1038/nm1533. [DOI] [PubMed] [Google Scholar]
- Darabi R, Arpke RW, Irion S, Dimos JT, Grskovic M, Kyba M.et al. (2012Human ES- and iPS-derived myogenic progenitors restore dystrophin and improve contractility upon transplantation in dystrophic mice Cell Stem Cell 10610–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudenege S, Lebel C, Huot NB, Dufour C, Fujii I, Gekas J.et al. (2012Myoblasts derived from normal hESCs and dystrophic hiPSCs efficiently fuse with existing muscle fibers following transplantation Mol Ther 202153–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salani S, Donadoni C, Rizzo F, Bresolin N, Comi GP., and, Corti S. Generation of skeletal muscle cells from embryonic and induced pluripotent stem cells as an in vitro model and for therapy of muscular dystrophies. J Cell Mol Med. 2012;16:1353–1364. doi: 10.1111/j.1582-4934.2011.01498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar A, Domev H, Ben-Yosef O, Tzukerman M, Zeevi-Levin N, Novak A.et al. (2012Multipotent vasculogenic pericytes from human pluripotent stem cells promote recovery of murine ischemic limb Circulation 12587–99. [DOI] [PubMed] [Google Scholar]
- Shani M, Faerman A, Emerson CP, Pearson-White S, Dekel I., and, Magal Y. The consequences of a constitutive expression of MyoD1 in ES cells and mouse embryos. Symp Soc Exp Biol. 1992;46:19–36. [PubMed] [Google Scholar]
- Zhao T, Zhang ZN, Rong Z., and, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–215. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]


