Abstract
Multiple sclerosis (MS) is the most common autoimmune demyelinating disease, affecting millions of individuals worldwide. In the last two decades, many therapeutic options for the treatment of MS have become available, however they are limited in terms of effectiveness and some remain plagued by safety issues. The currently available treatment options target relapsing remitting forms of MS and are not effective against the more progressive forms of the disease. These limitations highlight a significant unmet treatment need for MS. In experimental autoimmune encephalomyelitis (EAE) studies from our laboratory, we have previously shown, using a number of complement mutant and transgenic mice, that inhibition of the alternative complement pathway and the C3 convertase confers significant protection from disease. We report here that targeted inhibition of complement activation using complement receptor 2 (CR2)-conjugated inhibitors significantly attenuates EAE. Administration of CR2-Crry (blocks all complement pathways at C3 activation) and CR2-fH (specifically blocks the alternative pathway) just prior to and during the onset of EAE blocks progression of both acute and chronic disease. These data indicate that inhibition of complement may offer an effective therapeutic approach to treating both acute and chronic forms of demyelinating disease through blocking the alternative pathway or complement convertases.
Keywords: experimental autoimmune encephalomyelitis, neuroimmunology complement, immunology, autoimmune disease
Introduction
Multiple sclerosis remains one of the most common demyelinating diseases worldwide affecting millions of individuals [10, 13]. Progress has been made in recent years with respect to the development of disease-modifying therapies for MS, including IFN-β, glatiramir acetate, natalizumab (Tysabri) and fingolimod with others in the developmental pipeline [7, 34, 41, 50]. Despite their widespread use, all of these MS immunotherapeutic reagents have limitations in terms of overall clinical effectiveness within the relapsing-remitting population or, serious safety concerns [18–21, 23, 38]. Although there are several MS therapeutics in development or clinical trials, none show greater efficacy than the currently available therapeutics for relapsing remitting MS and few of these target progressive MS, which currently has no therapeutic options [7, 41, 50]. Thus despite the current clinical successes with disease-modifying MS drugs, there are still significant unmet needs for progressive forms of disease [15, 39].
Complement has been implicated in the pathology of MS and experimental autoimmune encephalomyelitis (EAE) for nearly forty years (reviewed in [4, 24, 26, 31, 32]). In early studies, animals treated with cobra venom factor (CVF), which transiently depletes serum complement activity, had reduced disease severity and CNS infiltration and inflammation [24, 25, 27, 31, 32, 35] demonstrating for the first time the potential therapeutic benefit of inhibiting complement activation in demyelinating disease. In subsequent studies, a soluble version of the complement receptor type 1 (sCR1) was used to treat EAE. sCR1 inactivates the C3 and C5 convertases and effectively blocks complement activation through all three activation pathways (reviewed in [22, 28]). sCR1 significantly reduced both inflammation and demyelination, but did not completely suppress disease [36]. Attempts to use sCR1 in human clinical studies were abandoned due in part to difficulty in properly expressing a large protein (>150kDa) with over 40 disulfide bridges. These initial studies raised the questions: could a more targeted approach that inhibits only one complement pathway or effector molecule provide insight into potential complement therapeutics for MS and what is the best target to pursue? Through the use of complement mutant mice, our laboratory and others have demonstrated that EAE is significantly attenuated on deletion of factor B or C3, key alternative pathway components [30, 46]) or on deletion of the complement receptors for iC3b (CR3 and CR4) [8, 9], but not through other complement pathways or proteins [4]. The results of these animal studies set the stage for proof of concept studies to determine if alternative pathway inhibitors would be effective in attenuating EAE. For these studies, we employed the recently described complement therapeutic agents CR2-Crry or CR2-fH [17, 43]. These agents specifically target sites of complement activation through the amino-terminal domain encoding the iC3b/C3d binding site from the complement receptor type 2 (CR2) and inhibit complement convertase activity through a carboxy-terminal domain containing either the complement receptor-1 related gene/protein y (Crry) or the amino-terminal domains of mouse factor H. CR2-Crry inhibits the C3 and C5 convertases generated by any of the complement activation pathways, while CR2-fH specifically inhibits the alternative pathway. We report here that both CR2-Crry and CR2-fH significantly inhibit MOG-induced EAE, particularly in the chronic phase of disease. These results suggest that inhibiting complement activation through the alternative pathway may be therapeutically useful for progressive forms of demyelinating disease.
Materials and Methods
Mice
Inbred C57BL/6 mice, originally from The Jackson Laboratory (Bar Harbor, ME), were from our own colony. All studies were performed with approval from the UAB IACUC.
CR2-Crry and CR2-fH preparation
The recombinant fusion proteins CR2-Crry and CR2-fH were produced and purified as previously described [2, 17]. Protein purity was assessed by SDS gel electrophoresis and complement inhibitory activity was evaluated by zymosan assay [17].
Induction of active and adoptive transferred EAE and CR2-Crry and CR2-fH treatment
For active EAE, mice were immunized as previously described [47] on day -1 with 250 ng of PT (i.p.) and on day 0 with CFA emulsion containing 1 mg heat- inactivated Mycobacterium tuberculosis and 250 μg MOG peptide35–55 (Biosynthesis, Inc., Lewisville, TX). On day 1 mice received a second PT injection and progression of EAE clinical signs were monitored daily for 30 days using a clinical scale ranging from 0 to 6 as follows: 0, asymptomatic; 1, loss of tail tone; 2, flaccid tail; 3, incomplete paralysis of one or two hind limbs; 4, complete hind limb paralysis; 5, moribund; 6, dead. Only mice with a score of at least 2 (flaccid tail) observed for 2 or more consecutive days were judged to have onset of EAE. A cumulative disease index (CDI) was calculated from the sum of the daily clinical scores observed between day 7 and day 30. All mice regardless of disease status were included in the CDI calculations. For transferred EAE, spleens of control donors were removed two to three weeks following induction of active EAE, and prepared as previously described [47]. Adoptive transfer EAE was induced by injecting ~5×106 purified T cells (i.p.) into wild type recipient mice and scored as described above. At various time points after induction of either active or transferred EAE, mice were injected i.p. with PBS (control group), CR2-Crry or CR2-fH as delineated in the Results section.
Statistics
Statistical significance between PBS, CR2-Crry and CR2-fH-treated mice for EAE onset, incidence and severity was calculated using the Student’s t-test (Prism 5, GraphPad Software, Inc.).
Results
Treatment with CR2-Crry or CR2-fH delays and attenuates EAE
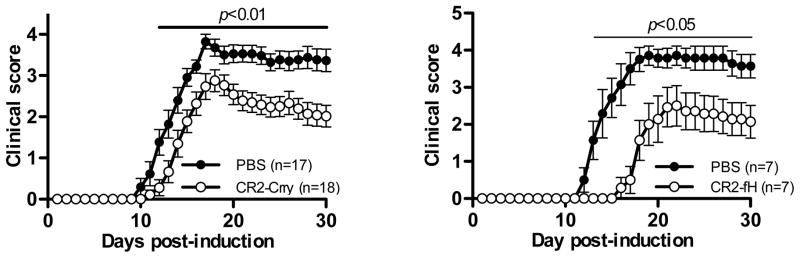
In preliminary EAE studies using CR2-Crry, we examined several dosing regimens and determined that two injections (500 μgs each injection) on days 7 and 12 were sufficient to attenuate EAE compared to PBS-treated controls. Disease severity was significantly reduced throughout the acute and chronic phases of disease (Fig. 1A, Table 1, days 12–30, p=0.01, Student’s t-test). The cumulative disease index in CR2-Crry-treated mice was reduced 35% compared to PBS-treated mice (CDI: 60 vs. 39). Treatment with CR2-Crry also delayed the onset of EAE (16 days vs. 13 days, p=0.021, Student’s t-test). The course of disease in CR2-Crry-treated mice is similar to what we reported for sCrry/GFAP mice in MOG-induced EAE in which a soluble form of Crry is produced in the CNS under the control of an astrocyte-specific promoter [11].
Figure 1. Clinical course of MOG-induced EAE in mice treated with CR2-Crry or CR2-fH.
A. Wild type mice were either treated with saline (n=17; black circles) or with CR2-Crry (n=18; open circles) after induction of EAE and the course of disease was monitored for 30 days. Mice were injected with 500 μgs of CR2-Crry on days 7 and 12-post immunization. Disease severity was significantly attenuated in antibody treated mice (day 12 to 30, p<0.01, Student’s t-test). Results shown are the mean of four experiments. B. Same as A except mice received 400μg of CR2-fH on days 7, 9, 11 and 13 (n=7; open circles) or PBS (n=7, black circles). Disease severity was significantly attenuated in CR2-Crry treated mice (day 13 to 30, p=0.05, Student’s t-test). Results shown are the mean of two experiments.
Table 1.
Active EAE phenotypes on treatment with CR2-Crry or CR2-fH.
| Inhibitor Treatment | CDIA | Disease OnsetB | Disease IncidenceC |
|---|---|---|---|
| PBS (n=17) | 60 | 13d | 100% |
| CR2-Crry (n=18) | 39* | 16d | 89% |
|
| |||
| PBS (n=7) | 61 | 14d | 100% |
| CR2-fH (n=7) | 28* | 20d | 86% |
Cumulative Disease Index (CDI) - mean of the sum of daily clinical scores observed between days 7 and 30.
Disease onset is defined as the first day of two consecutive days with a clinical score of 2 or greater.
Disease incidence is the percent of mice that displayed any clinical signs of disease.
p<0.05, control vs. inhibitor treated mice
We also performed EAE studies using CR2-fH, which specifically targets alternative pathway activity [3, 17]. Preliminary studies to determine the optimal dosing regimen demonstrated that more frequent administration of CR2-fH was required to delay and attenuate EAE compared to CR2-Crry treatment. We found that mice injected with 400μg of CR2-fH on days 7, 9, 11 and 13 post-induction developed significantly less severe EAE compared to PBS-treated controls (Fig. 1B, Table 1). Disease onset in CR2-fH treated mice was markedly delayed (20 days vs. 14 days, p=0.005, Student’s t-test). Similar to CR2-Crry-treated mice, we observed that disease severity was reduced throughout the acute and chronic phases of EAE (days 12–30, p=0.01, Student’s t-test) and the cumulative disease index was reduced over 50% (CDI: 28 vs. 61). Interestingly, shifting the treatment regimen earlier two days (days 5, 7, 9 and 11) resulted delayed disease, but little protection during the chronic phase of disease (data not shown). These data demonstrate that inhibition of complement by two different complement-targeted inhibitors can significantly attenuate an inflammatory and chronic form of EAE that is arguably reminiscent of progressive MS in humans [14, 44, 45].
Treatment with CR2-Crry prior to or after onset delays and attenuates transferred EAE
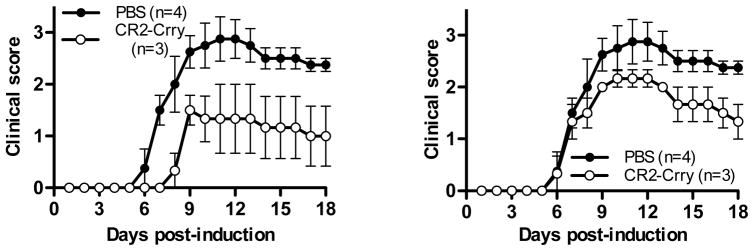
We extended our EAE studies with CR2-Crry to examine for protection in transferred EAE, which more closely mimics human demyelinating disease. For these studies we induced disease by transferring encephalitogenic T cells to wild type mice as previously described [46] after which they received either PBS or CR2-Crry (500 μgs/injection) on days 3, 5, 7 and 9 post-transfer. Similar to what we observed for active EAE, CR2-Crry-treated mice presented with delayed (13 days vs. 8 days) and attenuated EAE (CDI: 12 vs. 28.8) compared to PBS-treated mice (Fig. 2A, Table 2). Furthermore, the incidence of EAE was reduced by over 30% (100% vs. 67%). In a paired experiment, we also treated mice with CR2-Crry once they reached a clinical score of 1. In this treatment regimen, onset of EAE was not different compared to PBS-treated mice, however chronic disease was attenuated (Fig. 2B, Table 2; CDI 28.8 vs. 21).
Figure 2. Treatment with CR2-Crry prior to or after disease onset attenuates transferred EAE.
A. Transferred EAE was induced and monitored as in (A) but mice were either treated with PBS (n=4; black circles) or injected with 500 μgs of CR2-Crry on days 3, 5, 7 and 9-post immunization (n=3; open circles). B. In the same set of experiments as in A, a second group of mice were injected with 500 μgs of CR2-Crry on reaching a clinical score of 1 (n=3; open circles) (days 7, 9, 11, 13). Disease severity was significantly attenuated in CR2-Crry treated mice (p=0.002, Wilcoxon rank sign test).
Table 2.
Transferred EAE phenotypes on treatment with CR2-Crry before and after disease onset.
| Inhibitor Treatment | CDIA | Disease OnsetB | Disease IncidenceC |
|---|---|---|---|
| Saline control (n=4) | 28.8 | 8d | 100% |
| CR2-Crry (n=3) pre-onset | 12.2 | 13d | 67% |
| CR2-Crry (n=3) post-onset | 21 | 8d | 100% |
Cumulative Disease Index (CDI) - mean of the sum of daily clinical scores observed between days 0 and 30.
Disease onset is defined as the first day of two consecutive days with a clinical score of 2 or greater.
Disease incidence is the percent of mice that displayed any clinical signs of disease.
Discussion
The data we present here provides proof of concept that inhibition of complement using CR2-based inhibitors offers a viable therapeutic approach to the treatment of demyelinating disease such as MS. This concept is supported by numerous studies using complement mutant mice and a variety of complement inhibitor molecules [4, 16, 29]. Complement receptor 2-based inhibitors have also proven to be effective in a number of inflammatory disease settings including several in the CNS such as spinal cord injury [37], stroke [12] and macular degeneration [40]. Both CR2-Crry and CR2-fH function by binding to covalently attached C3d fragments within 30–60 minutes of administration and subsequently prevent continued local complement activation [17]. Both proteins have relatively short fluid-phase half-lives (8–9 hrs.) [1, 17], but the half-life of C3d-bound, CR2-based inhibitors in the CNS is currently unknown. Our data suggest that both inhibitors are functional for extended periods of time since clinical EAE scores remained attenuated for at least 30 days post disease induction (Fig. 1). The clinical course EAE we show here with CR2-Crry and CR2-fH treatment is remarkably similar to that reported for factor B- and C3-deficient mice during EAE [30, 46]. In both cases, disease is significantly attenuated in the acute phase and fails to progress to the severity levels seen in control animals. At present it is not known if attenuated disease severity during the chronic phase of EAE is due to increased inhibitor half-life while bound to C3d on the vascular endothelium and/or brain parenchymal surfaces. From a mechanistic point of view, limiting complement-mediated inflammation and tissue damage may prevent or lessen epitope spreading [49], thereby by reducing overall disease severity, and possibly contribute to complement-mediated tolerance mechanisms [33, 48].
Currently there are no disease-modifying drugs approved for clinical use that block complement alternative pathway activity. Although it seems counterintuitive to inhibit the alternative pathway as a therapeutic approach because of the subsequent increased risk of infectious disease, numerous animal model studies have shown significantly better outcome using this approach (reviewed in [16]). The use of CR2-targeted complement inhibitors allows increased anti-inflammatory efficacy without altering the host response to infectious agents [2, 42]. Additional support for a complement inhibitory approach comes from the clinical success using Eculizumab, a human monoclonal antibody directed to C5 that is used to treat paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome. This FDA-approved treatment has proven highly effective and very safe with respect to infection rates [5, 6] with the caveat that Eculizumab systemically inhibits complement but targets the terminal complement pathway. Taken together, these studies suggest that long-term use of targeted complement inhibitors, including alternative pathway inhibitors, in chronic inflammatory diseases such as MS, where treatment may be required for decades, are feasible and warrant further development.
Highlights.
Therapeutic approaches to treating demyelinating disease have unmet needs
Based on EAE studies with complement-deficient mice, complement represents a therapeutic target
CR2-based inhibitors are efficacious in blocking complement-mediated inflammation and damage
We show that treatment with CR2-based inhibitors is protective in murine demyelinating disease
Acknowledgments
This work was supported by NIH grants NS069365 to SRB and HL082485 to ST and VA Merit Award IO1BX001201 to ST.
Abbreviations
- CR2
complement receptor 2
- Crry
complement receptor related protein y
- fH
factor H
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Stephen Tomlinson, Email: tomlinss@musc.edu.
Scott R. Barnum, Email: sbarnum@uab.edu.
References
- 1.Atkinson C, Qiao F, Song H, Gilkeson GS, Tomlinson S. Low-dose targeted complement inhibition protects against renal disease and other manifestations of autoimmune disease in MRL/lpr mice. J Immunol. 2008;180:1231–1238. doi: 10.4049/jimmunol.180.2.1231. [DOI] [PubMed] [Google Scholar]
- 2.Atkinson C, Song H, Lu B, Qiao F, Burns TA, Holers VM, Tsokos GC, Tomlinson S. Targeted complement inhibition by C3d recognition ameliorates tissue injury without apparent increase in susceptibility to infection. The Journal of clinical investigation. 2005;115:2444–2453. doi: 10.1172/JCI25208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banda NK, Levitt B, Glogowska MJ, Thurman JM, Takahashi K, Stahl GL, Tomlinson S, Arend WP, Holers VM. Targeted inhibition of the complement alternative pathway with complement receptor 2 and factor H attenuates collagen antibody-induced arthritis in mice. J Immunol. 2009;183:5928–5937. doi: 10.4049/jimmunol.0901826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnum SR, Szalai AJ. Complement and demyelinating disease: No MAC needed? Brain Research Reviews. 2006;52:58–68. doi: 10.1016/j.brainresrev.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Brodsky RA. Advances in the diagnosis and therapy of paroxysmal nocturnal hemoglobinuria. Blood Rev. 2008;22:65–74. doi: 10.1016/j.blre.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brodsky RA, Young NS, Antonioli E, Risitano AM, Schrezenmeier H, Schubert J, Gaya A, Coyle L, de Castro C, Fu CL, Maciejewski JP, Bessler M, Kroon HA, Rother RP, Hillmen P. Multicenter phase 3 study of the complement inhibitor eculizumab for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Blood. 2008;111:1840–1847. doi: 10.1182/blood-2007-06-094136. [DOI] [PubMed] [Google Scholar]
- 7.Buck D, Hemmer B. Treatment of multiple sclerosis: current concepts and future perspectives. Journal of neurology. 2011;258:1747–1762. doi: 10.1007/s00415-011-6101-2. [DOI] [PubMed] [Google Scholar]
- 8.Bullard DC, Hu X, Adams JE, Schoeb TR, Barnum SR. p150/95 (CD11c/CD18) expression is required for the development of experimental autoimmune encephalomyelitis. The American journal of pathology. 2007;170:2001–2008. doi: 10.2353/ajpath.2007.061016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bullard DC, Hu X, Schoeb TR, Axtell RC, Raman C, Barnum SR. Critical requirement of CD11b (Mac-1) on T cells and accessory cells for development of experimental autoimmune encephalomyelitis. J Immunol. 2005;175:6327–6333. doi: 10.4049/jimmunol.175.10.6327. [DOI] [PubMed] [Google Scholar]
- 10.Compston A. McAlpine’s multiple sclerosis. Elsevier/Churchill Livingstone; Philadelphia: 2006. [Google Scholar]
- 11.Davoust N, Nataf S, Reiman R, Holers MV, Campbell IL, Barnum SR. Central nervous system-targeted expression of the complement inhibitor sCrry prevents experimental allergic encephalomyelitis. J Immunol. 1999;163:6551–6556. [PubMed] [Google Scholar]
- 12.Elvington A, Atkinson C, Kulik L, Zhu H, Yu J, Kindy MS, Holers VM, Tomlinson S. Pathogenic natural antibodies propagate cerebral injury following ischemic stroke in mice. J Immunol. 2012;188:1460–1468. doi: 10.4049/jimmunol.1102132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flachenecker P, Stuke K. National MS registries. Journal of neurology. 2008;255(Suppl 6):102–108. doi: 10.1007/s00415-008-6019-5. [DOI] [PubMed] [Google Scholar]
- 14.Gold L, Linington C, Lassmann H. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain: a journal of neurology. 2006;129:1953–1971. doi: 10.1093/brain/awl075. [DOI] [PubMed] [Google Scholar]
- 15.Hawker K. Progressive multiple sclerosis: characteristics and management. Neurologic clinics. 2011;29:423–434. doi: 10.1016/j.ncl.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Holers VM, Thurman JM. The alternative pathway of complement in disease: opportunities for therapeutic targeting. Molecular immunology. 2004;41:147–152. doi: 10.1016/j.molimm.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Huang Y, Qiao F, Atkinson C, Holers VM, Tomlinson S. A novel targeted inhibitor of the alternative pathway of complement and its therapeutic application in ischemia/reperfusion injury. J Immunol. 2008;181:8068–8076. doi: 10.4049/jimmunol.181.11.8068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jain N, Bhatti MT. Fingolimod-associated macular edema: incidence, detection, and management. Neurology. 2012;78:672–680. doi: 10.1212/WNL.0b013e318248deea. [DOI] [PubMed] [Google Scholar]
- 19.Kieseier BC, Stuve O. A critical appraisal of treatment decisions in multiple sclerosis--old versus new. Nature reviews. Neurology. 2011;7:255–262. doi: 10.1038/nrneurol.2011.41. [DOI] [PubMed] [Google Scholar]
- 20.Kleinschmidt-Demasters BK, Miravalle A, Schowinsky J, Corboy J, Vollmer T. Update on PML and PML-IRIS Occurring in Multiple Sclerosis Patients Treated With Natalizumab. Journal of neuropathology and experimental neurology. 2012;71:604–617. doi: 10.1097/NEN.0b013e31825caf2c. [DOI] [PubMed] [Google Scholar]
- 21.Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. The New England journal of medicine. 2005;353:369–374. doi: 10.1056/NEJMoa051782. [DOI] [PubMed] [Google Scholar]
- 22.Lambris JD, Holers VM. Therapeutic interventions in the complement system. Humana Press; Totowa: 2000. p. 259. [Google Scholar]
- 23.Langer-Gould A, Atlas SW, Green AJ, Bollen AW, Pelletier D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. The New England journal of medicine. 2005;353:375–381. doi: 10.1056/NEJMoa051847. [DOI] [PubMed] [Google Scholar]
- 24.Levine S, Cochrane CG, Carpenter CB, Behan PO. Allergic encephalomyelitis: effect of complement depletion with cobra venom. Proc Soc Exp Biol Med. 1971;138:285–289. doi: 10.3181/00379727-138-35880. [DOI] [PubMed] [Google Scholar]
- 25.Linington C, Morgan BP, Scolding NJ, Wilkins P, Piddlesden S, Compston DA. The role of complement in the pathogenesis of experimental allergic encephalomyelitis. Brain: a journal of neurology. 1989;112:895–911. doi: 10.1093/brain/112.4.895. [DOI] [PubMed] [Google Scholar]
- 26.Lumsden CE. The immunogenesis of the multiple sclerosis plaque. Brain research. 1970;28:365–390. doi: 10.1016/0006-8993(71)90052-7. [DOI] [PubMed] [Google Scholar]
- 27.Morariu MA, Dalmasso AP. Experimental allergic encephalomyelitis in cobra venom factor-treated and C4-deficient guinea pigs. Annals of neurology. 1978;4:427–430. doi: 10.1002/ana.410040507. [DOI] [PubMed] [Google Scholar]
- 28.Morgan BP, Harris CL. Complement regulatory proteins. Vol. 1. Academic Press; San Diego: 1999. p. 382. [Google Scholar]
- 29.Morgan BP, Harris CL. Complement therapeutics; history and current progress. Molecular immunology. 2003;40:159–170. doi: 10.1016/s0161-5890(03)00111-1. [DOI] [PubMed] [Google Scholar]
- 30.Nataf S, Carroll SL, Wetsel RA, Szalai AJ, Barnum SR. Attenuation of experimental autoimmune demyelination in complement-deficient mice. J Immunol. 2000;165:5867–5873. doi: 10.4049/jimmunol.165.10.5867. [DOI] [PubMed] [Google Scholar]
- 31.Oldstone MB, Dixon FJ. Immunohistochemical study of allergic encephalomyelitis. The American journal of pathology. 1968;52:251–263. [PMC free article] [PubMed] [Google Scholar]
- 32.Pabst H, Day NK, Gewurz H, Good RA. Prevention of experimental allergic encephalomyelitis with cobra venom factor. Proc Soc Exp Biol Med. 1971;136:555–560. doi: 10.3181/00379727-136-35310. [DOI] [PubMed] [Google Scholar]
- 33.Paidassi H, Tacnet-Delorme P, Arlaud GJ, Frachet P. How phagocytes track down and respond to apoptotic cells. Critical reviews in immunology. 2009;29:111–130. doi: 10.1615/critrevimmunol.v29.i2.20. [DOI] [PubMed] [Google Scholar]
- 34.Pelletier D, Hafler DA. Fingolimod for multiple sclerosis. The New England journal of medicine. 2012;366:339–347. doi: 10.1056/NEJMct1101691. [DOI] [PubMed] [Google Scholar]
- 35.Piddlesden S, Lassmann H, Laffafian I, Morgan BP, Linington C. Antibody-mediated demyelination in experimental allergic encephalomyelitis is independent of complement membrane attack complex formation. Clin Exp Immunol. 1991;83:245–250. doi: 10.1111/j.1365-2249.1991.tb05622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piddlesden SJ, Storch MK, Hibbs M, Freeman AM, Lassmann H, Morgan BP. Soluble recombinant complement receptor 1 inhibits inflammation and demyelination in antibody-mediated demyelinating experimental allergic encephalomyelitis. J Immunol. 1994;152:5477–5484. [PubMed] [Google Scholar]
- 37.Qiao F, Atkinson C, Song H, Pannu R, Singh I, Tomlinson S. Complement plays an important role in spinal cord injury and represents a therapeutic target for improving recovery following trauma. The American journal of pathology. 2006;169:1039–1047. doi: 10.2353/ajpath.2006.060248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ransohoff RM, Natalizumab PML. Nat Neurosci. 2005;8:1275. doi: 10.1038/nn1005-1275. [DOI] [PubMed] [Google Scholar]
- 39.Reynolds R, Roncaroli F, Nicholas R, Radotra B, Gveric D, Howell O. The neuropathological basis of clinical progression in multiple sclerosis. Acta neuropathologica. 2011;122:155–170. doi: 10.1007/s00401-011-0840-0. [DOI] [PubMed] [Google Scholar]
- 40.Rohrer B, Long Q, Coughlin B, Wilson RB, Huang Y, Qiao F, Tang PH, Kunchithapautham K, Gilkeson GS, Tomlinson S. A Targeted Inhibitor of the Alternative Complement Pathway Reduces Angiogenesis in a Mouse Model of Age-related Macular Degeneration. Invest Ophthalmol Vis Sci. 2009 doi: 10.1167/iovs.08-2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saidha S, Eckstein C, Calabresi PA. New and emerging disease modifying therapies for multiple sclerosis. Annals of the New York Academy of Sciences. 2012;1247:117–137. doi: 10.1111/j.1749-6632.2011.06272.x. [DOI] [PubMed] [Google Scholar]
- 42.Schepp-Berglind J, Atkinson C, Elvington M, Qiao F, Mannon P, Tomlinson S. Complement-dependent injury and protection in a murine model of acute dextran sulfate sodium-induced colitis. J Immunol. 2012;188:6309–6318. doi: 10.4049/jimmunol.1200553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song H, He C, Knaak C, Guthridge JM, Holers VM, Tomlinson S. Complement receptor 2-mediated targeting of complement inhibitors to sites of complement activation. The Journal of clinical investigation. 2003;111:1875–1885. doi: 10.1172/JCI17348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steinman L, Zamvil SS. How to successfully apply animal studies in experimental allergic encephalomyelitis to research on multiple sclerosis. Annals of neurology. 2006;60:12–21. doi: 10.1002/ana.20913. [DOI] [PubMed] [Google Scholar]
- 45.Steinman L, Zamvil SS. Virtues and pitfalls of EAE for the development of therapies for multiple sclerosis. Trends in immunology. 2005;26:565–571. doi: 10.1016/j.it.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 46.Szalai AJ, Hu X, Adams JE, Barnum SR. Complement in experimental autoimmune encephalomyelitis revisited: C3 is required for development of maximal disease. Molecular immunology. 2007 doi: 10.1016/j.molimm.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Szalai AJ, Nataf S, Hu XZ, Barnum SR. Experimental allergic encephalomyelitis is inhibited in transgenic mice expressing human C-reactive protein. J Immunol. 2002;168:5792–5797. doi: 10.4049/jimmunol.168.11.5792. [DOI] [PubMed] [Google Scholar]
- 48.van Kooten C, Fiore N, Trouw LA, Csomor E, Xu W, Castellano G, Daha MR, Gelderman KA. Complement production and regulation by dendritic cells: molecular switches between tolerance and immunity. Molecular immunology. 2008;45:4064–4072. doi: 10.1016/j.molimm.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 49.Vanderlugt CL, Miller SD. Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nature reviews. Immunology. 2002;2:85–95. doi: 10.1038/nri724. [DOI] [PubMed] [Google Scholar]
- 50.Weber MS, Menge T, Lehmann-Horn K, Kronsbein HC, Zettl U, Sellner J, Hemmer B, Stuve O. Current treatment strategies for multiple sclerosis - efficacy versus neurological adverse effects. Current pharmaceutical design. 2012;18:209–219. doi: 10.2174/138161212799040501. [DOI] [PubMed] [Google Scholar]