Abstract
This Letter describes the hit-to-lead progression and SAR of a series of biphenyl acetylene compounds derived from an HTS screening campaign targeting the mGlu5 receptor. ‘Molecular switches’ were identified that modulated modes of pharmacology, and several compounds within this series were shown to be efficacious in reversal of amphetamine induced hyperlocomotion in rats after i.p. dosing, a preclinical model that shows similar positive effects with known antipsychotic agents.
The NMDA receptor hypofunction hypothesis is generally the favored pathophysiological model for the disease mechanism for schizophrenia.1,2 As a result, multiple approaches to enhance the glutamate/NMDA system continue to be pursued as a means to ameliorate the major symptom dimensions of the disease.1,2 Development of positive allosteric modulators (PAMs) of the group I metabotropic glutamate receptor mGlu5 continue to offer promise as one such approach.3-6 After nearly a decade since the identification of the first mGlu5 PAMs DFB (1),7 CPPHA (2),8,9 and CDPPB (3),10,11 several chemically distinct series have recently emerged including ADX47273 (4),12-14 a series of MPEP-based pyrimidines (5)15,16 and nicotinamide (6),17-18 alkoxy biphenyl amides (7)19 and a chemically distinct series of piperazines (8).20-21 These second generation PAMs offer several physiochemical advantages (e.g. solubility, unbound free fraction in biological relevant matrices) over the first generation PAMs (Fig. 1).
Figure 1.
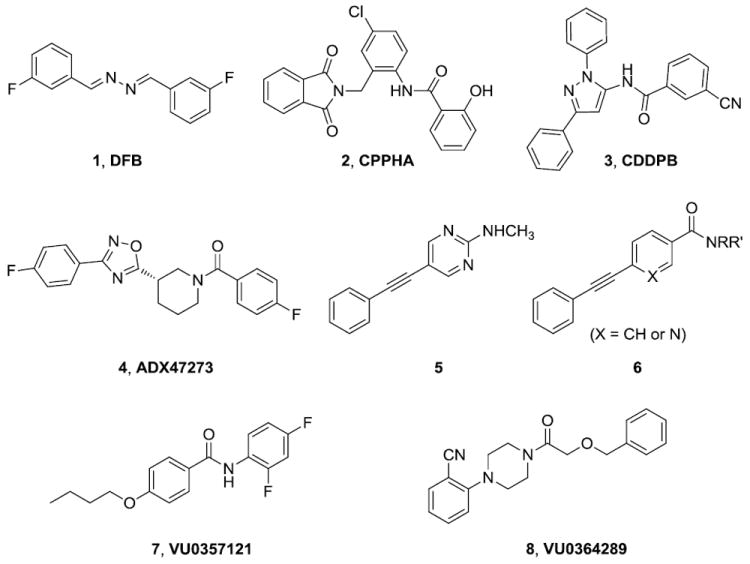
Prototype and recent mGlu5 positive allosteric modulators.
Utilizing an in-house developed triple-add functional calcium mobilization assay, we identified multiple modulators of mGlu5, including- agonists, antagonists and potentiators.17 This effort resulted in 1400 confirmed PAMs, including 63 with potency below 500 nM.17 Amongst these potent lead structures several, including VU0092273, were found to contain a biphenyl acetylene moiety with exceptional ligand efficiency (LE) approaching 0.5 (Fig. 2).17
Figure 2.
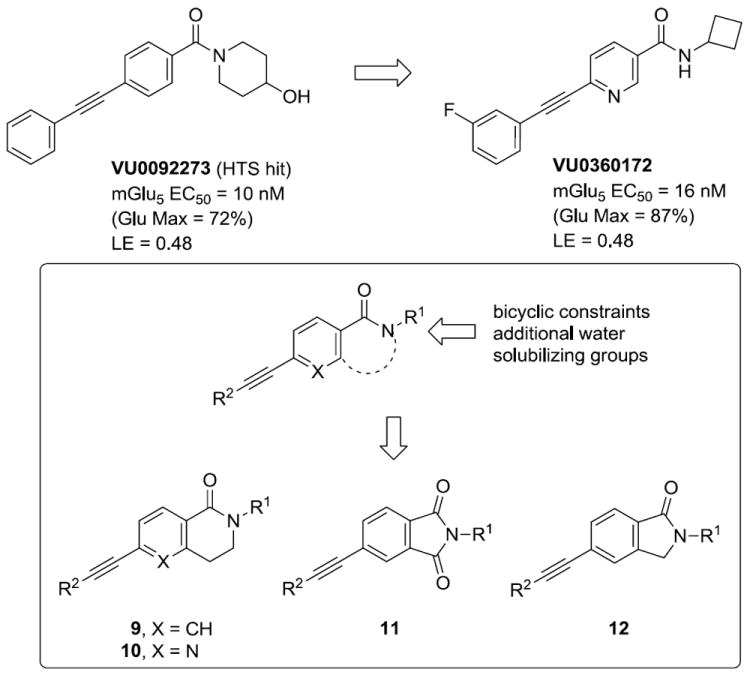
Nicotinyl amide and proposed bicyclic constraints 9-12.
An optimization campaign around VU0092273 followed, focusing on incorporation of water solubilizing groups, leading to VU0360172, the first orally active mGlu5 PAM to be efficacious in an in vivo preclinical antipsychotic model.17 In parallel to these studies, we investigated structurally constrained analogs of these phenyl and nicotinyl amides in order to understand their general activity as PAMs and also as a strategy to mitigate potential amidase activity that might metabolize amides similar to VU0092273 and VU0360172.17 In this Letter we describe the synthesis, SAR, and in vivo behavioral profile for three chemically distinct bicyclic scaffolds.22
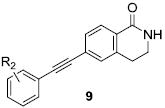
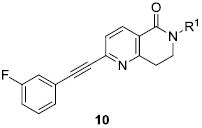
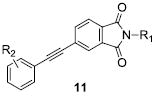
We envisioned initially exploring cyclic constraints of the linear amide scaffolds, VU0092273 and VU0360172, including dihydroisoquinolinones (9), dihydronaphthyridinones (10), phthalimides (11) and isoindolinones (12) (Fig. 2) in conjunction with evaluating alternate caps (R1) of the lactam NH and aryl moieties (R2). Synthetically all analogs were prepared from the parent bicylic scaffolds via a Sonagashira coupling reaction using the appropriate halogen precursors 13 and functionalized phenyl acetylene monomers (Scheme 1). N-alkyl congeners were subsequently prepared using standard alkylation conditions as shown.
Scheme 1.
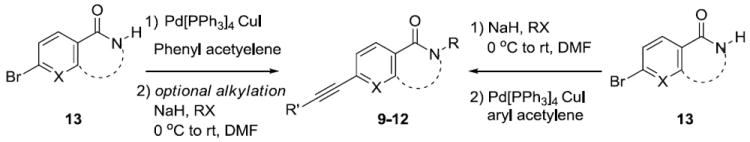
General routes utilized to prepare 9-12.
Initially, we prepared a library of dihydroisoquinolinones (9), wherein an unsubstituted phenyl moiety was held constant for R2, and the lactam NH was substituted with various R1 moieties. This bicyclic constraint proved to be highly beneficial, affording potent and efficacious PAMs and ago-PAMs, defined as PAMs with varying degrees of apparent agonist activity (Table 1). The NH congener 9a proved to be the most potent and efficacious (EC50 = 50 nM, 112% Glu Max, 10.8-fold shift) ago-PAM in this small library.
Table 1.
Structures and activities of bicyclic lactam mGlu5 PAMs 9a-i.
| ||||
|---|---|---|---|---|
| Cmpd | R1 | mGlu5 EC50 (nM)a | % Glu Maxb | Category |
| 9a | H | 50 | 112 | Ago-PAM |
| 9b | CH3 | 250 | 53 | PAM |
| 9c | n-Pr | 160 | 97 | Ago-PAM |
| 9d | n-Bu | 96 | 97 | Ago-PAM |
| 9e | Bn | 550 | 90 | Ago-PAM |
| 9f |
|
180 | 69 | Ago-PAM |
| 9g |
|
270 | 91 | Ago-PAM |
| 9h |
|
4,000 | 77 | PAM |
| 9i |
|
3,600 | 84 | PAM |
EC50s are the average of three determinations and are reproducible with a coefficient of variation of 0.3.
Determined at 30 μM test compound wherein %max vehicle is 10-30%.
Functionalization of the lactam NH afforded a number of potent PAMs and ago-PAMs with EC50s in the 96-600 nM range with good maximal responses (53-97% of the response elicited by a maximal concentration of glutamate, herein described as % GluMax) and leftward fold-shifts of a full glutamate concentration-response curve (5.7 - to 11.8x). Encouraged by the in vitro profile, several dihydroisoquinolinones (9) were scaled-up and evaluated in our tier one, single-point pharmacodynamic assay, amphetamine-induced hyperlocomotion (AHL), a standard preclinical assay predictive of antipsychotic acitivity.10,11 Compounds 9a, 9c and 9f all displayed significant reversal of amphetamine-induced hyperlocomotion at 30 mg/kg i.p., and Figure 3 shows representative data obtained with 9c, the N-propyl derivative.
Figure 3.
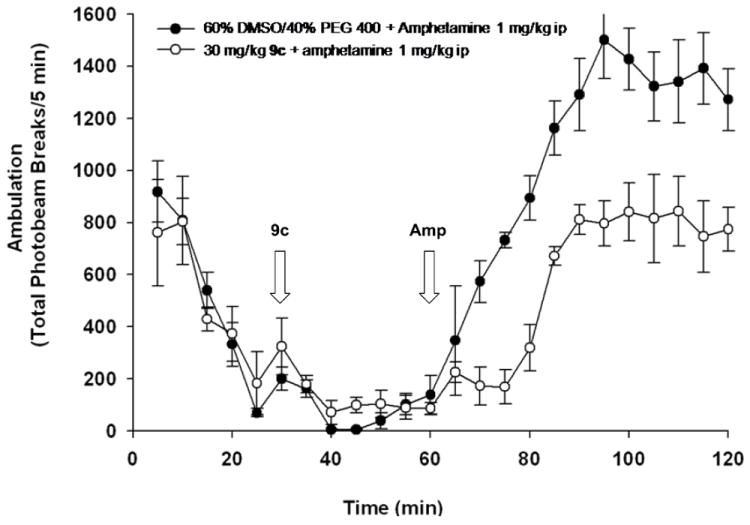
Reversal of amphetamine-induced hyperlocomotion with bicyclic Glu5 PAMs 9c at a dose of 30 mg/kg ip.
Based on these promising data, we then evaluated a library of analogs wherein we held the NH constant on the lactam, as in 9a, and surveyed a diverse group of functionalized aryl moieties in the R2 position (Table 2). Here, all analogs lose potency relative to 9a, but still afford potent PAMs and ago-PAMs such as the ortho-F congener 9m (EC50 = 150 nM, 80% Glu Max) and the meta-CH3 derivative 9k (EC50 = 170 nM, 76% Glu Max). Substituents in the para-position, such as para-OCH3 (9n) or para-Cl (9r) resulted in inactive compounds (mGlu5 EC50 >10 μM) whereas the smaller fluorine congener 9l afforded a 260 nM PAM. In our acyclic series, represented by VU0092273 and VU0360172,17 the meta-F derivative proved optimal, and thus far, the meta-position appeared the most amenable to substitution and afforded favorable DMPK disposition.17
Table 2.
Pendant Aryl SAR of Bicyclic PAMs 9i-t.
| ||||
|---|---|---|---|---|
| Cmpd | R2 | mGlu5 EC50 (nM)a | % Glu Maxb | Category |
| 9j | o-CH3 | 610 | 53 | PAM |
| 9k | m-CH3 | 170 | 76 | Ago-PA |
| 9l | p-F | 260 | 81 | PAM |
| 9m | o-F | 150 | 80 | Ago-PA |
| 9n | p-CH3O | >10,000 | ND | ND |
| 9o | m-CH3O | 3,400 | 58 | PAM |
| 9p | m-CH3, p-F | 4,900 | 53 | PAM |
| 9q | o-CI | 3,700 | 50 | PAM |
| 9r | p-CI | >10,000 | ND | ND |
| 9s | m-CI | 470 | 68 | PAM |
| 9t | p-F, o-F | 850 | 59 | PAM |
EC50s are the average of three determinations and are reproducible with a coefficient of variation of 0.3.
Determined at 30 μM test compound wherein %max vehicle is 10-30%.
While many of these novel PAMs were active in AHL, they required a DMSO-containing vehicle. Thus, future analogs were designed to incorporate a basic amine to improve physiochemical properties and enable salt formation to enable non-toxic vehicle formulations. Therefore, we elected to prepare a small library of dihydronaphthyridinones (10) wherein R2 was meta-F phenyl and R1 was varied (Table 3). Surprisingly, this effort uncovered an apparent ‘molecular switch.14-16 that modulated the mode of pharmacology – a first in this class of mGlu5 PAMs.22 Again PAMs, 10a, 10c, 10d and 10f, all lost potency and significant efficacy relative to 9a; however, the N-CH3 (10b) and N-cyclopropylmethyl (10e) congeners proved to be partial antagonists with IC50s of 170 nM and 660 nM, respectively. Thus, with slight structural changes, a reasonably potent PAM 10a, with a free NH, can be converted into a partial antagonist (10b) of comparable potency via N-methylation. These data once again highlight the challenges in developing SAR for MPEP-site allosteric ligands.
Table 3.
Structures and activities of dihydronaphthyridinones 10.
| ||||
|---|---|---|---|---|
| Cmpd | R1 | mGlu5 EC50 (nM)/IC50a | % Glu Maxb | Category |
| 10a | H | 290 | 72 | PAM |
| 10b | CH3 | 170 | 34 | Partial Antag |
| 10c | i-Bu | 54 | 40 | Weak PAM |
| 10d | n-Bu | 56 | 46 | Weak PAM |
| 10e |
|
660 | 34 | Partial Antag |
| 10f |
|
130 | 53 | PAM |
EC50s and IC50s are the average of three determinations and are reproducible with a coefficient of variation of 0.3.
Glu Max is relative to vehicle/EC20 (PAM) or vehicle/EC80 glutamate (antagonist) window.
In parallel, we were exploring the impact of constriction of the 6-membered lactam ring to the corresponding 5-membered homologs generating two-dimensional libraries of phthalimides (11) and isoindolinones (12). The chemistry to access these analogs was the same as that shown in Scheme 1, and allowed for diversity at both R1 and R2. As shown in Table 4, the library of phthalimides 11 was highly productive, affording PAMs with EC50s ranging from 5.9 nM to 5.7 μM. Compound 11a, wherein both R1 and R2 are H, represents the most potent mGlu5 PAM reported to date (EC50 = 5.9 nM, 104% Glu Max), and 11h, the ortho-fluorophenyl congener is comparable in potency (EC50 = 12 nM, 93% Glu Max). The introduction of a basic amine, as in 11b-11d, was unproductive, leading to 170- to >1,000-fold loss in potency relative to 11a. In addition, the meta-fluorophenyl derivative 11e was again potent (EC50 = 35 nM, 99% Glu Max), but in this series, 11f and 11g were also reasonable mGlu5 PAMs.
Table 4.
Structures and activities of phthalimides 11.
| |||||
|---|---|---|---|---|---|
| Cmpd | R1 | R2 | mGlu5 EC50 (nM)a | % Glu Maxb | Category |
| 11a | H | H | 5.9 | 104 | Ago-PAM |
| 11b |
|
H | 870 | 40 | PAM |
| 11c |
|
H | 900 | 76 | PAM |
| 11d |
|
H | 5,700 | 44 | pAM |
| 11e | H | m-F | 35 | 99 | Ago-PAM |
| 11f | H | m-F, p-F | 170 | 84 | PAM |
| 11g | H | p-F | 160 | 88 | PAM |
| 11h | H | o-F | 12 | 93 | Ago-PAM |
EC50s are the average of three determinations and are reproducible with a coefficient of variation of 0.3.
Determined at 30 μM test compound wherein %max vehicle is 10-30%.
Finally, we deleted one carbonyl of the phthalimide 11 to generate a small set of isoindolinone analogs 12. This modification also proved productive, affording mGlu5 PAMs in the 50 nM to 350 nM range (Table 5), but with diminished efficacy relative to phthalimide analogs 11. As anticipated, the unfunctionalized congener 12a (EC50 = 51 nM, 69% Glu Max) and the meta-fluorophenyl analog 12c (EC50 = 66 nM, 71% Glu Max) proved to be the best in this series. However, unlike the 6-membered lactam series, both the ortho- and para-fluorophenyl derivative were reasonable mGlu5 PAMs (EC50s of 200 nM and 350 nM, respectively).
Table 5.
Structures and activities of isoindolinones 12.
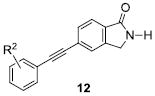
| ||||
|---|---|---|---|---|
| Cmpd | R2 | mGlu5 EC50 (nM)a | % Glu Maxb | Category |
| 12a | H | 51 | 69 | Ago-PAM |
| 12b | o-F | 200 | 67 | PAM |
| 12c | m-F | 66 | 71 | PAM |
| 12d | p-F | 350 | 60 | PAM |
EC50s are the average of three determinations and are reproducible with a coefficient of variation of 0.3.
Determined at 30 μM test compound wherein %max vehicle is 10-30%.
In summary, we introduced three types of cyclic constraints into our acyclic amide scaffolds, VU0092273 and VU0360172, and developed four series, 9-12, of highly potent and efficacious (EC50s as low as 5.9 nM, >100% Glu Max) mGlu5 PAMs and ago-PAMs. Importantly, several novel compounds were centrally active and displayed significant reversal in an amphetamine-induced hyperlocomotion assay, a preclinical assay predictive of antipsychotic efficacy. In addition, we identified a subtle ‘molecular switch’ within scaffold 10 that engendered both PAM and partial antagonist activities. Key compounds with the 9-12 series are the subject of a comprehensive in vitro molecular pharmacology, electrophysiology, occupancy and in vivo pharmacology study that will be published shortly.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.Lindsley CW, Shipe WD, Wolkenberg SE, Theberge CR, Williams DL, Jr, Sur C, Kinney GG. Curr Topics in Med Chem. 2006;8:771–784. doi: 10.2174/156802606777057599. [DOI] [PubMed] [Google Scholar]
- 2.Meltzer HY. Biol Psychiatry. 1999;46:1321–1327. [PubMed] [Google Scholar]
- 3.Conn JP, Lindsley CW, Jones CK. Trends Pharmacol Sci. 2009;30:25–31. doi: 10.1016/j.tips.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams DL, Jr, Lindsley CW. Curr Topics in Med Chem. 2005;5:825–838. doi: 10.2174/1568026054750290. [DOI] [PubMed] [Google Scholar]
- 5.Conn PJ, Christopolous A, Lindsley CW. Nat Rev Durg Discov. 2009;8:41–51. doi: 10.1038/nrd2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ayala JE, Chen Y, Banko JL, Sheffler DJ, Williams R, Telk AN, Watson NL, Xiang Z, Zhang Y, Jones PJ, Lindsley CW, Olive MF, Conn PJ. Neuropsychopharmacology. 2009;34:2057–2071. doi: 10.1038/npp.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Brien JA, Lemaire W, Wittmann M, Jacobson MA, Ha SN, Lindsley CW, Sur C, Pettibone DJ, Conn J, Williams DL. Mol Pharmacol. 2003;64:731–740. doi: 10.1124/mol.64.3.731. [DOI] [PubMed] [Google Scholar]
- 8.O’ Brien JA, Lemaire W, Wittmann M, Jacobson MA, Ha SN, Wisnoski DD, Lindsley CW, Schaffhauser HJ, Rowe B, Sur C, Duggan ME, Pettibone DJ, Conn PJ, Williams DL. J Pharmacol Exp Ther. 2004;309:568–577. doi: 10.1124/jpet.103.061747. [DOI] [PubMed] [Google Scholar]
- 9.Zhao Z, Wisnoski DD, O’ Brien JA, Lemaire W, Williams DL, Jacobson MA, Wittman M, Ha SN, Schaffhauser H, Sur C, Pettibone DJ, Duggan ME, Conn PJ, Hartman GD, Lindsley CW. Bioorg Med Chem Lett. 2007;17:1386–1391. doi: 10.1016/j.bmcl.2006.11.081. [DOI] [PubMed] [Google Scholar]
- 10.Lindsley CW, Wisnoski DD, Leister WH, O’ Brien JA, Lemaire W, Williams DL, Burno M, Sur C, Kinney GG, Pettibone DJ, Tiller PR, Smith S, Duggan ME, Hartman GD, Conn PJ, Huff JR. J Med Chem. 2004;47:5825–5828. doi: 10.1021/jm049400d. [DOI] [PubMed] [Google Scholar]
- 11.Kinney GG, O’ Brien JA, Lemaire W, Burno M, Bickel DJ, Clements MK, Chen TB, Wisnoski DD, Lindsley CW, Tiller PR, Smith S, Jacobson MA, Sur C, Duggan ME, Pettibone DJ, Conn PJ, Williams DL. J Pharmacol Exp Ther. 2005;313:199–206. doi: 10.1124/jpet.104.079244. [DOI] [PubMed] [Google Scholar]
- 12.Le Poul E, Bessis AS, Lutgens R, Bonnet B, Rocher JP, Epping-Jordan M, Mutel V. 5th International Metabotropic Glutamate Receptors Meeting; Taormina, Italy. 2005. [Google Scholar]
- 13.Engers DW, Rodriguez AL, Williams R, Hammond AS, Venable D, Oluwatola O, Sulikowski GA, Conn PJ, Lindsley CW. Chem Med Chem. 2009;4:505–511. doi: 10.1002/cmdc.200800357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamb JP, Engers DW, Niswender CM, Rodrigeuz AL, Venable DF, Conn PJ, Lindsley CW. Bioorg Med Chem Lett. doi: 10.1016/j.bmcl.2010.11.119. available online: 10.1016/j.bmcl.2010.11.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma S, Rodriguez A, Conn PJ, Lindsley CW. Bioorg Med Chem Lett. 2008;18:4098–4101. doi: 10.1016/j.bmcl.2008.05.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma S, Kedrowski J, Rood JM, Smith RL, Jones CK, Rodriguez AL, Conn PJ, Lindsley CW. J Med Chem. 2009;52:4103–4106. doi: 10.1021/jm900654c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez AL, Grier MD, Jones CK, Herman EJ, Kane AS, Smith RL, Williams R, Zhou Y, Marlo JE, Days EL, Blatt TN, Jadhav S, Menon U, Vinson PN, Rook JM, Stauffer SR, Niswender CM, Lindsley CW, Weaver CD, Conn PJ. Mol Pharm. 2010;78:1105–1123. doi: 10.1124/mol.110.067207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conn PJ, Lindsley CW, Weaver CW, Rodriguez AL, Niswender CM, Jones CK, Williams R. Benzamide derivatives as mGluR5 positive allosteric modulators and their preparation, pharmaceutical compositions and use in the treatment of diseases. 2008 WO 151184. [Google Scholar]
- 19.Hammond AS, Rodriguez AL, Townsend SD, Niswender CM, Gregory KJ, Lindsley CW, Conn PJ. ACS Chem Neurosci. 2010;1:702–716. doi: 10.1021/cn100051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Y, Manka J, Rodriguez AL, Weaver CD, Jones CK, Conn PJ, Lindsley CW, Stauffer SR. ACS Med Chem Lett. 2010;1:433–438. doi: 10.1021/ml100181a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiong H, Brugel TA, Balestra M, Brown DG, Brush KA, Hightower C, Hinkley L, Hoesch V, Kang J, Koether GM, McCauley JP, Jr, McLaren FM, Panko LM, Simpson TR, Smith RW, Woods JM, Brockel B, Chhajlani V, Gadient RA, Spear N, Sygowski LA, Zhang M, Arora J, Breysse N, Wilson JM, Isaac M, Slassi A, King MM. Bioorg Med Chem Lett. 2010;20:7381–7384. doi: 10.1016/j.bmcl.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 22.For studies on a closely related bicyclic ketone series of 7,8-dihydroquinolin-5(6H)-one mGlu5 modulators see: Vandejevs M, Jatzke C, Renner S, Müller S, Hechenberger M, Bauer T, Klochkova A, Pyatkin I, Kazyulkin D, Aksenova E, Shulepin S, Timonina O, Haasis A, Gutcaits A, Parsons CG, Kauss V, Weil T. J Med Chem. 2008;51:634–647. doi: 10.1021/jm0611298.


