Abstract
Background
Patients with bipolar disorder are known to be at high risk of premature death. Comorbid cardio-vascular diseases are a leading cause of excess mortality, well above the risk associated with suicide. In this review, we explore comorbid medical disorders, highlighting evidence that bipolar disorder can be effectively conceptualized as a multi-systemic inflammatory disease.
Methods
We conducted a systematic PubMed search of all English-language articles recently published with bipolar disorder cross-referenced with the following terms: mortality and morbidity, cardio-vascular, diabetes, obesity, metabolic syndrome, inflammation, auto-antibody, retro-virus, stress, sleep and circadian rhythm.
Results
Evidence gathered so far suggests that the multi-system involvement is present from the early stages, and therefore requires proactive screening and diagnostic procedures, as well as comprehensive treatment to reduce progression and premature mortality. Exploring the biological pathways that could account for the observed link show that dysregulated inflammatory background could be a common factor underlying cardio-vascular and bipolar disorders. Viewing bipolar disorder as a multi-system disorder should help us to re-conceptualize disorders of the mind as “disorders of the brain and the body”.
Limitations
The current literature substantially lacks longitudinal and mechanistic studies, as well as comparison studies to explore the magnitude of the medical burden in bipolar disorder compared to major mood disorders as well as psychotic disorders. It is also necessary to look for subgroups of bipolar disorder based on their rates of comorbid disorders.
Conclusions
Comorbid medical illnesses in bipolar disorder might be viewed not only as the consequence of health behaviors and of psychotropic medications, but rather as an early manifestation of a multi-systemic disorder. Medical monitoring is thus a critical component of case assessment. Exploring common biological pathways of inflammation should help biomarkers discovery, ultimately leading to innovative diagnostic tools, new methods of prevention and personalized treatments.
Keywords: Bipolar disorder, Cardiovascular disease, Mortality, Inflammation, Staging, Prevention
1. Introduction
Bipolar disorder is known to be associated with substantial functional impairment, high health care costs, and also premature mortality (Roshanaei-Moghaddam and Katon, 2009). The recent WHO update (2008) highlights the global burden of bipolar disorder to be the fourth highest burden throughout both high as well as low and middle income countries. The morbidity, mortality and personal suffering associated with bipolar disorder are not simply the result of psychiatric symptoms, but are also the consequence of a wide range of psychiatric and medical comorbid disorders. Emerging data show that bipolar disorder is associated with highly prevalent co-occurring psychiatric and substance use disorders, ranging from 57% to 74% (Bauer et al., 2005), but also with medical comorbidities which occur in over 80% of bipolar patients (Kilbourne et al., 2004, Kupfer, 2005). Patients with these co-occurring disorders experience worse prognosis with less favorable response to treatment, unemployment and thus higher cost than those without comorbidity (Angst et al., 2002; Tsai et al., 2005, Williams et al., 2011).
Despite this very important medical burden, under-recognition and inattention to physical diseases and their risk factors still prevails. As bipolar I patients are almost always treated only in mental health settings, most psychiatrists, physicians and health policy makers are not aware that these comorbid medical disorders are probably more prevalent in bipolar disorder that in any other major psychiatric disorders.
As the detection of these highly prevalent comorbid medical disorders could drastically change mental health organization by reinforcing the links with medical care, by modifying the education of psychiatrists, and by opening up new avenues of research, this article reviews research related to medical comorbid disorders in bipolar disorder. We thus conducted a systematic PubMed search of all English-language articles recently published with bipolar disorder cross-referenced with the following terms: mortality and morbidity, cardio-vascular, diabetes, obesity, metabolic syndrome, immuno-inflammatory, auto-antibody/auto-immunity, retro-virus, stress, sleep and circadian rhythm. In this report we have explored the issue of comorbid medical disorders in bipolar disorder asking three major questions related first, to the magnitude of these comorbid disorders, second, to the staging and timing of occurrence of these disorders across the life-span, and third, describing some of the possible mechanisms underlying these co-occurring disorders in order to ascertain whether the patho-physiology of bipolar disorder itself explains the clustering of medical disorders.
2. Methods
2.1. Is there evidence of excess medical co-morbidity and mortality in bipolar disorder?
A growing number of studies have demonstrated that patients with bipolar disorder are at high risk of premature death, unrelated to suicide. Excess mortality rates due to medical causes are between 1.5 and 3 times higher in adults with bipolar disorder compared to the general population (Correll, 2008), higher than those with major depression (Roshanaei-Moghaddam and Katon, 2009). This has been observed across diverse cultural and socioeconomic backgrounds (Ohaeri and Akani, 2011). Among patients with severe and chronic mental illnesses, most attention has focused on patients with schizophrenia, leading to the erroneous assumption that this disorder bears the highest burden of medical morbidity and mortality. Recent reports have shown that patients with bipolar disorder have similar and sometimes higher mortality rates due to general medical disorders. Life expectancy at birth, an indicator of all-cause mortality, has been estimated roughly 10 and 11 years shorter for men and women with bipolar disorder compared to the non-mentally ill general population (Chang et al., 2011). These figures are comparable to the estimated 14 and 9.8 years lost for men and women with schizophrenia. A recent study conducted on the Danish Civil Registration System found life-expectancy to be 13.6 years shorter for bipolar men and 12.1 years for bipolar women (Laursen, 2011) when compared to that of the surrounding general population. This increased mortality rate covers all natural causes of deaths, including cardio-vascular, cerebro-vascular, other medical disorders and external causes of death (Roshanaei-Moghaddam and Katon, 2009).
Cardio-vascular mortality is known to be a leading cause of excess mortality in bipolar disorder, well above the risk associated with un-natural causes of death such as suicide or accidents. Although the lifetime history of suicide attempts is elevated (36.3%) in bipolar disorder (Novick et al., 2010), the case fatality is not as high as previously claimed (Dutta et al., 2007). Overall, the increased cardiovascular mortality is between 1.5 and 2.5 fold when compared to the general population (Ahrens et al., 1995; Osby et al., 2001). Weiner et al. (2011) reviewed all the studies published in the past 25 years in which rates of cardiovascular mortality for bipolar inpatients could be extracted and estimated that the Standardized Mortality Ratio was 1.89. We will therefore focus on reviewing the extant literature on cardiovascular risk factors and diseases, a leading cause of excess death in bipolar disorder.
The “metabolic syndrome” is a constellation of metabolic abnormalities that predispose to cardiovascular disease and is highly prevalent in patients with bipolar disorder compared to the general population. In 2001, the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (NCEP ATP III) formulated an operational definition of this syndrome based on the presence of three or more of the following characteristics: abdominal obesity (waist circumference); hypertriglyceridemia; low high-density lipoprotein cholesterol (HDL-C), or being on an antilipemic agent; high blood pressure, or being on an antihypertensive agent; and fasting hyperglycemia, or being on a antiglycemic agent (NCEP, 2001). The presence of the metabolic syndrome confers substantial additional risk for cardiovascular events and diabetes, above and beyond the risk that would be predicted by simply adding the individual risk factors (Laaksonen et al., 2002; Lakka et al., 2002). The increased risk for metabolic syndrome amongst bipolar individuals is now documented in twelve countries from Europe, Australia, Asia, North and South America (McIntyre et al., 2010). Bipolar patients are more frequently affected by metabolic syndrome than the general population: from 36 to 49% compared to 32% in the general population in US studies and from 19% in Belgium to 27% in Italy compared to 8–17% in the general population in Europe (Salvi et al., 2011). While there is data to suggest differential liability of drug contribution associated with metabolic syndrome status and that bipolar patients with a metabolic syndrome have a less favorable response to treatment, adverse course and outcome, and are at greater risk for suicidality (Cardenas et al., 2008).
This high cardio-vascular mortality might be explained by the fact that bipolar patients have several cardio-vascular risk factors such as obesity, hypertension, diabetes and hyperlipidemia (Garcia-Portilla et al., 2009). In the USA, as many as 75% of patients with bipolar disorder are overweight, compared to the reported 60% (McElroy et al., 2002) of the general population and nearly half of them have a BMI in the obese range (Fiedorowicz et al., 2008). Since 1987, the prevalence of hypertension has been reported to be elevated (14%) in bipolar patients, compared to normal population (5.6%) and to unipolar depression (5%) (Yates and Wallace, 1987). This was replicated in several studies in USA and in Europe (see for review, Salvi et al., 2011 and Weiner et al., 2011). While the largest study involving 25,339 bipolar patients and 113,698 controls found an increased rate of new-onset cases of hypertension among bipolar patients compared to general population and to schizophrenic cases (Johannessen et al., 2006). The association between diabetes and bipolar disorder has again been reported nearly a century ago (Raphael and Parsons, 1921) and since then, it has been estimated that bipolar patients have diabetes 3 times more than expected (9.9% to versus 3.3%, Cassidy et al., 1999; 17.2% versus 15.6%, Kilbourne et al., 2004). Finally, evidence indicates that between half and one third of bipolar patients have increased hypertryglyceridemia compared to normals (Fagiolini et al., 2005; Fiedorowicz et al., 2008).
Is this association explained by factors specific to bipolar disorder or is it only a mere consequence of unhealthy lifestyle and long term exposure to medications? The most widely-studied modifiable pathways through which bipolar disorder is thought to increase cardio-vascular risk include health behaviors (e.g., smoking, physical inactivity, poor diet, and obesity), psychosocial functioning (e.g., occupational status, income, and education) and lifelong medication exposure. However, the association between cardiovascular risk factors and bipolar disorders remains significant after controlling for these co-factors which strongly suggests that additional and specific mechanisms have yet to be identified (Fiedorowicz et al., 2009; Goldstein et al., 2009; Murray et al., 2009). Moreover, increased cardiovascular risk factors have been found in first episode drug-naive patients (Maina et al., 2008) than in patients with schizophrenia who were equally medicated (Birkenaes et al., 2007; Correll et al., 2008; Johannessen et al., 2006) and than in patients with unipolar depression (Angst et al., 2002; Perris and d’Ellia, 1996; Tsuang et al., 1980). In addition, this increase has been documented long before the widespread use of atypical antipsychotics, tricyclic antidepressant and lithium (Weeke et al., 1987). Furthermore, treatment with mood stabilizers, including atypical antipsychotics, has been suggested to be protective against early natural death (Müller-Oerlinghausen et al., 2003; Müller-Oerlinghausen et al., 2005; Tsai et al., 2005) including returning the Standardized Mortality Ratio associated with cardiovascular mortality to unity (Ahrens et al., 1995).
These pieces of evidence thus support the concept that the rates of obesity, diabetes, dyslipidemia, hypertension, smoking and metabolic syndrome, all serious cardio-vascular disease risk factors, are elevated in bipolar disorder compared to the general population. On top of the medical burden associated with these disorders, bipolar patients with these comorbid medical disorders appear to have greater psychopathology and suicidality, increased relapse and re-hospitalization (Fagiolini et al., 2005). More studies are however needed to confirm that the magnitude of this medical burden is higher in bipolar disorder than in other severe psychiatric disorders. It is also necessary to search for possible subgroups of bipolar disorder, such as young patients, who might have higher rates of comorbid disorders and who might thus need to be specifically monitored and treated.
2.2. Timing and staging of medical comorbid disorders across the life span
Since most of the studies conducted so far to support the association between comorbid medical and bipolar disorders are largely based on cross-sectional analysis, the temporal association between them remains unknown. This lack of information impedes the proper understanding of their exact role in the evolution of bipolar disorders. In general medicine, staging models are used to fully delineate chronic and complex disorders such as cancer, diabetes and cardiovascular diseases, and play a critical part of prognosis formulation, appropriate treatment planning and follow up. The most prominent example of the staging approach is the ‘tumor, node metastasis’ system, which describes the progression of the illness, defines prognosis and guides appropriate treatment selection. Severe mental disorders can be conceptualized in the same manner as cancer or diabetes, with an early phase, progression to a chronic course and different levels of systemic involvement. The concept of staging in psychiatry, initially described by Fava and Kellner (1993), was then developed in the pioneering work of McGorry in schizophrenia. Importantly, the corresponding stages in psychosis – the prodrome, the first episode, and the chronic phase – are mirrored by cognitive and brain anatomical changes and improved by specific treatments strategies adapted to each phase (McGorry et al., 2010). Staging models have only recently been proposed (Berk et al., 2007) and used in research in bipolar disorders (Kapczinski et al., 2009 Vieta et al., 2011). However, we advocate that medical burden should be incorporated into future staging model, as it is correlated with duration of illness, independently of age (Soreca et al., 2008), and might be explained by shared mechanisms and validated by shared bio-markers.
In order to include the occurrence of medical disorders and risk factors into the staging model of bipolar disorder, accurate description of their onset and longitudinal course is needed. The extant literature suggests that medical burden is part of the early stages of bipolar disorder, particularly for patients with onset at a very young age. For example, all-cause mortality, excluding suicide/homicide, is highest in early onset bipolar disorder (Roshanaei-Moghaddam and Katon, 2009). Not surprisingly, patients with adolescent-onset bipolar disorder also have more medical comorbid disorders than youth with other mental disorders. Over 70% of youth with bipolar disorder receive treatment for chronic medical disorders such as cardiovascular, gastro intestinal/hepatic, neurologic, musculo skeletal and respiratory diseases (Evans-Lacko et al., 2009). Moreover, nearly 30% of youth with bipolar disorder receive treatment for 2 or more medical disorders (Jerrell et al., 2010). In the USA, the prevalence of obesity in adolescent-onset bipolar disorder is above 40%, which is 20% greater than its prevalence in non-bipolar youth (Goldstein et al., 2008). Preliminary evidence shows that patients with adolescent onset bipolar disorder have higher rates of pre-existing somatic disorders, including obesity (OR=1.58) and hypertension (OR=2.93) (Jerrell et al., 2010).
In mid-life bipolar disorder, several mechanisms may contribute to the elevated rate of medical disorders, such as lifestyle factors and medication side effects (Goldstein et al., 2008). Many of these patients present cardiovascular diseases and hypertension over a decade earlier than in age-matched non-bipolar adults (Goldstein et al., 2009). It is also reported that cardio-vascular disorders are correlated with the duration of bipolar disorder, independently of the age at assessment (Soreca et al., 2008). Interestingly, medical illnesses have not been found at increased frequency among geriatric patients with bipolar disorder (Gildengers et al., 2008; Tsai et al., 2009)., This apparent paradox can be explained by the “survivor effect”; it is possible either that patients who survive the age peak of mortality do not incur a further increase in medical burden compared with non-bipolar age-matched samples, or, that patients who live into late life represent a healthier subgroup.
Longitudinal studies are needed to identify the different trajectories of medical burden in order to be incorporated into the disease staging process. The evidence gathered so far suggests that the multi-system involvement is present from the early stages, and therefore requires proactive screening and diagnostic procedures, as well as comprehensive treatment to reduce progression and premature mortality. Viewing bipolar disorder as a multi-system disorder should also help us to re-conceptualize disorders of the mind as “disorders of the brain and the body”. Exploring the biological mechanisms that explain the comorbidity between bipolar disorder and medical disorder should provide strong bases for the discovery of biomarkers which should ultimately lead to innovative diagnostic tools as well as new methods of prevention and personalized treatments.
2.3. Bipolar disorder: “A multi-systemic inflammatory disease”?
So far, the best-studied pathways through which bipolar disorder was thought to increase cardio-vascular risk included health behaviors, psychosocial functioning and lifelong medication exposure (Lasser et al., 2000). Until recently, a lot of emphasis has been put on the fact that psychotropic medication contributes to cardiovascular risk factors (Parsons et al., 2009). Lithium can cause weight gain and adversely influence glucose metabolism, valproic acid is associated to weight gain and insulin resistance, second generation anti-psychotics are associated to hyperlipidemia, increased risk with diabetes, and weight gain though the extent of weight gain depends on which antipsychotic is used (Newcomer, 2009). It should however be stressed that the increased mortality rate in bipolar predate modern pharmacologic treatments (Weiner et al., 2011). In addition, the fact that the association between cardiovascular risk factors and bipolar disorder remains significant after controlling for these co-factors strongly suggests that mechanisms specific to bipolar disorder itself have yet to be identified (Fiedorowicz et al., 2009; Goldstein et al., 2009; Murray et al., 2009). The relationship between depression and comorbid medical disorder, in particular cardio-vascular disease, has been the subject of research for more than 30 years. However, systematic assessment of depression during cardio-vascular disorder is still not widely performed in clinical settings (Frasure-Smith and Lespérance, 2006). One way to change attitudes is to review research explaining the relation between the two and to search for biological mechanisms to account for the observed link.
Inflammation has been shown to be crucial throughout atherosclerosis from endothelial dysfunction to plaque rupture and thrombosis (Corrado et al., 2010); a number of studies also suggest that inflammation may be implicated in the pathophysiology of bipolar disorder (for review see, Goldstein et al., 2009). The data supporting the hypothesis that inflammation could be a common factor underlying both cardio-vascular and bipolar disorder is important to be reviewed. We will examine whether these two apparently separate, but highly comorbid disorders can be viewed as manifestations of shared physiological mechanism leading to “a multi-systemic inflammatory disease”. Evidence reveals that chronic, mild inflammation in the periphery and in the brain occurs during bipolar disorder (reviewed in Goldstein et al., 2009; Hamdani et al., 2012), while dysregulated systemic and brain inflammatory processes also trigger and propagate atherosclerosis, hypertension, diabetes and obesity (Drake et al., 2011, Ross, 1999). Cytokines have been shown to access the brain and interact with every patho-physiologic domain relevant to bipolar disorder. Animal models have shown that peripheral cytokines reach the brain thanks to different mechanisms including leaky brain barrier, active transport, activation of endothelial cells, binding to cytokine receptors (see Miller et al., 2009 for review). Levels of pro-inflammatory cytokines like interleukin-1 (IL-1), IL-2, IL-4, IL-6 and tumor necrosis factor-α (TNF-α) are elevated during mania, while IL-6 is elevated during depression (Brietzke et al., 2009). Evidence of a stage-related impact on cytokines is suggested by the fact that pro-inflammatory cytokines IL-6 and TNF-α are elevated both during the early and late stage, while the anti-inflammatory IL-10 is increased only in the early phase of bipolar disorder (Berk et al., 2011). These pro-inflammatory cytokines, as well as C-reactive-Protein (CRP), are also known to have pro-atherogenic effect (Maes et al., 2011) which indeed would provide an explanation for the association between bipolar and cardio-vascular disorder. Moderate increased levels of C-reactive-Protein (CRP), and in particular hsCRP, are highly correlated to manic episodes (Dickerson et al., 2007) while high hsCRP is also a risk factor for cardio vascular disorder (Greenland et al., 2001).
Over the last two decades, it has been shown that inflammatory processes and neural immune interactions are involved in the pathophysiology of major depression (Maes et al., 2011), these data also shed light on how to explain the plausible link between increased levels of cytokines and mood states in bipolar disorder. A pro-inflammatory state is known to activate the tryptophan and serotonin-degrading-enzyme, indoleamine 2–3 dioxygenase (IDO), which has been found elevated in the plasma of bipolar patients (Mynt et al., 2007). Activation of this enzyme leads to increased consumption of tryptophan, thus reducing the availability of serotonergic neurotransmission, as well as inducing the production of detrimental tryptophan catabolites with neurotoxic effects (Muller and Schwartz, 2007). It has also been shown that the activity of dopaminergic system is reduced in response to inflammation (Morón et al., 2003) while cytokines enhance the re-uptake of monoamine neuro-transmitters thereby reducing their intra-synaptic concentrations in the brain (Zhu et al., 2006).
In addition to the direct effect of inflammation on mono-amines depletion and on initiation and maintenance of atherosclerosis, inflammation is also known to induce oxidative and nitrosative stress, including damage to lipids, DNA and proteins, mitochondrial dysfunction and consequently increasing apoptosis, cell membrane damage and protein aggregation (for review see Maes et al., 2011). Stage-dependant changes in oxidative stress have been reported in late stage more than in early stage patients, which may be part of a progressive failure of compensatory mechanisms over time, leading to cognitive decline and resistant bipolar disorder and may in part underlie the staging process (Berk et al., 2011).
The pro-inflammatory cytokines also induce decrease in neurotrophins, and in particular diminished levels of Brain-Derived-Neurotrophic-Factor (BDNF) leading to decrease neuronal repair, decrease in neurogenesis and an increased activation in glutamatergic pathway which also contributes to neuronal apoptosis (for review, see Leonard, 2010). It is noteworthy that serum BDNF has been associated both with changes in mood states in bipolar disorder (Fernandes et al., 2010) as well as in coronary heart diseases (Lorgis et al., 2010).
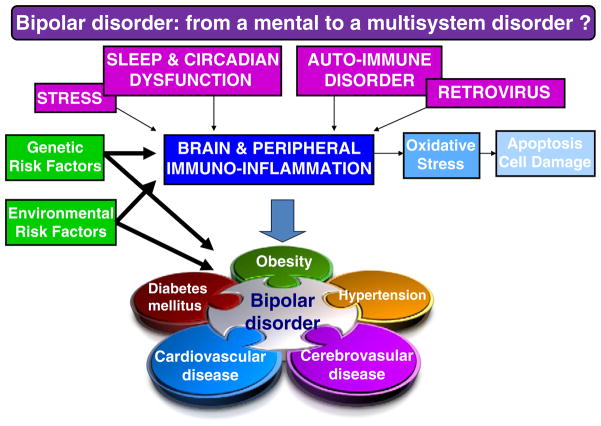
Pro-inflammatory processes appear to clearly have detrimental effect both on mood as well as and on atherosclerosis and may explain the co-occurrence between bipolar disorder and cardio-vascular disorder. Furthermore, this abnormal biological reaction may be seen as a shared final pathway induced by different candidate pathological mechanisms, each having been observed in bipolar disorder. So far, there has been little research on the pathways through which bipolar disorder may promote or maintain inflammation. We review briefly some of the mechanisms which may plausibly induce this abnormal inflammatory pathway such as disruptions of sleep and circadian rhythms, stress, as well as the more recently described phenomena of auto-immune dysfunction and retro-virus activation. For each of these potential pathways, causality may also be bi-directional and it is very likely that each of the proposed mechanisms might be induced or regulated by genetic, environmental and/or gene×environment risk factor interactions (McCaffery et al., 2006; Rutten and Mill, 2009). Fig. 1 presents the potential causes and consequences of this abnormal inflammatory reaction, showing how it could explain the comorbidity between bipolar and cardio-vascular disorder.
Fig. 1.
Different mechanisms that might cause abnormal immuno-inflammation and inactivity with genetic susceptible background and environmental factors will lead to bipolar disorder best conceptualized as a multi-system disorder.
2.4. Sleep and circadian abnormalities
Abnormalities of circadian rhythms and of sleep/wake cycles are considered as core phenotype of bipolar disorder: sleep disturbances are observed very early in bipolar disorders, long before the occurrence of the first mood episode, and are prominent symptoms during mood states and in recovered patients (Harvey, 2008). Sleep loss is also prevalent in obesity, metabolic syndrome, diabetes, and atherosclerosis. Short and long self reported sleep durations are associated to an increased risk of coronary events (Ayas et al., 2003). Sleep is a powerful regulator of immune function by fostering adaptative immune response (Lange et al., 2010). Loss of sleep prevents these immunosupportive actions, while prolonged sleep loss induces an inflammation response characterized by increased in leukocytes and neutrophil counts, cytokine seum levels and C-reactive protein (Mullington et al., 2009). These data thus highlight another common pathway, observed both in bipolar disorder and in cardio-vascular disorder, and possibly leading and/or reinforcing abnormal inflammation.
2.5. Stress
An association between stressful events and episode recurrences has repeatedly been found in bipolar patients (Etain et al., 2008). Psychological stress also may activate inflammatory responses in the brain (Wager-Smith and Markou, 2011). The theoretical model frames the depressive episode as being a repair response to stress induced neuronal microdamage that can grade into a chronic neuroinflammatory condition. Cardiovascular damage and atherogenic changes could be a by-product of this process. One of the mechanisms whereby psychosocial stress influences both peripheral and central inflammatory cascade, is coordinated by autonomic nervous system. Thus, the release of noradrenaline and adrenaline follows the activation of the sympathetic system and induces the activation of both alpha and beta adrenoreceptors on immune cells thereby initiating the release of pro-inflammatory cytokines via the nuclear factor-kappa-beta (NF-kB) cascade (Leonard, 2010). The brain is now known to be directly influenced by peripherally derived cytokines and gluco-corticoids as well as immune cells, which can access the brain through leaky blood–brain barrier and/or by activation of endothelial cells that line the cerebral vasculature, or bind to cytokine receptors (Raison et al., 2006).
2.6. Auto-immune disorders
A relationship between auto-immune disorders and bipolar disorder has been reported as early as 1888. Patients with bipolar disorder tend to develop organ-specific autoimmunity as shown, for example, by thyro-peroxidase antibodies (TPO-Abs) associated with thyroid failure, by antibodies to H/KAT-Pase associated with atrophic gastritis and by GAD65A, isoform of glutamic acid decarboxylase which is a marker of type-I diabetes (Vonk et al., 2007). Recently, manic episodes with psychotic symptoms were observed during acute encephalitis with antibodies directed in particular against extracellular domains of the glutamatergic NMDA receptor (Dalmau et al., 2011). In addition, it has recently been reported that gastrointestinal processing of food antigens such as bovine caseins and wheat glutens is altered in bipolar disorder. Bipolar patients have been reported to have increased antibodies to gliadin, a glycoprotein derived from the ingestion of gluten from wheat (Dickerson et al., 2011) or to casein activation, particularly during mania (Severance et al., 2011). Further studies are needed to determine if gastro-intestinal process affecting digestion, epithelial permeability, and immune overactivity are involved in bipolar disorder. Presence of these auto-antibodies might even precede the onset of bipolar disorder, as an increased prevalence of Multiple Sclerosis, thyrotoxicosis, ulcerative colitis, psoriasis and rheumatoid arthritis has been reported in unaffected relatives of patients with BD (Eaton et al., 2010).
2.7. Retro-virus activation
It is very unlikely that viral infections can directly cause bipolar disorder, but they may initiate a cascade of events leading to dys-regulation of the immuno-inflammatory system. This cascade involves a complex interaction of other environmental and genetic factors (see Hamdani et al., 2012). Pre-natal exposure to viruses, e.g. during influence epidemics, can increase the risk of developing an affective disorder in the offspring of women who were pregnant at the time, especially if the exposure occurred in the 2nd trimester (Scott et al., 2006). Such prenatal exposure may lead to activation of retro-viruses such as the Human Endogenous Retrovirus (HERVs). The retro-virus envelope (HERV-W ENV) has neuro-toxic as well as pro-inflammatory properties (Griffiths, 2001), thus contributing to a chronic inflammatory state affecting neurodevelopment. Thus, HERV-W abnormal expression might cause neuronal excitotoxicity and neurotoxicity, yielding neuronal loss associated with cognitive impairment and inflammatory phenomenon, as well as an oligodendrocytes death and subsequent demyelination (Antony et al., 2004). These HERV-W elements are part of the human genome, can be triggered by environmental agents such as Influenza virus and can be associated with seasonal infections “risk” during pregnancy and can then recombine, retro-transpose, or simply transpose within host’s genome. These elements can also be triggered later in life by Herpes viridae primary infections during early adulthood, or with Epstein–Barr virus and Herpes simplex viruses (Sutkowski et al., 2001). This potential pathogenic cascade could largely been applied to bipolar disorder as the expression of these retroviruses, as well as dys-immunity and seropositivity to microbial agents have been found in the disease. HERV-W RNA over-expression was evidenced in postmortem brains of bipolar patients (Weis et al., 2007). Infection with herpes simplex virus type 1 has been found associated with cognitive deficits in bipolar disorder (Dickerson et al., 2004).
In conclusion, several candidate mechanisms may underlie the pro-inflammatory pathways inherent to both bipolar and cardio-vascular disorders. Their respective implication and possible interactions remain to be tested in future research.
3. Results
This review highlights evidence that the magnitude of early and severe medical burden in bipolar disorder is an indication that it might better be viewed as a multi-system disorder. Secondly, it asks whether inflammation might plausibly explain the link between bipolar and cardio-vascular disorders. Although there are gaps in the evidence to support this hypothesis, the review provides an important first step in framing critical questions that could be tested in future research and changes that could be implemented in clinical practice.
What are the research implications of this hypothetical link? We propose that common inflammatory processes could underpin both bipolar and cardio-vascular disorders. However, we know very little of the specificity of these shared pathways as these associations have also been described in other mood and psychotic disorders (Berk et al., 2011). More research is thus needed to explore if immuno-inflammatory processes are general risk factors for stress and severe mental disorders, or if they are unique to bipolar disorder. For example, the role of inflammation in the pathophysiology of suicide has been suggested based on the analysis of plasma or cerebrospinal fluid of suicidal patients (Janelidze et al., 2011, Lindquist et al., 2009) or on post mortem brain tissue obtained from suicide victims (Tonelli et al., 2008) revealing abnormalities of cytokines in suicide. As suicide overlaps with bipolar disorder, further studies are needed to explore if inflammatory changes are specific to bipolar disorders, or to a subtype of bipolar patients with suicidal behavior, or to suicidality. In particular, studies of central or peripheral levels of cytokines of bipolar patients who committed suicide need to be performed.
In addition, further studies will be needed to explore if inflammatory changes are specific to bipolar patients in terms of their occurrence at a very early stage of the disorder, their interaction with specific genetic and/or environmental risk factor and/or their induction by mechanisms specifically observed in bipolar disorder. Clearly, carefully conducted prospective studies are needed to further explore these important issues. In particular, prospective studies of children and adolescents with severe psychiatric disorders, (i.e. bipolar disorders, recurrent depression and schizophrenia) that include those with comorbid psychiatric and medical disorders, are needed to allow comparisons of trajectories and to disentangle shared and separate biological pathways and mechanisms. Furthermore, if, as shown in some studies (Dickerson et al., 2007), these abnormal inflammatory markers appear very early in the course of the disorder, further research can examine whether they can be viewed as risk factors for bipolar and cardio vascular disorder. For example, in a prospective design, elevated level of CRP has been shown to be a risk marker for depression and cardio-vascular diseases (Pasco et al., 2010), whilst the predominant mood abnormality in bipolar disorders is depression, no equivalent data is available for bipolar disorder. Furthermore, studies are needed to examine whether the inflammatory diathesis can predict the occurrence of bipolar disorder. High-risk populations, such as offspring of bipolar parents, need to be followed up to address this issue. A single study examining inflammation among adolescent and young-adult offspring of parents with BP found that a pro-inflammatory gene expression signature was more prevalent among BP offspring compared to control offspring, particularly among offspring with mood disorders (Padmos et al., 2008), but no data on comorbid cardio vascular disorder or risk factor was mentioned. Clinical research could target predictors of treatment response. To date, we lack reliable biological predictors of response, but an example is a study of the use of aspirin in schizophrenia that suggested that efficacy was greater in patients having high levels of inflammation (Laan et al., 2010). No such studies have been conducted in bipolar disorder, and even less in bipolar and comorbid cardio vascular disorder. Overall, additional research is needed to enable us to better understand the implication of these mediators and to discover new drug targets based on their anti-inflammatory actions (Berk et al., 2011).
What is the take home message for clinicians? Currently, bipolar patients are undertreated for co-occurring medical disorders and are less likely to be monitored and appropriately treated for cardio-vascular risk factors than the general population (Garcia-Portilla et al., 2009; Weiner et al., 2011). Evidence synthesized in this paper, points to the fact that multi-system involvement is the rule, rather than the exception in bipolar disorders. Furthermore, the findings reviewed demonstrate that from its very early stages, bipolar disorder is associated with high medical burden, especially cardio-vascular disorders. We therefore propose that a modern conceptualization of bipolar disorder is that it represents a multi-systemic disease.
Our operational model frames mood symptoms in bipolar disorder as one manifestation of an underlying biological vulnerability that underpins a whole host of clinical manifestations ranging from somatic symptoms (for example fatigue and pain), affective oscillations, cognitive impairment to cardiovascular diseases. We also suggest that, in contrast to health behavior, iatrogenic or ‘set point’ theory models, the co-occurrence of cardio-vascular and bipolar disorders could be viewed as multi-system expressions of an inflammatory disease process. As in other medical disorders with progressive systemic involvement, defining stages of bipolar disorder will help formulate accurate screening, diagnostic and treatment algorithms. However, it is relevant to extent staging beyond psychiatric symptoms and co-morbidities to include medical co-morbidities. It is particularly important to raise awareness among clinicians that medical illnesses are not merely a consequence of a chaotic and unpredictable disorder and of long term use of psychotropic medications, but rather an early manifestation of a multi-systemic disorder. This means that medical screening and physical monitoring is not merely good clinical practice in the assessment of patients prior to the introduction of certain treatments, nor simply a vehicle for safety monitoring for maintenance treatment (Ng et al., 2009; NICE guideline, 2006), but is a critical component of the initial case assessment and has a vital role in the development of a personalized treatment program for each person presenting with bipolar disorder. Such a model is advocated in France within the FondaMental Foundation network of bipolar centers and has been adopted across Europe by the ENBREC (European Network of Bipolar Expert Centers) clinical and research colloborative group (Henry et al., 2011). Such careful assessment and documentation will both improve the clinical understanding of the links between physical and mental disorders, but opens up avenues for much-needed prospective research to understand the complex temporal relationships and shared and/or separate underlying causal mechanisms.
Acknowledgments
Role of funding source
This research was supported by “Institut National de la Santé et de la Recherche Médicale” (INSERM), the FondaMental Foundation (Fondation de Coopération Scientifique de Recherche et de Soins en Santé Mentale) and by grants from the National Institute of Mental Health MH081003, DA027508-03 and from the Agence Nationale pour la Recherche, (ANR; NEURO 2009, V.I.P. project).
We thank Kim Bauer and Kasey Dickenson who assisted us with literature search and preparation and proof-reading of the manuscript.
Footnotes
Conflict of interest
All the authors declare they have no conflict of interest with regard to this paper.
References
- Ahrens B, Müller-Oerlinghausen B, Schou M, Wolf T, Alda M, Grof E, Grof P, Lenz G, Simhandl C, Thau K, et al. Excess cardiovascular and suicide mortality of affective disorders may be reduced by lithium prophylaxis. J Affect Disord. 1995;33 (2):67–75. doi: 10.1016/0165-0327(94)00074-j. [DOI] [PubMed] [Google Scholar]
- Angst F, Stassenm HH, Clayton PJ, Angst J. Mortality of patients with mood disorders: follow up of 34–38 years. J Affect Disord. 2002;68:167–181. doi: 10.1016/s0165-0327(01)00377-9. [DOI] [PubMed] [Google Scholar]
- Antony JM, van Marle G, Opii W, Butterfield DA, Mallet F, Yong VW, Wallace JL, Deacon RM, Warren K, Power C. Human endogenous retrovirus glycoprotein-mediated induction of redox reactants causes oligodendrocyte death and demyelination. Nat Neurosci. 2004;7 (10):1088–1095. doi: 10.1038/nn1319. [DOI] [PubMed] [Google Scholar]
- Ayas NT, White DP, Manson JE, Stampfer MJ, Speizer FE, Malhotra A, Hu FB. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163 (2):205–209. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- Bauer MS, Altshuler L, Evans DR, Beresford T, Williford WO, Hauger R. Prevalence and distinct correlates of anxiety, substance, and combined comorbidity in a multi-site public sector sample with bipolar disorder. J Affect Disord. 2005;85:301–315. doi: 10.1016/j.jad.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Berk M, Hallam KT, McGorry P. The potential utility of a staging model as a course specifier: a bipolar disorder perspective. J Affect Disord. 2007;100:279–280. doi: 10.1016/j.jad.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Berk M, Kapczinski F, Andreazza AC, Dean OM, Giorlando F, Maes M, Yucel M, Gama CS, Dodd S, Dean B, Magalhaes PVS, Amminger P, McGorry P, Malhi GS. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Behav Rev. 2011;35:804–817. doi: 10.1016/j.neubiorev.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Birkenaes AB, Opjordsmoen S, Brunborg C, Engh JA, Jonsdottir H, Ringen PA, Simonsen C, Vaskinn A, Birkeland KI, Friis S, Sundet K, Andreassen OA. The level of cardiovascular risk factors in bipolar disorder equals that of schizophrenia: a comparative study. J Clin Psychiatry. 2007;68 (6):917–923. doi: 10.4088/jcp.v68n0614. [DOI] [PubMed] [Google Scholar]
- Brietzke E, Sterz L, Fernades BS, Kauer Sant’Anna M, Mascarenhas M, Escoteguy Vargas A, Chies JA, Kapzinski F. Comparison of cytokine levels in depressed, manic and euthymic patients with bipolar disorder. J Affect Disord. 2009;116:214–217. doi: 10.1016/j.jad.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Cardenas J, Frye MA, Marusak SL, Levander EM, Chirichigno JW, Lewis S, Nakelsky S, Hwang S, Mintz J, Altshuler LL. Modal subcomponents of metabolic syndrome in patients with bipolar disorder. J Affect Disord. 2008;106:91–97. doi: 10.1016/j.jad.2007.05.030. [DOI] [PubMed] [Google Scholar]
- Cassidy F, Ahearn E, Carroll BJ. Elevated frequency of diabetes mellitus in hospitalized manic-depressive patients. Am J Psychiatry. 1999;156:1417–1420. doi: 10.1176/ajp.156.9.1417. [DOI] [PubMed] [Google Scholar]
- Chang CK, Hayes RD, Perera G, Broadbent MT, Fernandes AC, Lee WE, Hotopf M, Stewart R. Life expectancy at birth for people with serious mental illness and other major disorders from a secondary mental health care case register in London. PLoS One. 2011;6 (5):e19590. doi: 10.1371/journal.pone.0019590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrado E, Rizzo M, Coppola G, Fattouch K, Novo G, Marturana I, Ferrara F, Novo S. An update on the role of markers of inflammation in atherosclerosis. J Atheroscler Thromb. 2010;17 (1):1–11. doi: 10.5551/jat.2600. [DOI] [PubMed] [Google Scholar]
- Correll CU, Frederickson AM, Kane JM, Manu P. Equally increased risk for metabolic syndrome in patients with bipolar disorder and schizophrenia treated with second-generation antipsychotics. Bipolar Disord. 2008;10:788–797. doi: 10.1111/j.1399-5618.2008.00625.x. [DOI] [PubMed] [Google Scholar]
- Correll CU. Elevated cardiovascular risk in patients with bipolar disorder: when does it start and where does it lead? J Clin Psychiatry. 2008;69 (12):1948–1952. doi: 10.4088/jcp.v69n1214. [DOI] [PubMed] [Google Scholar]
- Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10:63–74. doi: 10.1016/S1474-4422(10)70253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson FB, Boronow JJ, Stallings C, Origoni AE, Cole S, Krivogorsky B, Yolken RH. Infection with herpes simplex virus type 1 is associated with cognitive deficits in bipolar disorder. Biol Psychiatry. 2004;55 (6):588–593. doi: 10.1016/j.biopsych.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Dickerson F, Stallings C, Origoni A, Boronow J, Yolken RH. Elevated serum levels of C-reactive protein are associated with mania symptoms in outpatients with bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31 (4):952–955. doi: 10.1016/j.pnpbp.2007.02.018. [DOI] [PubMed] [Google Scholar]
- Dickerson F, Stallings C, Origoni A, Vaughan C, Khushalani S, Alaedini A, Yolken R. Markers of gluten sensitivity and celiac disease in bipolar disorder. Bipolar Disord. 2011;13:52–58. doi: 10.1111/j.1399-5618.2011.00894.x. [DOI] [PubMed] [Google Scholar]
- Drake C, Boutin H, Jones MS, Denes A, McColl BW, Selvarajah JR, Hulme S, Georgiou R, Hinz R, Gerhard A, Vail A, Prenant C, Julyan P, Maroy R, Brown G, Smigova A, Herholz K, Kassiou M, Crossman D, Francis S, Proctor S, Russel JC, Hookins SL, Tvrrell P, Rothweil N, Alllan S. Brain inflammation is induced by co-morbidities and risk factor for stroke. Brain Behav Immun. 2011;25:1113–1122. doi: 10.1016/j.bbi.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta R, Boydell J, Kennedy N, VAN Os J, Fearon P, Murray RM. Suicide and other causes of mortality in bipolar disorder: a longitudinal study. Psychol Med. 2007 Jun;37(6):839–847. doi: 10.1017/S0033291707000347. [DOI] [PubMed] [Google Scholar]
- Eaton WW, Pedersen MG, Nielsen PR, Mortensen PB. Autoimmune diseases, bipolar disorder, and non-affective psychosis. Bipolar Disord. 2010;12 (6):638–646. doi: 10.1111/j.1399-5618.2010.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etain B, Heny C, Bellivier F, Mathieu F, Leboyer M. Beyond genetics: childhood affective trauma in bipolar disorder. Bipolar Disord. 2008;10:867–876. doi: 10.1111/j.1399-5618.2008.00635.x. [DOI] [PubMed] [Google Scholar]
- Evans-Lacko S, Zeber J, Gonzales J, Olvera R. Medical comorbidity among youth diagnoses with bipolar disorder in the United States. J Clin Psychiatry. 2009;70 (10):1461–1466. doi: 10.4088/JCP.08m04871. [DOI] [PubMed] [Google Scholar]
- Fagiolini A, Frank E, Scott JA, Turkin S, Kupfer DJ. Metabolic syndrome in bipolar disorder: findings from the Bipolar Disorder Center for Pennsylvanians. Bipolar Disord. 2005;5:424–430. doi: 10.1111/j.1399-5618.2005.00234.x. [DOI] [PubMed] [Google Scholar]
- Fava GA, Kellner R. Staging: a neglected dimension in psychiatric classification. Acta Psychiatr Scand. 1993;87:225–230. doi: 10.1111/j.1600-0447.1993.tb03362.x. [DOI] [PubMed] [Google Scholar]
- Fernandes BS, Gama CS, Walz JC, Cerser KM, Fries GR, Colpo G, Aguiar B, Pfasffenseller B, Kauer-Sant’Anna M, Kapczinski F. Increased neurotrophin-3 in drug free subjects with bipolar disorder during manic and depressive episodes. J Psychiatr Res. 2010;44 (9):561–565. doi: 10.1016/j.jpsychires.2009.11.020. [DOI] [PubMed] [Google Scholar]
- Fiedorowicz JG, Palagummi NM, Forman-Hoffman VL, Miller DD, Haynes WG. Elevated prevalence of obesity, metabolic syndrome, and cardiovascular risk factors in bipolar disorder. Ann Clin Psychiatry. 2008;20:131–137. doi: 10.1080/10401230802177722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedorowicz JG, Solomon DA, Endicott J, Leon AC, Li C, Rice JP, Coryell WH. Manic/hypomanic symptom burden and cardiovascular mortality in bipolar disorder. Psychosom Med. 2009;71 (6):598–606. doi: 10.1097/PSY.0b013e3181acee26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasure-Smith N, Lespérance F. Recent evidence linking coronary heart disease and depression. Can J Psychiatry. 2006;51:730–737. doi: 10.1177/070674370605101202. [DOI] [PubMed] [Google Scholar]
- Garcia-Portilla MP, Saiz PA, Bascaran MT, Martínez AS, Benabarre A, Sierra P, Torres P, Montes JM, Bousoño M, Bobes J. Cardiovascular risk in patients with bipolar disorder. J Affect Disord. 2009;115 (3):302–308. doi: 10.1016/j.jad.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Gildengers AG, Whyte EM, Drayer RA, Soreca I, Fagiolini A, Kilbourne AM, Houck PR, Reynolds CF, III, Frank E, Kupfer DJ, Mulsant BH. Medical burden in late-life bipolar and major depressive disorders. Am J Geriatr Psychiatry. 2008;16 (3):194–200. doi: 10.1097/JGP.0b013e318157c5b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein B, Birmaher B, Axelson D, Goldstein T, Esposito-Smythers C, Strobre M, Hunt J, Leonard H, Gill M, Lyengar S, Grimm C, Yang M, Ryan N, Keller M. Preliminary findings regarding overweight and obesity in pediatric bipolar disorder. J Clin Psychiatry. 2008;69 (12):1953–1959. doi: 10.4088/jcp.v69n1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BI, Kemp DE, Soczynska JK, McIntyre RS. Inflammation and the phenomenology, pathophysiology, comorbidity, and treatment of bipolar disorder. J Clin Psychiatry. 2009;70 (8):1078–1090. doi: 10.4088/JCP.08r04505. [DOI] [PubMed] [Google Scholar]
- Griffiths DJ. Endogenous retroviruses in the human genome sequence. Genome Biol. 2001;2 (6):1017. doi: 10.1186/gb-2001-2-6-reviews1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, Foster E, Hlatky MA, Hodgson JM, Kushner FG, Lauer MS, Shaw LJ, Smith SC, Jr, Taylor AJ, Weintraub WS, Wenger NK, Jacobs AK, Smith SC, Jr, Anderson JL, Albert N, Buller CE, Creager MA, Ettinger SM, Guyton RA, Halperin JL, Hochman JS, Griffiths DJ. Endogenous retroviruses in the human genome sequence. Genome Biol. 2001;2(6):1017–1017.5. doi: 10.1186/gb-2001-2-6-reviews1017. reviews. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdani N, Tamouza R, Leboyer M. Immuno-inflammatory markers of bipolar disorder: a review of evidence. Front Biosci. 2012;4:2170–2182. doi: 10.2741/e534. (Elite Ed) [DOI] [PubMed] [Google Scholar]
- Harvey AG. Sleep and circadian rhythms in bipolar disorder: seeking synchrony, harmony, and regulation. Am J Psychiatry. 2008;165 (7):820–829. doi: 10.1176/appi.ajp.2008.08010098. [DOI] [PubMed] [Google Scholar]
- Henry C, Etain B, Mathieu F, Raust A, Vibert JF, Scott J, Leboyer M. A French network of bipolar expert centres: a model to close the gap between evidence-based medicine and routine practice. J Affect Disord. 2011;(1–3):358–363. doi: 10.1016/j.jad.2010.11.013. [DOI] [PubMed] [Google Scholar]
- Janelidze S, Mattei D, Westrin A, Träksman-Bendz L, Brundin L. Cytokine levels in the blood may distinguish suicide attempters from depressed patients. Brain, behavior and immunity. 2011;25:335–339. doi: 10.1016/j.bbi.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Jerrell J, McIntyre R, Tripathi A. A cohort study of the prevalence and impact of comorbid medical conditions in pediatric bipolar disorder. J Clin Psychiatry. 2010;11:1518–1525. doi: 10.4088/JCP.09m05585ora. [DOI] [PubMed] [Google Scholar]
- Johannessen L, Strudsholm U, Foldager L, Munk-Jørgensen P. Increased risk of hypertension in patients with bipolar disorder and patients with anxiety compared to background population and patients with schizophrenia. J Affect Disord. 2006;95:13–17. doi: 10.1016/j.jad.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Kapczinski F, Videira Dias V, Kauer Sant’Anna M, Noronha Frey B, Grassi-Oliveira R, Colom F, Berk M. Clinical implication of a staging model for bipolar disorders. Expert Rev Neurother. 2009;7:957–966. doi: 10.1586/ern.09.31. [DOI] [PubMed] [Google Scholar]
- Kilbourne AM, Cornelius JR, Han X, Pincus HA, Shad M, Salloum I, Conigliaro J, Haas GL. Burden of general medical conditions among individuals with bipolar disorder. Bipolar Disord. 2004;6:368–373. doi: 10.1111/j.1399-5618.2004.00138.x. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ. The increasing medical burden in medical disorder. JAMA. 2005;20:2528–2530. doi: 10.1001/jama.293.20.2528. [DOI] [PubMed] [Google Scholar]
- Laaksonen D, Lakka HM, Niskanen L, Kaplan G, Salonen J, Lakka T. Metabolic syndrome and development of diabetes mellitus: application and validation of recently suggested definitions of the metabolic syndrome in a prospective cohort study. Am J Epidemiol. 2002;156:1070–1077. doi: 10.1093/aje/kwf145. [DOI] [PubMed] [Google Scholar]
- Laan W, Grobbee DE, Selten J-P, Heijnen CJ, Kahn RS, Burger H. Adjuvant aspirin therapy reduces symptoms of schizophrenia spectrum disorders: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2010;71 (5):520–527. doi: 10.4088/JCP.09m05117yel. [DOI] [PubMed] [Google Scholar]
- Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- Lange T, Dimitrov S, Born J. Effects of sleep and circardian rhythm on the human immune system. Ann NY Acad Sci. 2010;1193:48–59. doi: 10.1111/j.1749-6632.2009.05300.x. [DOI] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhnader S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: a population-based prevalence study. JAMA. 2000;284:2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Laursen TM. Life expectancy among persons with schizophrenia or bipolar affective disorder. Schizophr Res. 2011;131 (1–3):101–104. doi: 10.1016/j.schres.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Leonard BE. The concept of depression as a dysfunction of the immune system. Curr Immunol Rev. 2010;3:205–212. doi: 10.2174/157339510791823835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist D, Janelidze S, Hagell P, Erhardt S, Samuelsson M, Minthon L, Hansson O, Björqvist M, Traskman-Bendz L, Brundin L. Inter-leukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol Psychiatry. 2009;66:287–292. doi: 10.1016/j.biopsych.2009.01.030. [DOI] [PubMed] [Google Scholar]
- Lorgis L, Amoureux S, de Maistre E, Sicard P, Bejot Y, Zeller M, Vergely C, Sequeira-Le-Grand A, Lagrost AC, Nerchoud J, Cottin Y, Rohcette L. Serum brain derived neurotrophic facrot and platelet activation evaluated by soluble-P-selectin and solubre CD-40-ligand in patients with acute myocardial infarction. Fundam Clin Pharmacol. 2010;4:525–530. doi: 10.1111/j.1472-8206.2009.00790.x. [DOI] [PubMed] [Google Scholar]
- Maes M, Ruckoanich P, Chang YS, Mahanonda N, Berk M. Multiple aberrations in shared inflammatory and oxidative & nitrosative stress (IO&NS) pathways explain the co-association of depression and cardiovascular disorder (CVD) and the increased risk for CVD and due mortality in depressed patients. Prog Neuro Psychopharmacol Biol Psychiatry. 2011;35:769–783. doi: 10.1016/j.pnpbp.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Maina G, Salvi V, Vitalucci A, D’Ambrosio V, Bogetto F. Prevalence and correlates of overweight in drug-naïve patients with bipolar disorder. J Affect Disord. 2008;110 (1–2):149–155. doi: 10.1016/j.jad.2007.12.233. [DOI] [PubMed] [Google Scholar]
- McCaffery JM, Frasure-Smith N, Dube MP, Théroux P, Rouleau G, Duan Q, Lesperance F. Common genetic vulnerability to depressive symptoms and coronary artery disease: a review and development of candidate genes related to inflammation and serotonin. Psychosom Med. 2006;68:187–200. doi: 10.1097/01.psy.0000208630.79271.a0. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Frye MA, Suppes T, Dhavale D, Keck PE, Jr, Leverich GS, Altshuler L, Denicoff KD, Nolen WA, Kupka R, Grunze H, Walden J, Post RM. Correlates of overweight and obesity in 644 patients with bipolar disorder. J Clin Psychiatry. 2002;63:207–213. doi: 10.4088/jcp.v63n0306. [DOI] [PubMed] [Google Scholar]
- McGorry P, Nelson B, Goldstone S, Yung A. Clinical staging: a heuristic and practical strategy for new research and better health and social outcomes for psychotic and related mood disorders. Can J Psychiatry. 2010;55 (8):486–497. doi: 10.1177/070674371005500803. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Danilewiz M, Liauw S, Kemp D, Nguyen H, Kahn L, Kucyi A, Soczynska J, Woldeyohannes H, Lachowski A, Kim B, Nathanson J, Alsuwaidan M, Taylor V. Bipolar disorder and metabolic syndrome: an international perspective. J Affect Disord. 2010;126:366–387. doi: 10.1016/j.jad.2010.04.012. [DOI] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009 May 1;65(9):732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morón JA, Zakharova I, Ferrer JV, Merrill GA, Hope B, Lafer EM, Lin ZC, Wang JB, Javitch JA, Galli A, Shippenberg TS. Mitogen activated protein kinase regulates dopamine transporter surface expression and dopamine transport capacity. J Neurosci. 2003;23:8480–8488. doi: 10.1523/JNEUROSCI.23-24-08480.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller N, Schwartz MJ. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol Psychiatry. 2007;12:988–1000. doi: 10.1038/sj.mp.4002006. [DOI] [PubMed] [Google Scholar]
- Müller-Oerlinghausen B, Berghöfer A, Ahrens B. The antisuicidal and mortality-reducing effect of lithium prophylaxis: consequences for guidelines in clinical psychiatry. Can J Psychiatry. 2003;48 (7):433–439. doi: 10.1177/070674370304800702. [DOI] [PubMed] [Google Scholar]
- Müller-Oerlinghausen B, Felber W, Berghöfer A, Lauterbach E, Ahrens B. The impact of lithium long-term medication on suicidal behavior and mortality of bipolar patients. Arch Suicide Res. 2005;9 (3):307–319. doi: 10.1080/13811110590929550. [DOI] [PubMed] [Google Scholar]
- Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cadiovascular, inflammatory and metabolic consequences of sleep deprivation. Progr Cardiovasc Dis. 2009;51:294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray DP, Weiner M, Prabhakar M, Fiedorowicz JG. Mania and mortality: why the excess cardiovascular risk in bipolar disorder? 2009;11(6):475–480. doi: 10.1007/s11920-009-0072-3. [DOI] [PubMed] [Google Scholar]
- Mynt AM, Kim YK, Yerkerk R, Sharpe S, Steinbusch H, Leonard B. Kynurenine pathway in major depression: evidence of impaired neuroprotection. J Affect Disord. 2007;98 (1–2):143–151. doi: 10.1016/j.jad.2006.07.013. [DOI] [PubMed] [Google Scholar]
- NCEP. Executive summary: the Third Report of The National Cholesterol Education Program. Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- Newcomer JW. Comparing the safety and efficacy of atypical antipsychotics in psychiatric patients with comorbid medical illnesses. J Clin Psychiatry. 2009;70 (3):30–36. doi: 10.4088/JCP.7075su1c.05. [DOI] [PubMed] [Google Scholar]
- Ng F, Mammen OK, Wilting I, Sachs GS, Ferrier IN, Cassidy F, Beaulieu S, Yatham LN, Berk M. International Society for Bipolar Disorders. The International Society for Bipolar Disorders (ISBD) consensus guidelines for the safety monitoring of bipolar disorder treatments. Bipolar Disord. 2009;11 (6):559–595. doi: 10.1111/j.1399-5618.2009.00737.x. [DOI] [PubMed] [Google Scholar]
- NICE Guideline Development Group. The Management of Bipolar Disorder in Adults, Children and Adolescents in Primary and Secondary Care. National Collaborating Centre for Mental Health; UK: 2006. [PubMed] [Google Scholar]
- Novick DM, Swartz HA, Frank E. Suicide attempts in bipolar I and bipolar II disorder: a review and meta-analysis of the evidence. Bipolar Disord. 2010 Feb;12(1):1–9. doi: 10.1111/j.1399-5618.2009.00786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohaeri JU, Akani DP. Metabolic syndrome in severe mental disorders. Metab Snyd Relat Disord. 2011;9 (2):91–98. doi: 10.1089/met.2010.0053. [DOI] [PubMed] [Google Scholar]
- Osby U, Brandt L, Correia N, Ekbom A, Sparén P. Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry. 2001;58:844–850. doi: 10.1001/archpsyc.58.9.844. [DOI] [PubMed] [Google Scholar]
- Padmos RC, Hillergers MH, Knijff EM, Vonk R, Bouvy A, Stall F, de Ridder DD, Kupka RW, Noeln WA, Drexhage HA. A discriminating messenger RNA signature for bipolar disorder formed by aberrant expression of inflammatory genes in monocytes. Arch Gen Psychiatry. 2008;65 (4):395–407. doi: 10.1001/archpsyc.65.4.395. [DOI] [PubMed] [Google Scholar]
- Parsons B, Alllison D, Loebel A, Williams K, Giller E, Romano S, Siu C. Weight effects associated with antipsychotics: a comprehensive database analysis. Schizophr Res. 2009;120:103–110. doi: 10.1016/j.schres.2008.09.025. [DOI] [PubMed] [Google Scholar]
- Pasco JA, Nicholson GC, Williams LJ, Jacka FN, Henry MJ, Kotowicz MA, Schneider HG, Leonard BE, Berk M. Association of high-sensitivity C-reactive protein with de novo major depression. Br J Psychiatry. 2010;197 (5):372–377. doi: 10.1192/bjp.bp.109.076430. [DOI] [PubMed] [Google Scholar]
- Perris C, d’Ellia G. A study of bipolar (manic-depressive) and unipolar recurrent depressive psychoses. Acta Psychiatrica Scand. 1996;164:172–189. [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raphael T, Parsons JP. Blood sugar studies in dementia praecox and manic-depressive insanity. Arch Neurol Psychiatry. 1921;5:687–709. [Google Scholar]
- Roshanaei-Moghaddam B, Katon W. Premature mortality from general medical illnesses among persons with bipolar disorder: a review. Psychiatr Serv. 2009;60 (2):147–156. doi: 10.1176/ps.2009.60.2.147. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis is an inflammatory disease. Am Heart J. 1999;138:S419–S420. doi: 10.1016/s0002-8703(99)70266-8. [DOI] [PubMed] [Google Scholar]
- Rutten BP, Mill J. Epigenetic mediation of environmental influences in major psychotic disorders. Schizophr Bull. 2009;35 (6):1045–1056. doi: 10.1093/schbul/sbp104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi V, D’Ambrosio V, Rosso G, Bogetto F, Maina G. Age-specific prevalence of metabolic syndrome in Italian patients with bipolar disorder. Psychiatry Clin Neurosci. 2011;65:47–54. doi: 10.1111/j.1440-1819.2010.02160.x. [DOI] [PubMed] [Google Scholar]
- Scott J, McNeill Y, Cavanagh J, Cannon M, Murray R. Exposure to obstetric complications and subsequent development of bipolar disorder: systematic review. Br J Psychiatry. 2006;189:3–11. doi: 10.1192/bjp.bp.105.010579. [DOI] [PubMed] [Google Scholar]
- Severance EG, Dickerson FB, Halling M, Krivogorsky B, Haile L, Yang S, Stallings CR, Origoni AE, Bossis I, Xiao J, Dupont D, Haasnoot W, Yolken RH. Subunit and whole molecule specificity of the anti-bovine casein immune response in recent onset psychosis and schizophrenia. Schizophr Res. 2011;118 (1–3):240–247. doi: 10.1016/j.schres.2009.12.030. [DOI] [PubMed] [Google Scholar]
- Soreca I, Fagiolini A, Frank E, Houck PR, Thompson WK, Kupfer DJ. Relationship of general medical burden, duration of illness, and age in patients with bipolar I disorder. J Psychiatr Res. 2008;42 (11):956–961. doi: 10.1016/j.jpsychires.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Sutkowski N, Conrad B, Thorley-Lawson DA, Huber BT. Epstein–Barr virus transactivates the human endogenous retrovirus HERV-K18 that encodes a superantigen. Immunity. 2001;15 (4):579–589. doi: 10.1016/s1074-7613(01)00210-2. [DOI] [PubMed] [Google Scholar]
- Tsai SY, Kuo CJ, Chung KH, Huang YL, Lee HC, Chen CC. Cognitive dysfunction and medical morbidity in elderly outpatients with bipolar disorder. Am J Geriatr Psychiatry. 2009;17 (12):1004–1011. doi: 10.1097/JGP.0b013e3181b7ef2a. [DOI] [PubMed] [Google Scholar]
- Tonelli LH, Stiller J, Rujescu D, Giegling I, Schneider B, Maurer K, Schnabel A, Moller HJ, Chen HH, Postolache TT. Elevated cytokine expression in the orbitofrontal cortex of victims of suicide. Acta Psychiatr Scand. 2008;117:198–206. doi: 10.1111/j.1600-0447.2007.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SY, Lee CH, Kuo CJ, Chen CC. A retrospective analysis of risk and protective factors for natural death in bipolar disorder. J Clin Psychiatry. 2005;66:1586–1591. doi: 10.4088/jcp.v66n1215. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Woolson RF, Fleming JA. Premature deaths in schizophrenia and affective disorders. An analysis of survival curves and variables affecting the shortened survival. Arch Gen Psychiatry. 1980;37 (9):979–983. doi: 10.1001/archpsyc.1980.01780220017001. [DOI] [PubMed] [Google Scholar]
- Vieta E, Reinares M, Rosa AR. Staging bipolar disorder. Neurotox Res. 2011;19:279–285. doi: 10.1007/s12640-010-9197-8. [DOI] [PubMed] [Google Scholar]
- Vonk R, van der Schot AC, Kahn RS, Nolen WA, Drexhage HA. Is autoimmune thyroiditis part of the genetic vulnerability (or an endo-phenotype) for bipolar disorder? Biol Psychiatry. 2007;62 (2):135–140. doi: 10.1016/j.biopsych.2006.08.041. [DOI] [PubMed] [Google Scholar]
- Wager-Smith K, Markou A. Depression: a repair response to stress-induced neuronal microdamage that can grade into a chronic neuroinflammatory condition? Neurosci Biobehav Rev. 2011;35:742–764. doi: 10.1016/j.neubiorev.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeke A, Juel K, Vaeth M. Cardiovascular death and manic-depressive psychosis. J Affect Disord. 1987;13:287–292. doi: 10.1016/0165-0327(87)90049-8. [DOI] [PubMed] [Google Scholar]
- Weiner M, Warren L, Fiedorowicz JG. Cardiovascular morbidity and mortality in bipolar disorder. Ann Clin Psychiatry. 2011;23 (1):40–47. [PMC free article] [PubMed] [Google Scholar]
- Weis S, Llenos IC, Sabunciyan S, Dulay JR, Isler L, Yolken R, Perron H. Reduced expression of human endogenous retrovirus (HERV)-W GAG protein in the cingulate gyrus and hippocampus in schizophrenia, bipolar disorder, and depression. J Neural Transm. 2007;114 (5):645–655. doi: 10.1007/s00702-006-0599-y. [DOI] [PubMed] [Google Scholar]
- Williams MD, Shah ND, Wagie AE, Wood DL, Frye MA. Direct costs of bipolar disorder versus other chronic conditions: an employer-based health plan analysis. Psychiatr Serv. 2011;62 (9):1073–1078. doi: 10.1176/ps.62.9.pss6209_1073. [DOI] [PubMed] [Google Scholar]
- World Health Organization. The Global Burden of Disease. 2008. [Google Scholar]
- Yates WR, Wallace R. Cardiovascular risk factors in affective disorder. J Affect Disord. 1987;12:129–134. doi: 10.1016/0165-0327(87)90004-8. [DOI] [PubMed] [Google Scholar]
- Zhu CB, Blakely RD, Hewlett WA. The proinflammatory cytokines IL-1 beta and TNF-alpha activates serotonin transporters. Neuropsychopharmacology. 2006;31:2121–2131. doi: 10.1038/sj.npp.1301029. [DOI] [PubMed] [Google Scholar]