Abstract
Human embryonic stem cells (hESCs) hold promise for the treatment of many human pathologies. For example, hESCs and the neuronal stem cells (NSCs) and neurons derived from them have significant potential as transplantation therapies for a variety of neurodegenerative diseases. Two concerns about the use of hESCs and their differentiated derivatives are their ability to function and their ability to resist neoplastic transformation in response to stresses that inevitably arise during their preparation for transplantation. To begin to understand how these cells handle genotoxic stress, we examined the responses of hESCs and derived NSCs and neurons to ionizing radiation (IR). Undifferentiated hESCs were extremely sensitive to IR, with nearly all the cells undergoing cell death within 5–7 hours. NSCs and neurons were substantially more resistant to IR, with neurons showing the most resistant. Of interest, NSCs that survived IR underwent cellular senescence and acquired astrocytic characteristics. Unlike IR-treated astrocytes, however, the NSC-derived astrocytic cells that survived IR did not display the typical pro-inflammatory, pro-carcinogenic senescence-associated secretory phenotype. These findings suggest distinct genotoxic stress-responses of hESCs and derived NSC and neuronal populations, and suggest that damaged NSCs, while failing to function, may not cause local inflammation.
Keywords: Neuronal stem cells, neurons, astrocytes, senescence, apoptosis
INTRODUCTION
Human embryonic stem cells (hESCs) are pluripotent cells derived from the inner cells mass of blastocysts. Because of their pluripotency and capacity for indefinite self-renewal [1], hESCs can, in theory and when appropriately differentiated, serve as an unlimited source of cells for basic research and clinical applications, including treatments for some incurable diseases in which certain cell types are lost [2]. For instance, neural stem cells (NSCs) and neurons derived from hESCs have therapeutic potential for the treatment of neurodegenerative diseases such as the Parkinson’s disease [3]. There are challenges in using such cells therapeutically, including the risk of stem cells undergoing neoplastic transformation. To ensure safety and efficacy, it is critical to maintain hESCs and their derivatives as pure populations with minimal genetic defects. Therefore, understanding how hESCs and their differentiated progeny respond to stressors such as DNA damage is important.
Ionizing radiation (IR) damages DNA and causes well-characterized cellular responses [4,5,6]: temporary cell cycle arrest, senescence or apoptosis. IR can produce DNA double stranded breaks (DSBs), one of the most catastrophic types of genomic lesions, but can also generate reactive oxygen species [6,7,8]. The response of cells to IR differs depending on the cell type, differentiation stage, radiation dose and other variables.
When IR creates severe or irreparable DNA damage, the DNA damage response (DDR) triggers the potent tumor suppressor mechanisms of apoptosis or cellular senescence [4,5,6]. Apoptosis, or programmed cell death, eliminates damaged cells in a controlled manner that minimizes tissue disruption and inflammation [9]. Cellular senescence, by contrast is a stable permanent cell cycle arrest that occurs not only when cells experience DNA damage, but also in response to mitogenic stresses such as activated oncogenes [10]. Senescent cells also secrete many biological active factors, a phenomenon termed the senescence-associated secretory phenotype (SASP) [11,12]. SASP components include a number of growth factors, proteases and pro-inflammatory cytokines such as IL-6 and IL-8. In some contexts (e.g., tissue repair), the SASP is beneficial, but in others (e.g., tumor progression) the SASP can be deleterious, largely by virtue of its pro-inflammatory nature [13,14]. High doses of IR induce human fibroblasts to undergo senescence with secretion of IL-6 and IL-8 as a consequence of the DNA damage and constitutive DDR signaling [15,16].
Here, we exposed hESCs (the H9 strain) and their derivatives (NSCs and neurons) to high doses (10 Gy) of IR. Whereas hESCs died within a few hours, as reported [17,18,19,20], surviving NSCs underwent senescence and displayed features of astrocytes. Interestingly, these surviving senescent NSCs did not display hallmarks of the SASP otherwise detected in similarly treated astrocytes.
MATERIAL AND METHODS
Cell culture
H9 hESCs were cultured in Knockout DMEF/F12 with Knockout Serum Replacement (Invitrogen) and basic fibroblast growth factor (bFGF) on irradiated (60 Gy) mouse embryonic fibroblasts (MEFs) feeder cells. hESCs were passaged using collagenase IV, as described [21], and used at passage ~30. For feeder-free cultures, hESCs were cultured on growth factor-reduced Geltrex (Invitrogen) in MEF conditioned media. To derive NSCs, hESC colonies were harvested after collagenase treatment, cultured in suspension as embryoid bodies (EBs) for 8 d in DMEM with 20% fetal bovine serum, and in neural induction medium (DMEM/F12, N2 supplement (Invitrogen), 25 ng/ml bFGF) for 7 d on plates coated with Geltrex. Rosettes were manually isolated using stretched glass pipettes, dissociated into single cells and expanded in neural expansion medium [3]. To differentiate NSCs into neurons, bFGF was replaced with 20 ng/ml brain-derived neurotrophic factor and 10 µM Rock inhibitor Y27632 for 6 d, then cells were cultured with 5 µM all-trans retinoic acid, 0.5 mM dibutyryl cAMP and 0.5 µM valpromide for 3 d. HCA2 cells have been described [11,15,16]. Human astrocytes were obtained from GlobalStem.
Immunostaining
Cells were seeded in multi-well chamber slides, fixed in 4% paraformaldehyde for 10 min at room temperature (RT), permeabilized with 0.5% Triton-X 100, washed, incubated in 10% goat serum for 1 hr at RT or overnight at 4° C, washed, and incubated for 1 hr at RT or overnight at 4° C with primary antibodies: goat anti-Oct4 (Santa Cruz), rabbit anti-nestin (BD Biosciences), rabbit anti-Sox1 (Abcam) or mouse anti-MAP2 (Sigma). After washing, slides were incubated with Alexa conjugated secondary antibodies for 30–45 min at RT. Nuclear DNA was stained with DAPI in mounting media (Vectasheild). CellProfiler image analysis software was used to score cells.
Ionizing radiation
Cells were seeded in 4-well chambers, 96-well plates or 6-cm dishes and irradiated at 320 kv and 10 mA to achieve a dose of 10 Gy. Mock-irradiated cells were placed in the irradiator without power for an equivalent interval. After IR, fresh media were added.
MultiTox-Fluor Multiplex Cytotoxicity Assay (MCA)
The multiplex cytotoxicity system MCA (Promega) was used to simultaneously determine cell viability and cytotoxicity [22]. Cells seeded in 96-well plates were cultured for 2–4 d depending upon the cell type. After IR, reagent was added to cells for 30 min. Data were collected as relative fluorescence units (RFU) using a fluorescent plate reader.
SA-β-Gal assay
Senescence Detection Kit (Biovision) was used to measure senescence of cells seeded at 1×105 in 6-well plates, essentially as instructed by the manufacturer.
BrdU incorporation
Cells were seeded in 4-well chamber slides and incubated with BrdU for 1 hr using a kit (Promega). Cells were washed and fixed with 4% paraformaldehyde, permeabilized with 0.5% Triton X-100, incubated for 30 min to 1 hr with BrdU enzyme mix, and stained according to the manufacturer’s instructions.
ELISA
Conditioned medium (CM) was prepared by washing cells 3 times in PBS followed by incubation in serum-free DMEM for 24 h. CM was filtered and stored at −80° C. Cell numbers were determined in every experiment. ELISAs were performed using AlphaLISA IL-6 Immunoassay Research (PerkinElmer). Data were normalized to cell number and expressed in pg/1000 cells.
Western blotting
Cells were washed with PBS, scraped in 200 µl of 5% sodium dodecyl sulfate, passed through a needle and centrifuged at 2000 rpm for 5 min. Supernatants were assayed for protein concentration using the BCA protein assay kit (Pierce). Proteins were separated on 4–12% polyacrylamide gels (Biorad) and transferred to a PVDF membrane (Millipore). Membranes were blocked in 5% milk powder and incubated with primary antibody for 2 h at room temperature or overnight at 4° C. Membranes were washed with 1X tris buffered saline tween-20 (TBS-T), incubated with HRP conjugated secondary antibodies (Invitrogen), washed with TBS-T, and detected using an Enhanced Electrochemoluminescence kit (GE Healthcare). Primary antibodies were mouse anti-tubulin (Sigma) and rabbit anti-nestin (Abcam).
Reverse transcription quantitative PCR (RT-qPCR)
Total RNA was isolated using the RNeasy Mini kit (Qiagen), and 1 µg was used to prepare cDNA using cDNA Reverse Transcription kit (Applied Biosystems). qPCR was performed using universal probe library dyes (Roche). Primers for IL-6 were: forward (GCCCAGCTATGAACTCCTTCT) and reverse (GAAGGCAGCAGGCAACAC); primers for IL-8 were: forward (AGACAGCAGAGCACACAAGC) and reverse (ATGGTT CCTTCCGGTGGT); primers for IL-1α were: forward (GGTTGAGTTTAAGCCAATCCA) and reverse (TGCTGACCTAGGCTTGATGA); primers for IL-5 were: forward (GGTTTG TGCAGCCAAAGAT) and reverse (TCTTGGCCCTCATTCTCACT). For quantification, the 2−ΔΔCp method was used to determine relative expression level normalized against β-actin.
TRAP (Telomeric Repeat Amplification Protocol) assay
We used the TRAPEZE Telomerase Detection kit (Intergen) to measure telomerase activity. 2 × 106 cells were lysed in 1X CHAPS lysis buffer. PCR was performed using the GeneAmp PCR System 9700 (Applied Biosystems) and following conditions: 30° C for 30 min, 94° C for 30 sec, 59° C for 30 sec and 33 cycles. PCR reaction products were separated on PAGE gels for 1.5 hrs at 400 volts. Gels were stained with SYBR green and scanned using a variable mode imager Typhoon 8610 (Molecular Dynamics) to detect the intensity of the TRAP products.
RESULTS
Characterization of ESCs, NSCs and neurons
To confirm the homogeneity of the H9 hESC population, we immunostained the cells for Oct4 and Sox2, two markers of pluripotent stem cells (Sup. Fig. 1A panels a–c). The ratios of Oct4-and Sox2-positive to DAPI (nuclear stain)-positive cells was 96%. To confirm the differentiation to NSCs, we stained for the NSC markers nestin (Sup. Fig. 1A panels d–f) and Sox1 (Sup. Fig. 1A panels g–i). More than 98% of the cells expressed both markers. To confirm the differentiation to neurons, we stained for the neuronal marker MAP2 (Sup. Fig. 1A panels j–l). Approximately 87% of cells expressed this marker. We also tested the cells for telomerase activity and confirmed that hESCs, but not the differentiated NSCs, expressed high telomerase activity, as expected [20] (Sup. Fig. 1B). These results confirmed the lineage of the cells used in subsequent experiments.
ESCs, NSCs and neurons show different susceptibility to IR
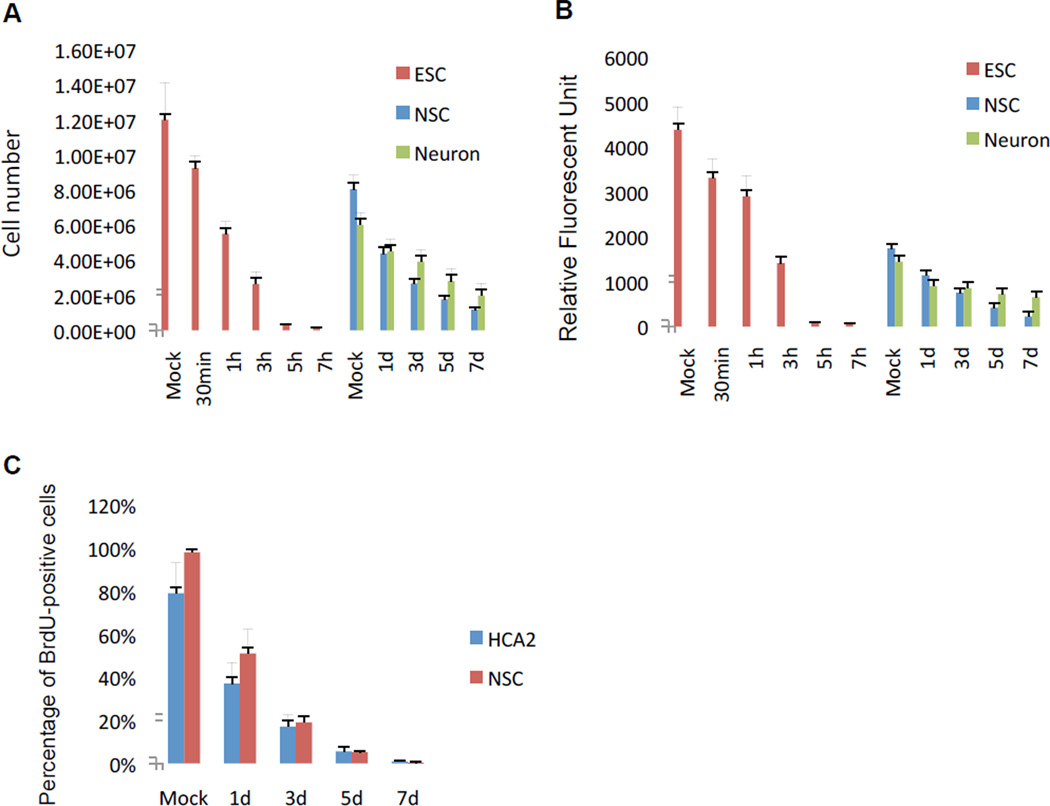
We exposed hESCs, NSCs and neurons to 10 Gy IR, and quantified survival by both cell count and the MCA viability assay (see Material and Methods). When irradiated, >99% of hESCs died within 5–7 h (Fig. 1A and B; Sup. Fig. 2A). NSCs survived longer (>50% survival after 24 h), with ~20% of the cells viable 7 d after IR exposure (Fig. 1A and B; Sup. Fig. 2B). Neurons were the most resistant to IR, with ~50% survival 7 d after IR exposure (Fig. 1A and B; Sup. Fig. 2B). Cell counting and the MCA assay gave similar assessments of viability (compare A and B in Fig. 1). Thus, ESCs were exquisitely sensitivity to high dose IR, as expected [18,19,20,23], with NSCs showing less sensitivity and neurons showing the least sensitivity.
Figure 1. Survival of human ESCs, NSCs and neurons after exposure to IR.
(A) Human ESCs, NSCs and neurons were X-irradiated (10 Gy). At the indicated intervals after IR, surviving cells were quantified using a Coulter counter. Mock = cells placed in the irradiator for the interval needed to deliver 10 Gy, but without power.
(B) Survival of the cell populations in A was assessed using the MultiTox-fluor Multiplex Cytotoxicity Assay (MCA).
(C) BrdU incorporation was determined by immunostaining 3 d after HCA2 fibroblasts and NSCs were mock-irradiated or exposed to 10 Gy IR. The percentage of BrdU positive cells was determined by counting and using the CellProfiler.
NSCs show features of cell senescence after IR
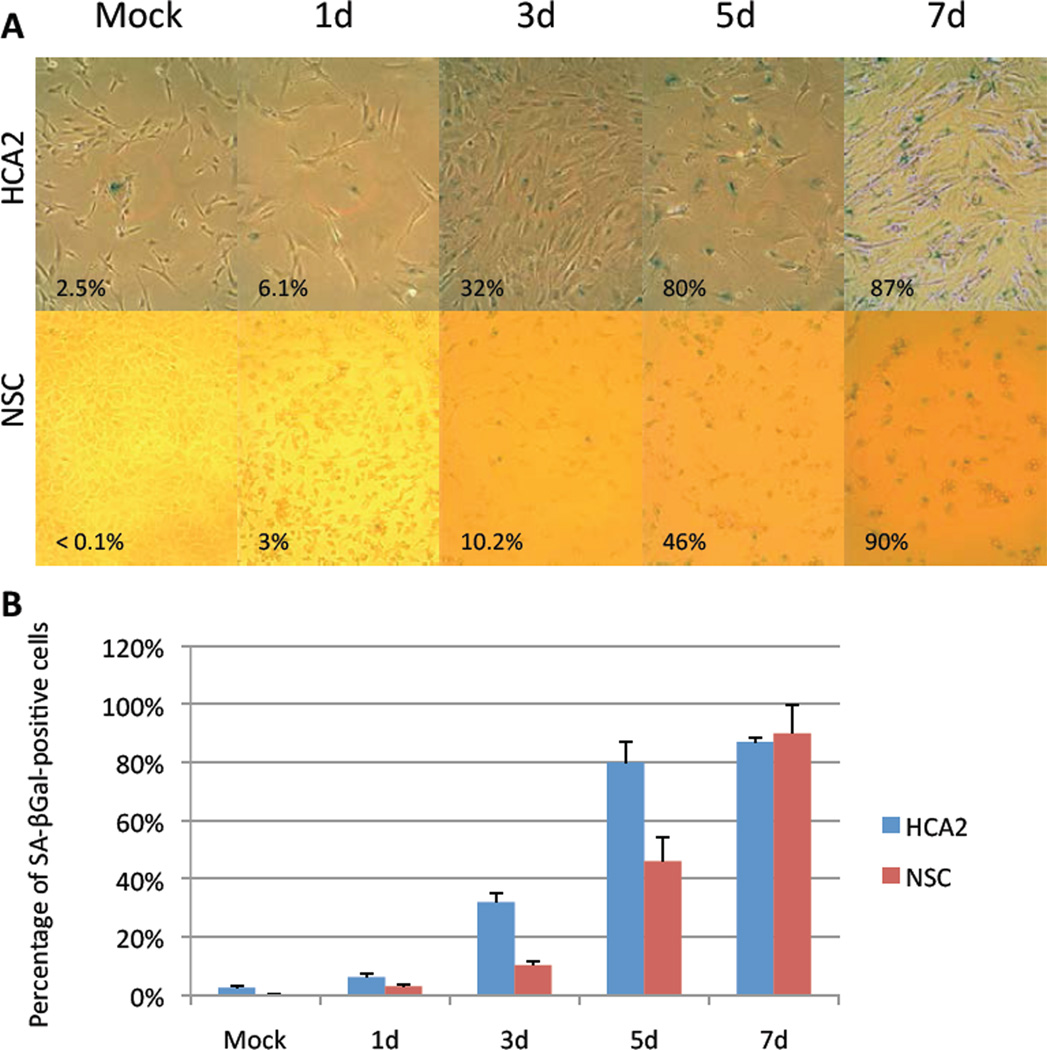
Because many cell types, including fibroblasts, undergo senescence in response to IR [10], we asked whether NSCs or neurons that survived IR expressed hallmarks of senescence (and we used human foreskin fibroblasts (HCA2) as a positive control). Cell proliferation, as determined by BrdU incorporation, rapidly declined in response to IR in both HCA2 cells and NSCs (Fig. 1C), as expected of senescent cells. As expected of post-mitotic cells, neurons did not incorporate BrdU before or after IR (data not shown). In addition, HCA2 cells and NSCs expressed senescence-associated beta-galactosidase (SA-Bgal), an established senescence marker [24]. The percentage of SA-Bgal-positive NSCs increased from <0.1% in mock-irradiated cells to ~90% of the surviving cells 7 d after IR, a level comparable to HCA2 cells (2.5% in mock-irradiated cells; 87% 7 d after IR) (Figs. 2A and B). Interestingly, neurons did not express this senescence marker, whether before or after IR (data not shown).
Figure 2. Expression of the senescence marker SA-BGal in surviving NSCs.
(A) SA-BGal expression was detected in HCA2 cells and NSCs at the indicated intervals after exposure to 10 Gy IR by histochemical staining.
(B) The fraction of SA-BGal-positive HCA2 cells and NSCs in A was determined by counting and using the CellProfiler.
Surviving NSCs do not express lineage specific markers
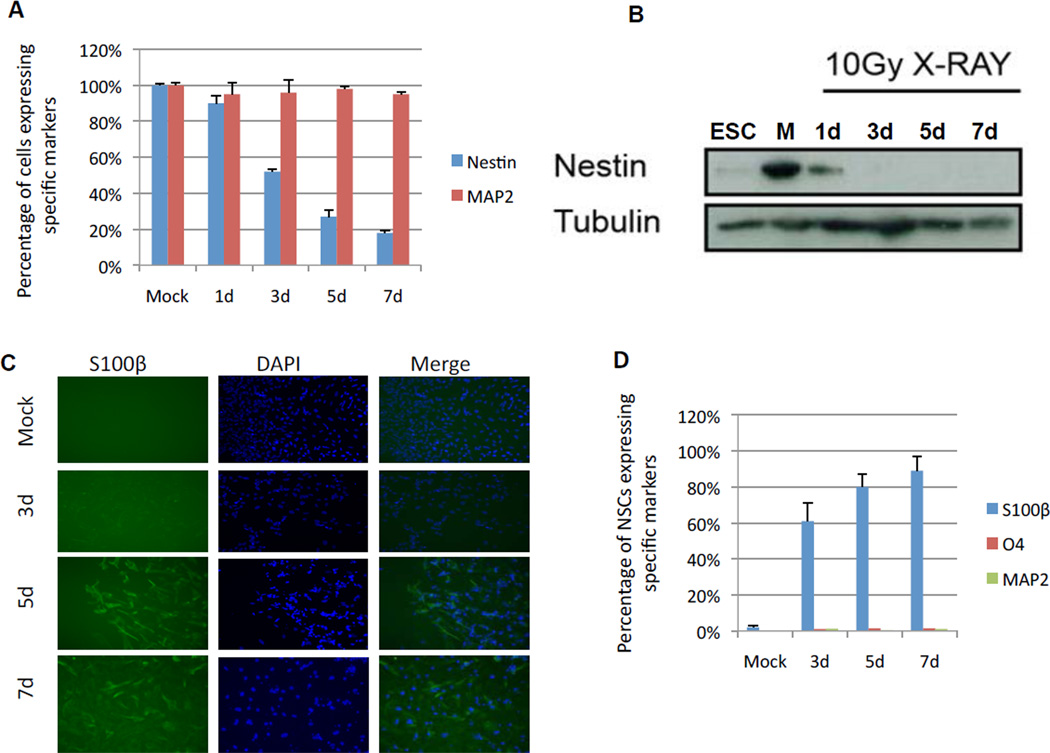
To determine whether the neurons and NSCs that survived IR retained their differentiation or stem cell identities, we immunostained the surviving cells for lineage specific markers. We used MAP2 to identify neurons, nestin to identify NSCs, S100β to identify astrocytes and O4 to identify oligodendrocytes [25,26,27]. Irradiated neurons maintained their differentiation marker, i.e., expression of MAP2 (Fig. 3A). By contrast, the surviving NSCs progressively lost nestin expression (<20% nestin-positive cells 7 d after irradiation) (Fig. 3A). This decrease in nestin expression was confirmed by western blotting (Fig. 3B). Conversely, >80% of surviving NSCs expressed the astrocytic marker S100β (Fig. 3C and D). None of the irradiated NSCs expressed markers of oligodendrocytes (O4) or neurons (MAP2) (Fig. 3D).
Figure 3. Loss of nestin and acquisition of S100β expression in surviving NSCs after IR.
(A) NSCs (blue) and neurons (red) were immunostained for nestin and MAP2 respectively at the indicated intervals after exposure to 10 Gy IR.
(B) Western blots analysis confirming the decrease in nestin expression after NSCs were exposed to IR. Tubulin served as a loading control.
(C) NSCs were immunostained for S100β at the indicated intervals after IR.
(D) Quantification of the percentage of NSCs that express S100β, O4 and MAP2 at the indicated intervals after IR.
Surviving NSCs do not exhibit a secretory phenotype
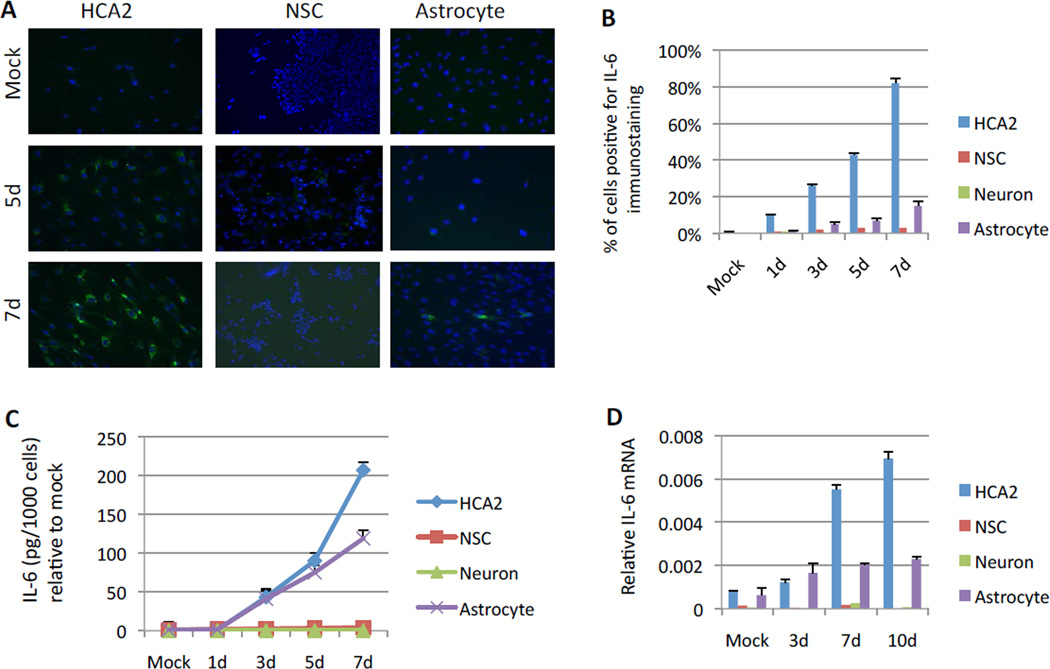
Our results suggest that NSCs that survive irradiation differentiate into astrocytes and undergo senescence. However, despite staining positive for SA-Bgal, the surviving cells did not secrete IL-6, a prominent component of the SASP [28,29]. We determined IL-6 expression and secretion in these cells using immunostaining, ELISA and quantitative PCR, all with consistently negative results (Fig. 4A–D). This lack of IL-6 expression contrasted sharply with the robust IL-6 expression and secretion shown by irradiated HCA2 cells and homogeneous human astrocyte cultures (Fig. 4A–D). We also examined the expression of two other SASP factors, IL-8 and IL-1α [11,30]. Like IL-6, only fibroblasts and astrocytes expressed these factors to a substantial extent (Sup. Fig. 3A–E), although IL-8 was more robustly expressed by fibroblasts. Neither fibroblasts nor astrocytes expressed substantial levels of IL-5, which is not a SASP factor [11]. Irradiated neurons, which did not express SA-β-Gal (data not shown), also did not express substantial levels of IL-6, IL-8 or IL-1α (Fig. 4B–D; Sup. Fig. 3B–E). Interestingly, among the four cell types, only neurons expressed substantial levels of IL-5, and this expression increased >5-fold after irradiation (Sup. Fig. 3E). These results suggest that surviving NSCs, which acquired some astrocytic characteristics after IR, do not display the full phenotype of astrocytes, i.e., the ability to secrete SASP factors upon senescence.
Figure 4. Measurement of IL-6 expression and secretion in surviving NSCs.
(A) Expression of IL-6 in HCA2 cells and NSCs was determined by immunostaining at the indicated intervals after exposure to IR.
(B) The percentage of HCA2 cells, NSCs, neurons and astrocytes with positive immunostaining for IL-6 was determined by counting and using the CellProfiler at the indicated intervals after IR.
(C) IL-6 secretion by HCA2 cells, NSCs, neurons and astrocytes at the indicated intervals after IR was determined by ELISA.
(D) IL-6 mRNA levels in HCA2 cells, NSCs, neurons and astrocytes at the indicated intervals after IR were determined by quantitative PCR.
DISCUSSION
Embryonic stem cells have the potential to provide novel therapies and treatments for a wide variety of diseases and disorders [2,31]. However, their safety and efficacy will require the maintenance of pure and functional populations of stem cells and their progeny [31]. Here, we investigated the responses of hESCs and NSCs and neurons derived from them to a major stressor, i.e., DNA damage caused by ionizing radiation (IR).
hESCs require an undamaged genome in order to function properly during embryonic development [32,33]. In response to a mild genotoxic stress such as low dose IR, hESCs repair DNA damage with efficiencies that are similar to those of somatic cells. However, in response to high dose IR, hESC cells undergo apoptosis [23]. Our data indicate that >90% of hESCs die 5–7 h following exposure 10 Gy IR. These results suggest that high dose IR saturates the DNA repair capacities of hESCs.
After hESCs differentiate, DNA repair capacity and stress defense mechanisms appear to decline [34]. For example, hESC-derived NSCs undergo cell death and display elevated levels of reactive oxygen species following exposure to 5 Gy irradiation [35]. We likewise found that a large proportion of NSCs undergo cell death after exposure to 10 Gy IR, but a sizeable fraction survived. An even greater fraction of neurons survived 10 Gy IR. Thus, although hESC have a robust capacity to repair DNA damage and tolerate stress [34], they appear to be more sensitive to IR than NSCs and neurons.
Interestingly, most of the surviving NSCs displayed features of cellular senescence, which was not the case for surviving neurons. Following IR, few NSCs incorporated BrdU, and the percentage of cells positive for SA-Bgal, a senescence marker [24], increased to levels comparable those detected in senescent HCA2 fibroblasts [15]. Although surviving neurons expressed the neuronal marker MAP2 after IR, only a small percentage of surviving NSCs expressed the NSC marker nestin.
NSCs give rise to neurons, astrocytes and oligodendrocytes, three major cell types in the mammalian central nervous system [25,26,27]. We therefore tested the possibility that exposure of NSCs to IR triggered differentiation toward one of these cell types. A significant portion of surviving NSCs expressed the astrocytic marker S100β 5–7 d after IR. None of the surviving NSCs expressed markers of oligodendrocytes or neurons. These data suggest severe genotoxic stress can promote astrocytic differentiation in NSCs. In central nervous system, glial scars that appear after injuries contain a heterogeneous population of cells that includes astrocytes [36]. Our results suggest that a severe stress such as IR may promote NSCs to differentiate into astrocytes that function in the injury response.
As previously described [11,28,29], cells induced to senescence by genotoxic stress secrete pro-inflammatory factors, a phenomenon known as the senescence-associated secretory phenotype (SASP). We found that the SASP factors IL-6, IL-8 and IL-1α were secreted by IR-induced senescent human fibroblasts and, interestingly, astrocytes. However, these factors were not secreted by the surviving senescent NSCs, despite expression of the astrocyte marker. Thus, these astrocyte-like NSCs may correspond to an intermediate phenotype, but one that may not cause inflammation. Further studies will be necessary to better define this cell population.
Supplementary Material
hESCs and their progeny, NSCs and neurons, were exposed to ionizing radiation.
Upon irradiation, most hESCs died within 5–7 hours.
Surviving NSCs underwent senescence and displayed features of astrocytes.
Surviving NSCs did not display the secretory phenotype expressed by pure astrocytes.
This study is to better understand the stress-responses of hESCs and their progeny.
ACKNOWLEDGEMENTS
This work was supported by research grants from the National Institutes of Health (AG09909, AG017242 and CA126540), the Department of Defense (BC096367), and the California Institute for Regenerative Medicine (CL1-00501).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ying QL, Nichols J, Chambers I, Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- 2.Lerou PH, Daley GQ. Therapeutic potential of embryonic stem cells. Blood Rev. 2005;19:321–331. doi: 10.1016/j.blre.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Swistowski A, Peng J, Han Y, Swistowska AM, Rao MS, Zeng X. Xeno-free defined conditions for culture of human embryonic stem cells, neural stem cells and dopaminergic neurons derived from them. PLoS One. 2009;4:e6233. doi: 10.1371/journal.pone.0006233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wahl GM, Carr AM. The evolution of diverse biological responses to DNA damage: insights from yeast and p53. Nature Cell Biol. 2001;3:277–286. doi: 10.1038/ncb1201-e277. [DOI] [PubMed] [Google Scholar]
- 5.Parplys AC, Petermann E, Petersen C, Dikomey E, Borgmann K. DNA damage by X-rays and their impact on replication processes. Radiother Oncol. 2012;102:466–471. doi: 10.1016/j.radonc.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Valerie K, Yacoub A, Hagan MP, Curiel DT, Fisher PB, Grant S, Dent P. Radiation-induced cell signaling: inside-out and outside-in. Mol Cancer Ther. 2007;6:789–801. doi: 10.1158/1535-7163.MCT-06-0596. [DOI] [PubMed] [Google Scholar]
- 7.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 8.Klaunig JE, Wang Z, Pu X, Zhou S. Oxidative stress and oxidative damage in chemical carcinogenesis. Toxicol Appl Pharmacol. 2011;254:86–99. doi: 10.1016/j.taap.2009.11.028. [DOI] [PubMed] [Google Scholar]
- 9.Edinger AL, Thompson CB. Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol. 2004;16:663–669. doi: 10.1016/j.ceb.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nature Rev Molec Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 11.Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coppe JP, Patil CK, Rodier F, Krtolica A, Beausejour C, Parrinello S, Hodgson G, Chin K, Desprez PY, Campisi J. A human-like senescence-associated secretory phenotype is conserved in mouse cells dependent on physiological oxygen. PLoS ONE. 2010;5:e9188. doi: 10.1371/journal.pone.0009188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campisi J. Cellular senescence: putting the paradoxes in perspective. Curr Opin Genet Dev. 2011;21:107–112. doi: 10.1016/j.gde.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol. 2011;192:547–556. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodier F, Coppe JP, Patil CK, Hoeijmakers WA, Munoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodier F, Munoz DP, Teachenor R, Chu V, Le O, Bhaumik D, Coppe JP, Campeau E, Beausejour C, Kim SH, Davalos AR, Campisi J. DNA-SCARS: Distinct nuclear structures that sustain damage-induced senescence growth arrest and inflammatory cytokine secretion. J Cell Sci. 2011;124:68–81. doi: 10.1242/jcs.071340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Filion TM, Qiao M, Ghule PN, Mandeville M, van Wijnen AJ, Stein JL, Lian JB, Altieri DC, Stein GS. Survival responses of human embryonic stem cells to DNA damage. J Cell Physiol. 2009;220:686–592. doi: 10.1002/jcp.21735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miura T, Mattson MP, Rao MS. Cellular lifespan and senescence signaling in embryonic stem cells. Aging Cell. 2004;3:333–343. doi: 10.1111/j.1474-9728.2004.00134.x. [DOI] [PubMed] [Google Scholar]
- 19.Qin H, Yu T, Qing T, Liu Y, Zhao Y, Cai J, Li J, Song Z, Qu X, Zhou P, Wu J, Ding M, Deng H. Regulation of apoptosis and differentiation by p53 in human embryonic stem cells. J Biol Chem. 2007;282:5842–5852. doi: 10.1074/jbc.M610464200. [DOI] [PubMed] [Google Scholar]
- 20.Zeng X, Rao MS. Human embryonic stem cells: long term stability, absence of senescence and a potential cell source for neural replacement. Neuroscience. 2007;145:1348–1358. doi: 10.1016/j.neuroscience.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 21.Ezashi T, Das P, Roberts RM. Low O2 tensions and the prevention of differentiation of hES cells. Proc Natl Acad Sci U S A. 2005;102:4783–4788. doi: 10.1073/pnas.0501283102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mor G, Montagna MK, Alvero AB. Modulation of apoptosis to reverse chemoresistance. Methods Mol Biol. 2008;414:1–12. doi: 10.1007/978-1-59745-339-4_1. [DOI] [PubMed] [Google Scholar]
- 23.Filion TM, Qiao M, Ghule PN, Mandeville M, van Wijnen AJ, Stein JL, Lian JB, Altieri DC, Stein GS. Survival responses of human embryonic stem cells to DNA damage. J Cell Physiol. 2009;220:586–592. doi: 10.1002/jcp.21735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith OM, Peacocke M, Campisi J. A novel biomarker identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taupin P, Gage FH. Adult neurogenesis and neural stem cells of the central nervous system in mammals. J Neurosci Res. 2002;69:745–749. doi: 10.1002/jnr.10378. [DOI] [PubMed] [Google Scholar]
- 26.Price J, Williams BP. Neural stem cells. Curr Opin Neurobiol. 2001;11:564–567. doi: 10.1016/s0959-4388(00)00250-6. [DOI] [PubMed] [Google Scholar]
- 27.Kennea NL, Mehmet H. Neural stem cells. J Pathol. 2002;197:536–550. doi: 10.1002/path.1189. [DOI] [PubMed] [Google Scholar]
- 28.Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freund A, Orjalo A, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Molec Med. 2010;16:238–248. doi: 10.1016/j.molmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orjalo A, Bhaumik D, Gengler B, Scott GK, Campisi J. Cell surface IL-1α is an upstream regulator of the senescence-associated IL6/IL-8 cytokine network. Proc Natl Acad Sci USA. 2009;106:17031–17036. doi: 10.1073/pnas.0905299106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Maynard S, Swistowska AM, Lee JW, Liu Y, Liu ST, Da Cruz AB, Rao M, de Souza-Pinto NC, Zeng X, Bohr VA. Human embryonic stem cells have enhanced repair of multiple forms of DNA damage. Stem Cells. 2008;26:2266–2274. doi: 10.1634/stemcells.2007-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hong Y, Cervantes RB, Tichy E, Tischfield JA, Stambrook PJ. Protecting genomic integrity in somatic cells and embryonic stem cells. Mutat Res. 2007;614:48–55. doi: 10.1016/j.mrfmmm.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 34.Saretzki G, Walter T, Atkinson S, Passos JF, Bareth B, Keith WN, Stewart R, Hoare S, Stojkovic M, Armstrong L, von Zglinicki T, Lako M. Downregulation of multiple stress defense mechanisms during differentiation of human embryonic stem cells. Stem Cells. 2008;26:455–464. doi: 10.1634/stemcells.2007-0628. [DOI] [PubMed] [Google Scholar]
- 35.Acharya MM, Lan ML, Kan VH, Patel NH, Giedzinski E, Tseng BP, Limoli CL. Consequences of ionizing radiation-induced damage in human neural stem cells. Free Radic Biol Med. 2010;49:1846–1855. doi: 10.1016/j.freeradbiomed.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 36.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.