Abstract
The primary aim of this study was to test the hypothesis that adolescent binge drinkers, but not lighter drinkers, would show signs of impairment on tasks of affective decision-making as measured by the Iowa Gambling Test (IGT), when compared to adolescents who never drank.
We tested 207 10th grade adolescents in Chengdu City, China, using two versions of the IGT, the original and a variant, in which the reward/punishment contingencies were reversed. This enables one to distinguish among different possibilities of impaired decision-making, such as insensitivity to long-term consequences, or hypersensitivity to reward. Furthermore, we tested working memory capacity using the Self-ordered Pointing Test (SOPT). Paper and pencil questionnaires were used to assess drinking behaviors and school academic performance.
Results indicated that relative to never-drinkers, adolescent binge drinkers, but not other (ever, past 30-day) drinkers, showed significantly lower net scores on the original version of the IGT especially in the latter trials. Furthermore, the profiles of behavioral performance from the original and variant versions of the IGT were consistent with a decision-making impairment attributed to hypersensitivity to reward. In addition, working memory and school academic performance revealed no differences between drinkers (at all levels) and never-drinkers. Logistic regression analysis showed that after controlling for demographic variables, working memory, and school academic performance, the IGT significantly predicted binge-drinking.
These findings suggest that a “myopia” for future consequences linked to hypersensitivity to reward is a key characteristic of adolescents with binge-drinking behavior, and that underlying neural mechanisms for this “myopia” for future consequences may serve as a predisposing factor that renders some adolescents more susceptible to future addictive behaviors.
Keywords: Executive function, Affective control, Reward, Working memory, Adolescent drinking, Iowa Gambling Test
1. Introduction
Although some studies have investigated the role of the Pre-frontal Cortex (PFC) in the vulnerability to experimentation with alcohol, and the acquisition of alcohol disorders in adolescents, most of these studies have focused on the role of the “cold” cognitive aspects of executive functions (EF) in alcohol use, which have been linked to the dorsolateral sector of the prefrontal cortex (DLPC) (Finn, Mazas, Justus, & Steinmetz, 2002; Hartley, Elsabagh, & File, 2004; Sher, 2006; Thush & Wiers, 2007;Zeigler et al., 2005; Zetteler, Stollery, Weinstein, & Lingford-Hughes, 2006). Relatively few studies have addressed the “hot” cognitive aspects of EF in alcohol and other substance use, which have been more linked to the orbital/ventromedial sector of the prefrontal cortex (OFC/VMPC) (Overman et al., 2004). In this study, we examined the affective decision-making ability measured by the Iowa Gambling Test (IGT) in adolescent drinkers. The IGT has been shown to tax aspects of decision-making that are guided by affect and emotions (Bechara, 2003; Turnbull, Evans, Bunce, Carzolio, & O’Connor, 2005). We hypothesized that binge drinkers would show relatively poor measures of affective decision-making relative to other drinkers and/or never drinkers. The rationale was that poor affective decision-making, as measured by the IGT, would predispose individuals to poorly controlled substance use, as they become more likely to be lured by immediate reward, or more oblivious to the negative future consequences of their choice.
Much evidence shows that there are protracted maturational changes in top-down PFC systems relative to subcortical systems during the transitional period of adolescence (Giedd, 2004; Gogtay et al., 2004; Spear, 2000; Toga, Thompson, & Sowell, 2006). However, the PFC is relatively large, and its functions are heterogeneous (Faw, 2003; Kolb, Pellis, & Robinson, 2004). These “supervisory” functions of PFC can be further categorized into cognitive control or “cold” EF, which flexibly regulates thoughts and actions in the presence of competing goals (Durston & Casey, 2006; Miller & Cohen, 2001), and affective control or “hot” EF, which strategically controls feelings in the service of a goal (Dahl, 2003; Kerr & Zelazo, 2004). Studies in neuroimaging and neuropsychology have shown that cognitive and affective control associate with different but interacting subregions of the PFC—the DLPC and the OFC/VMPC, respectively (Kringelbach, 2005; Miller & Cohen, 2001; O’Doherty, Kringelbach, Rolls, Hornak, & Andrews, 2001; Oya et al., 2005). Development studies have indicated that maturation of the OFC/VMPC, and especially the frontal pole (e.g., Brodmann’s Area 10) might be a developmentally distinct process from maturation of other regions of the frontal lobe, and cognitive control functions might mature earlier than do affective control functions (Crone & van der Molen, 2004; Hooper, Luciana, Conklin, & Yarger, 2004). This immature period of the OFC/VMPC perhaps explains why adolescents are often capable of understanding the risks and consequences of their actions, despite making disadvantageous decisions (Cauffman & Steinberg, 2000), which suggests that cognitive control functions are already in place, whereas affective control is still developing (Steinberg, 2005). Thus, examining the affective control functions in adolescents might be important to understanding their reward-seeking behavior, such as alcohol consumption.
Affective control is essential for adequate functioning and is likely to have an effect on a number of behaviors in which positive and negative affective consequences must be acted on adaptively. Among several useful measures to assess affective control functions (Bechara, Damasio, Damasio, & Anderson, 1994; Elliott, Friston, & Dolan, 2000; Ernst et al., 2004; Rogers et al., 1999), we employed a widely used measure— Iowa Gambling Test (IGT) (Bechara et al., 1994) —to assess the affective decision-making ability of a group of Chinese adolescents. Compared to other tasks, which assess brain functions related to the calculation of probability or expected value, IGT requires participants to learn and infer from their past experience (such as reward and punishment encountered during the task) about outcome probabilities (Bechara, 2004a, 2004b). Affective and emotional systems play a critical role in such learning processes. The decision-making of neurologically developed and intact participants is guided by an emotional signal that assigns negative value for the disadvantageous choices and positive value for advantageous choices, thereby leading behavior towards long term favorable options. Several developmental studies have demonstrated that there is significant and steady improvement on the IGT or IGT analogous tasks during early adolescence to adulthood (Crone & van der Molen, 2004; Hooper et al., 2004; Overman, 2004). These findings parallel other studies, which show that the prefrontal cortex may not develop fully until the age of 21 (Giedd, 2004; Gogtay et al., 2004). Although all adolescents might still be undergoing developmental changes in the prefrontal region (i.e., having a “premature” prefrontal cortex), and by and large be mostly susceptible to making suboptimal or “risky” decisions compared to adults, we investigated in this study the individual variability among the adolescents at similar age. We attempted to identify those who might be at a higher risk for making bad decisions, which potentially may translate into real life risky behaviors, such as alcoholism and drug dependence. It remains to be determined whether a relatively abnormal poor decision-making capacity as measured by the IGT will serve as an early neurocognitive marker that may help identify at-risk adolescent individuals. This early identification could be useful for prevention.
The IGT has two versions: the original and the variant. In the original version, the disadvantageous decks of cards yield higher immediate rewards but unpredictable and larger delayed punishments, while the advantageous decks provide lower immediate gains but unexpected and smaller future losses. In the variant version, the schedules of reward and punishment contingencies are reversed, so that the advantageous decks yield immediate losses but even higher future rewards, and the disadvantageous decks offer lower immediate punishments but even lower long-term rewards. The variant version was developed to address the question of whether hypersensitivity to immediate reward and/or insensitivity to long-term consequences might account for the choice of the disadvantageous decks on the original version (Bechara, Tranel, & Damasio, 2000; Bechara, Dolan, & Hindes, 2002). For example, although patients with VMPC brain damage and the Substance Dependence Individuals (SDI) showed similar disadvantageous choices on the original version, they performed very differently on the variant version. VMPC patients still performed badly but one large subgroup of SDI per-formed normally (Bechara, Tranel et al., 2000; Bechara et al., 2002). These results were interpreted to suggest that although both VMPC patients and SDI were “myopic” for the future, the underlying mechanisms were different: VMPC patients were insensitive for future consequences, positive or negative (Bechara, Tranel et al., 2000; Bechara et al., 1994) while some SDI were hypersensitive to immediate reward (Bechara et al., 2002).
Because hot and cold executive functions diverge in their maturational trends and may have different implications for binge-drinking, cognitive performance associated with cold cognition is also important to consider. One of the specific and most well-researched cognitive functions in this domain is working memory. Good working memory helps individuals keep competing considerations “online” (Kane & Engle, 2002), even whenfaced with other demands on cognitive resources (e.g., carrying on a conversation, considering peer’s opinions). Without good working memory, multiple considerations are not as likely to be kept active or “online” for any decisions, and explicit memory retrieval is less effective (De Neys, Schaeken, & d’Ydewalle, 2005a, 2005b; Kane & Engle, 2000). Therefore, a smaller subset of learned effects (only the most spontaneously activated ones) is available to influence behavior.
In this study, we used the Self-ordered Pointing Test (SOPT) (Peterson, Pihl, Higgins, & Lee, 2002) to assess working memory capacity, utilizing a task developed by Petrides and Milner (Petrides & Milner, 1982). The task is feasible for use in field research with adolescent participants. This task requires in each trial, an individual to memorize a maximum number of 12 items, either visually or phonologically encoded, and hold them “online” for further operations. Because there are 6 trials of the SOPT, the maximum capacity is not required in the first trial but the amount of information increases cumulatively over the course of each trial. This process resembles that of transient online storage (Perry et al., 2001), or active monitoring and retrieving of the increasing amount of information (Petrides, 1995) in the concept of working memory. This task has been linked to neural activity within the Dorsolateral Prefrontal Cortex (DLPC) (Petrides, Alivisatos, Meyer, & Evans, 1993) and has been used to assess working memory capacity in several studies (Chaytor & Schmitter-Edgecombe, 2004; Chey, Lee, Kim, Kwon, & Shin, 2002; Pukrop et al., 2003; Ward, Shum, McKinlay, Baker-Tweney, & Wallace, 2005). Moreover, studies have shown that working memory is highly related to general cognitive functions such as reading, mathematics, and reasoning (Colom, Rebello, Palacios, Juan-Espinosa, & Kyllonen, 2004;Engle, Cantor, & Carullo, 1992; Jarrold & Towse, 2006). Therefore, we also ask the participants to report their school academic performance.
In this study, we focus on binge-drinking behavior because binge-drinking in youth represents poorly controlled alcohol consumption and is highly predictive of alcohol abuse and dependence in the future (Bonomo, Bowes, Coffey, Carlin, & Patton, 2004; Jennison, 2004). Our primary hypothesis is that binge drinkers will demonstrate signs of worse decision-making than never-drinkers, as reflected by lower scores on the original version of the IGT. Since hyperactivity of the reward system in the adolescent brain has been implicated in their risky behaviors (Ernst et al., 2005; May et al., 2004), we predict that the binge drinkers will perform normally on the variant version of the IGT, which indicates that the “myopia” for the future among binge drinkers is due to their hypersensitivity to reward. Furthermore, studies have reported that decision-making and working memory functions have an asymmetrical relationship (Bechara, Damasio, Tranel, & Anderson, 1998; Bechara, Damasio, & Damasio, 2000), i.e., poor working memory related to dorsolateral prefrontal cortex damage can compromise decision-making, but poor decision-making related to OFC/VMPC damage can occur independent of any working memory deficits. Developmental studies have suggested that maturation of the OFC/VMPC, might be a developmentally distinct process from maturation of other regions of the frontal lobe (Crone & van der Molen, 2004; Hooper et al., 2004). Other studies have shown that adolescents make disadvantageous decisions, yet they seem to have a mature capacity to reason and to explain reward probabilities (Crone, Jennings, & Van der Molen, 2004; Crone, Somsen, Van Beek, & Van Der Molen, 2004). Therefore, we anticipate that poor decision-making in binge drinkers is not due to deficits in their “cold” dorsolaterally mediated executive functions, and thus we expect normal scores on working memory and school academic performance, but poor performance on the original version of the IGT.
2. Methods
2.1. Sample
The data collected in this study support the Pacific Rim Transdisciplinary Tobacco and Alcohol Use Research Center investigation of the determinants of tobacco and alcohol use and abuse among youth in China. All research protocols and instruments were approved by the USC and Chengdu, China CDC IRB’s. With the assistance of the municipal Education Committee and the Chengdu Center for Disease Control and Prevention (CCDCP), in Chengdu City, Sichuan Province, four schools were recruited for the study. To ensure maximum variability across the student sample, two academic high schools, one of high- and one of low/middle academic status, and two vocational schools, one of middle- and one of low academic status, were selected. School administrators and teachers from the selected schools agreed to participate in the research after receiving a thorough explanation of the project from the CCDCP staff. One 10th grade class from each of the four schools was randomly selected, and a total of 223 students were invited to participate. Students voluntarily took part in the study and were told that they could discontinue their participation at any time. Out of that total, sixteen participants were excluded from the data analysis due to computer malfunctions or failure to complete the survey or follow instructions on the SOPT. The analytic data set included 207 participants (92.8% of total participants).
2.2. Measures
Study measures included two computer-assisted neurocognitive assessments and a paper-and-pencil self-report questionnaire. The questionnaires and instructions for each neuropsychological task were translated into Mandarin Chinese (the only language used in the surveys) and back-translated prior to use.
2.2.1. Iowa Gambling Task (IGT)
As described in previous studies (Bechara et al., 1994; Bechara, Damasio, Damasio, & Lee, 1999), the original and variant version of the IGT are computerized versions of the gambling task with an automated and computerized method for collecting data. In the Original Version of the IGT, four decks of cards labeled A’, B’, C’ and D’ are displayed on the computer screen. The backs of the cards resemble real decks of cards. The participant starts the task with a sum of make-believe money in his or her account (i.e. $2000), represented by a green bar that changes in length as the participant “wins” or “loses” money during the task. The subject is required to select one card at a time from one of the four decks. When the subject selects a card, a message is displayed on the screen indicating the amount of money the subject has won or lost. The pre-programmed schedules of gain and loss are controlled by the computer. Turning each card can bring an immediate reward of $100 in Decks A’ and B’ and $50 in Decks C’ and D’. As the game progresses, there are also unpredictable losses among the card selection. Total losses amount to $1250 in every 10 cards in Decks A’ and B’ compared to $250 in Decks C’ and D’. Decks A’ and B’ are equivalent in terms of overall net loss, and Decks C’ and D’ are equivalent in terms of overall net gain over the course of the trials. The difference is that in Decks A’ and C’, the punishment is more frequent but of smaller magnitude. Whereas in Decks B’ and D’, the punishment is less frequent but of greater magnitude. Thus, Decks A’ and B’ are disadvantageous because they yield high immediate gains but greater losses in the long run (i.e. net loss of $250 for every 10 cards), and Decks C’ and D’ are advantageous in that they yield lower immediate gains but smaller losses in the long run (i.e. net gain of $250 for every 10 cards). After the original version of the IGT is completed, its net score is obtained by subtracting the total number of selections from the disadvantageous decks (A’ + B’) from the total number selections from the advantageous decks (C’ + D’). The variant version of the IGT is the same as that used in the previous study (Bechara et al., 2002). The appearance and operation of this task is very similar to the original version, with exception of the order of the cards and the payment schedule. In this task, the punishment is immediate while the reward is delayed. Schedules of Decks E’ and G’ are designed to be advantageous in the way that the net difference between the immediate losses and future rewards in these decks increased in the positive direction across each block of 10 cards (i.e. toward larger gains). By contrast, the schedules of Decks F’ and H’ are designed to be disadvantageous in the manner in which the net difference in these decks increased in the negative direction across each block of 10 card (i.e. toward larger losses). Deck E: The immediate punishment is on average $100 for each selection of the first 10 cards. In this first block of 10 cards, there is one unpredictable reward of $1250. In each subsequent block, the average immediate punishment goes up $15 (i.e. $150 in 10 cards), while the magnitude of delayed reward increases $195 in each block. When one adds the rewards versus the penalties in each block, there is a net gain of $250 in the first block. The net gain goes up in increments of $45 in each subsequent block until it reaches $475 in the sixth block. Deck G: In the first block of 10 cards the immediate punishment averages $100 for each card selection. In this first block, there are five unpredictable delayed rewards ranging from $150 to $350 each (total $1250), amounting to a net gain of $250. In the remaining five blocks, immediate punishment remains the same, but the frequency of delayed reward drops gradually until it reaches 20% in the sixth block (i.e. an average drop of 6% in each block). The magnitude of these delayed rewards is adjusted so that the net gain increases by an average of $45 in each block, until it reaches a net gain of $475 in the sixth block. Overall, the net gain incurred by Deck G is equal to that of Deck E. Deck F: This deck parallels Deck G except in the opposite direction. In the first block of 10 cards, the immediate punishment is $50 on an average for each selection of a card. In this first block, there are five unpredictable rewards ranging from $25 to $75 each (total $250). The outcome is a net loss of $250. In the remaining five blocks, the immediate punishment remains the same, but the frequency of delayed reward drops gradually until it reaches 20% in the sixth block (an average drop of 6% in each block). The magnitude of these delayed rewards is adjusted so that the net loss increases by an average of $45 in each block, until it reaches $475 in the sixth block. Deck H: This deck parallels Deck E except in the opposite direction. In the first block of 10 cards, the immediate punishment is $50 on average. In this first block, there is one unpredictable reward of $250. In each subsequent block, the average immediate punishment goes up $5 (i.e. total $50), while the magnitude of reward increases only $5. The outcome is a net loss of $250 in the first block. The net loss goes up in increments of $45 in each subsequent block until it reaches $475 in the sixth block. Overall, the net loss incurred by Deck F is equal to H. The net score on the variant version of the IGT is calculated by subtracting the total number of selections from the disadvantageous Decks (F’ + H’) from the total number selections from the advantageous Decks (E’ + G’).
2.2.2. Self-ordered pointing test (SOPT)
We used a computerized version of the SOPT (Peterson et al., 2002) which was based upon a task originally developed by Petrides and Milner (1982). The SOPT has both verbal and non-verbal components with three trials of each. In the verbal component, subjects view pictures of concrete, nameable objects (clock, book, bus, etc.); whereas in the non-verbal component, subjects view abstract designs that are difficult to name or encode verbally. In each trial, 12 pages are presented sequentially, with each page depicting the same 12 pictures, but in a different spatial arrangement each time. Subjects are instructed to point to a different picture on each presentation. The total number of correct selections of different pictures represents the working memory score. There is a maximum possible score of 12 on each trial and a total of 72 for all 6 trials. In our study, the internal consistency across the 6 trials was 0.86.
2.2.3. Questionnaire measurements
2.2.3.1. Drinking behaviors
Ever drinking was assessed using the following item: During your life, on how many days have you had at least one drink of alcohol?” The response options ranged from “0 day” to “100 or more days”. Past 30-day drinking was assessed using the following item: “During the past 30 days, on how many days did you have at least one drink of alcohol?” The response options ranged from “0 day” to “All 30 days”. Binge-drinking was assessed using the following item: “During the past 30 days, on how many days did you have 4 or more drinks of alcohol in a row, that is, within a couple of hours? The response options range from “0 day” to “20 or more days”. Although 5 or more drinks for males and 4 or more drinks for females is typically taken as the definition of binge-drinking in western populations, we opted to define binge-drinking as 4 or more drinks for both males and females in this study because of the generally lower body mass of Chinese youth. These three questions were classified into four drinking categories of participants: never-drinkers who were defined as those who reported never having had one drink of alcohol in their life, ever-drinkers who were defined as those who had had at least one drink of alcohol in their life but not in the past 30 days, past 30-day drinkers who were defined as those who had had at least one drink of alcohol but did not consume 4 or more drinks of alcohol in a row in the past 30 days, and binge-drinkers who were defined as those who had had 4 or more drinks of alcohol in a row on at least one occasion in the past 30 days.
Drinking problems were assessed using the following item: “Indicate if any of the following things may have happened to you because of drinking alcohol within the past one year (mark all that apply).” The participants responded to the following 12 situations: “I was not able to do my homework or study for a test”, “I got into fights with other people (friends, relatives, strangers)”, “I went to school drunk”, “I caused shame or embarrassment to someone”, “I neglected my responsibilities”, “I was told by a friend, neighbor or relative to stop or cut down drinking”, “I felt that I needed more alcohol than I used to in order to get the same effect”, “I tried to control my drinking (for example: tried to drink only at certain times of the day or in certain places)”, “I missed a day (or part of a day) of school”, “I suddenly found myself in a place that I could not remember getting to”, “I passed out or fainted suddenly’, “I kept drinking when I promised myself not to”. Each response was assigned the score ‘1′ or ‘0′, which represent whether the corresponding response had been marked by participants or not, respectively. The score for drinking problems was the sum of the 12 items.
2.2.3.2. School academic performance
Students self-reported their academic performance in school by answering the following question: “What is your usual academic performance at your current school or the last school where you received grades?” The five response options ranged from: ‘Mostly A’s, or 90 or more points, or Superior’ to ‘Mostly F’s, or Below 60 points, or Failing’. A higher score represented a higher academic performance.
2.3. Procedures
Trained data collectors from the CCDCP and the University of Southern California provided written and verbal instructions to the students and administered the computer-based assessments and questionnaires in temporary computer labs set up at each school. Students completed the questionnaire and the computer-based assessments (the original and variant versions of the IGT and SOPT) during a 1-hour period. Students were provided with earphones to muffle any potentially distracting noises in the environment.
2.4. Data analysis
Data were analyzed with the Statistical Package for the Social Sciences for Windows, Version, 11.5.0 (SPSS Inc., Chicago, IL). Frequencies were generated to analyze demographic characteristics such as gender and school type and drinking variables of the entire sample and each category of drinkers. The gender, school type and drinking variables among different drinkers were analyzed by χ2 tests. The age among different drinkers was analyzed with ANOVA. Associations among drinking variables, the original IGT overall net score, working memory, and school academic performance were calculated as partial correlations (controlling for age, gender, and school type). For analyzing the profile of the IGT performance among drinkers we conducted between-within ANOVA tests with “Block” as within-subject factor. Differences of means on working memory performance were calculated using an independent sample t test. To reveal potential predictors of binge-drinking, logistic regression models were used with binge-drinking as the dependent variable and the original IGT overall net scores as the independent variable, conditioning on demographic characteristics, working memory and academic performance.
3. Results
Demographic and drinking characteristics of the sample are presented in Table 1. Both gender and school type were almost equally represented. The proportions of youth with drinking experience (ever + 30-day + binge) were not significantly different between males (64.1%) and females (51.9%) or between vocational (59.2%) and academic (56.7%) students in the whole sample (P > 0.1). Gender and school type also did not reveal any significant difference in drinkers (at any level) relative to never-drinkers (P > 0.1). However, there was significant age difference among drinkers (F(3,203) = 6.97 P = 0.001). Post hoc analysis showed the past 30-day drinkers were significantly older than other drinkers (never, ever, and binge) (P < 0.05).
Table 1.
Demographic characteristics of drinkers (N= 207)
| All (n) | Never-drinkers % (n) |
Ever-drinkers % (n) |
Past 30-day drinkers % (n) |
Binge-drinkers % (n) | Difference among drinkers | |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Female | 104 | 48.1 (50) | 25.0 (26) | 18.3 (19) | 8.7 (9) | χ2 (3)a = 3.77, P=29 |
| Male | 103 | 35.9(37) | 26.2 (27) | 25.2 (26) | 12.6 (13) | |
| Age (mean±S.D.) | 16.21±0.58 | 16.11±0.52 | 16.17±0.47 | 16.53±0.69 | 16.00±0.62 | F(3,203) = 6.97, P = 0.001 |
| School type | ||||||
| Academic | 104 | 43.3 (45) | 29.8 (31) | 20.2 (21) | 6.7 (7) | χ2(3)a = 4.74, P = 0.19 |
| Vocational | 103 | 40.8(42) | 21.4(22) | 23.3 (24) | 14.6 (15) | |
| How many days have at least one drink of alcohol during the past 30 days | ||||||
| 0 | 140 | 62.1 (87) | 37.9 (53) | χ2(2)b = 2.59, P = 0.27 | ||
| 1 or 2 days | 36 | 75.0 (27) | 25(9) | |||
| 3–9 days | 22 | 54.5 (12) | 45.5(10) | |||
| ≥10 days | 9 | 66.7 (6) | 333(3) | |||
| Drinking problems | ||||||
| 0 | 157 | 55.4(87) | 27.4(43) | 10.8 (17) | 6.4(10) | χ2(3) = 5.86b, P = 0.12 |
| 1 | 35 | 25.7(9) | 60(21) | 143(5) | χ3(3)c,d, P < 0.05 | |
| 2 | 11 | 9.1 (1) | 54.5 (6) | 36.4 (4) | ||
| 3 | 4 | 25(1) | 75(3) | |||
Comparing within never, ever, past 30-day and past 7-day drinkers.
Past 30-day vs. binge-drinkers.
Ever-drinkers vs. binge-drinkers.
Ever-drinkers vs. past 30-day drinkers. Percentage by row.
The number of days of having at least one drink of alcohol during the past 30-days was not significantly different between past 30-day and binge drinkers (P > 0.05). Both past 30-day drinkers and binge drinkers averaged 3–5 days of drinking in the past month. The highest number of drinking problems indicated by the past 30-day drinkers and binge-drinkers was 3. They did not differ in the number of drinking problems either. However, both past 30-day drinkers and binge-drinkers reported significantly more drinking problems than ever-drinkers (P < 0.05).
3.1. Partial correlations among drinking variables, original IGT, working memory and school academic performance
Table 2 reports partial correlations among drinking variables, original IGT, working memory, and school academic performance (adjusting for age, gender, and school type). The number of drinking days in the past month was significantly correlated with drinking problems (r = 0.41, P < 0.0001). Drinking days in the past month and drinking problems were not significantly correlated with the original IGT, working memory, or school academic performance (r < 0.1). Although there was a significant correlation as expected between working memory and school academic performance (r = 0.23, P < 0.001), the original IGT did not significantly correlate with either the working memory or school academic performance (r < 0.1).
Table 2.
Partial correlations among drinking variables, original IGT, working memory and school academic performance controlling for age, gender, and school type (N= 207)
| Measures |
||||
|---|---|---|---|---|
| 2 | 3 | 4 | 5 | |
| 1. Drinking days in past month | 0.41*** | −0.09 | −0.02 | −0.03 |
| 2. Drinking problems | – | −0.01 | −0.01 | 0.08 |
| 3. Original IGT | – | 0.03 | 0.07 | |
| 4. Working memory | – | 0.23** | ||
| 5. School academic performance | – | |||
Note: Results of two-tailed significance tests are denoted by superscripts.
P < 0.0001,
P < 0.05; IGT, Iowa Gambling Task.
3.2. Behavioral performance on the IGT
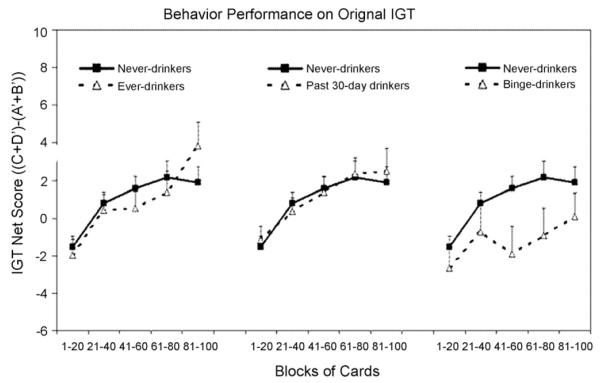
We subdivided the 100 card selections into five blocks of 20 cards each in the original version of the IGT. For each block, we counted the number of selections from Decks A’ and B’ (dis-advantageous) and the number of selections from Decks C’ and D’ (advantageous), and then derived a net score for that block ((C’ + D) – (A’ + B’)). A net score above zero implied that the participants were selecting cards advantageously, and a net score below zero implied disadvantageous selection. Fig. 1 presents the net scores as a function of group (never, ever, in the past 30 days, or binge drinkers) and block. The comparison of the plots shows that, as the task progressed, never-drinkers and ever-drinkers showed similar learning curves. They gradually switched their preferences toward the advantageous decks (C’ and D’) and away from the disadvantageous decks (A’ and B’), as reflected by increasingly positive net scores. A between-within ANOVA test did not reveal any significant difference in groups (never-drinkers vs. ever-drinkers) (F1,138 = 0.04; P = 0.85) or interaction between groups and blocks (F2.8,380.4 = 1.37; P = 0.24). A block effect (F2.8,380.4 = 10.98; P < 0.001) was significant after the Greenhouse-Geisser adjustment. The comparison of never-drinkers vs. past 30-day drinkers also revealed only a block effect (P < 0.001), and neither a group effect (never-drinkers vs. past 30-day drinkers) (F1,130 = 0.02; P = 0.88) nor an interaction (group by blocks) effect (F2.7,347.4 = 0.17; P = 0.95). These findings confirm that compared with never-drinkers, ever-drinkers and past 30-day drinkers did not differ in either their learning curves or overall net scores on the original version of the IGT.
Fig. 1.
The original version of the IGT net scores ((C’ + D’) – (A’ + B’)) by drinking status (never, ever, In the past 30 days, or binge-drinkers) across five blocks of 20 cards expressed as mean + S.E. Positive net scores reflect advantageous (non-impaired performance) while negative net scores reflect disadvantageous (impaired) performance.
Binge-drinkers showed a distinctly different pattern, however. Although binge-drinkers seemed to shift toward the good decks (decks with more favorable payoffs) initially, they did not select significantly more cards from the advantageous decks than from the disadvantageous ones even in the last block on the original version of the IGT (P > 0.05, net score in each block comparing to the score of 0). A between-within ANOVA test found a significant main effect for groups (never-drinkers vs. binge-drinkers) (F1,107 = 4.45; P < 0.05) and blocks (F2.5,272 = 3.29; P < 0.05) after the Greenhouse-Geisser adjustment. The interaction between groups and blocks was not significant.
Previous research showed that the IGT taps into two decision-making contexts, decisions under ambiguity in the first trials and decisions under risk in the latter trials (Brand, Recknor, Grabenhorst, & Bechara, 2007). We therefore computed the original IGT net score in the first 50 cards selected and last 50 cards selected. An independent sample t-test showed that binge-drinkers did not differ significantly in the first 50 cards selected compared to never-drinkers (mean ± S.D. = 3.91 13.05 vs. – 0.23 ± 10.93 respectively, P > 0.05). However, ± – binge-drinkers performed significantly worse relative to never-drinkers in the last 50 cards selected (mean S.D. = 2.19 15.03 5.13 respectively, – vs. ± 13.05, P < 0.05).±These results demonstrate ± that compared to never-drinkers, binge-drinkers showed worse IGT performance, as reflected by significantly lower overall IGT net scores especially in the latter trials of the original IGT.
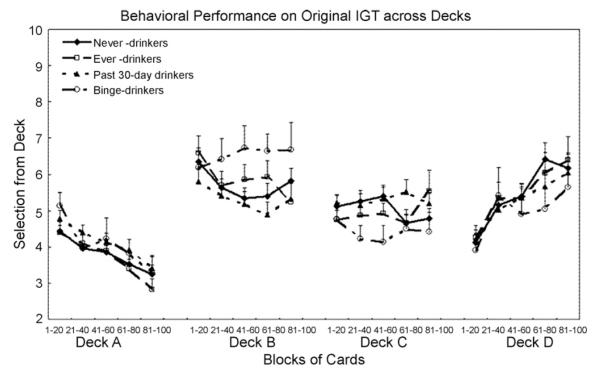
In light of more recent evidence reporting that individuals, especially adolescent girls, have a preference for decks with infrequent punishments (Decks B and D) (Overman et al., 2004), we looked further into patterns of scores from the four decks. In the whole sample, the 207 adolescents selected 44.44 9.54 cards (mean ± S.D.) from decks with infrequent punishments ± (Decks B and D) and 55.56 9.54 cards from decks with frequent punishment (Decks A±and C). The difference was significant (t206 = 8.38; P < 0.001). Then the question was whether the different performance on the IGT between binge drinkers and never drinkers could be explained by choosing decks with infrequent punishments versus decks with frequent punishments. The number of card selections from each deck is plotted by drinking status across the five blocks of 20 cards each in Fig. 2. The results show that compared to never drinkers, binge drinkers selected marginally significantly more from disadvantageous Deck B (F1,107 = 3.17; P < 0.08) and marginally significantly less from advantageous Deck C (F1,107 = 2.95; P < 0.09). However, the difference in card selections between decks with infrequent punishment (Decks B and D) and decks with frequent punishment (Decks A and C) was not significant in binge drinkers relative to never drinkers (t107 = 0.72; P = 0.48). There was no gender difference in such selections among the different groups of drinkers. Those results demonstrate that although adolescents chose significantly more cards from the decks with infrequent punishment (Decks B and D) than from the decks with frequent punishment (Decks A and C), the fact that binge drinkers performed worse on the IGT relative to never drinkers could not be explained by their preference for the decks with infrequent punishment.
Fig. 2.
The number of selections from each Deck in the original IGT by drinking status (never, ever, in the past 30 days, or binge-drinkers) across five blocks of 20 cards each expressed as mean + S.E.
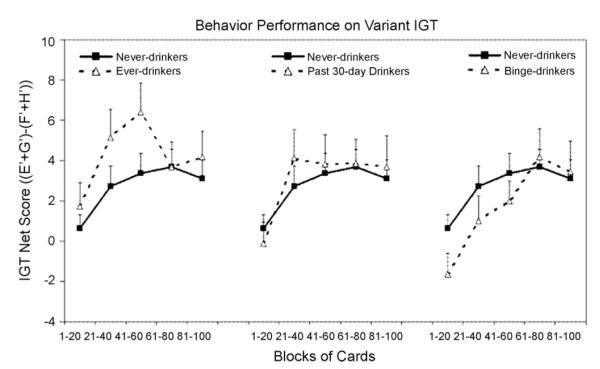
Fig. 3 presents the variant version of the IGT net scores by drinkers (drinking never, ever, in the past 30 days, or binge) across five blocks of 20 cards each. In this task, ever, past 30-day drinkers, and binge-drinkers all showed similar behavior performance compared to never drinkers. A between-within ANOVA test in groups (never-drinkers vs. ever-drinkers) revealed only a significant block effect (F2.9,403.2 = 5.81; P < 0.001) after the Greenhouse-Geisser adjustment, but did not reveal any significant difference in groups (never-drinkers vs. ever drinkers) (F1,138 = 1.69; P = 0.20) or interaction between groups and blocks (F2.9,403.2 = 1.09; P = 0.36). The comparison of never-drinkers vs. past 30-day drinkers also revealed only a block effect (F2.6,349.9 = 5.43; P < 0.001), and neither a group effect (never-drinkers vs. past 30-day drinkers) (F1,130 = 0.11; P = 0.73) nor an interaction (group by blocks) effect (F2.6,349.9 = 0.37; P = 0.83). Although binge drinkers performed poorly on the originally IGT, they performed normally on the variant IGT. The comparison of never-drinkers vs. binge drinkers revealed neither a group effect (never-drinkers vs. binge drinkers) (F1,107 = 0.45; P = 0.50) nor an interaction (group by blocks) effect (F2.8,302.5 = 0.65; P = 0.62) but only a block effect (F2.8,302.5 = 4.93; P < 0.001). Therefore, no significant difference in the main effect in the variant IGT of group or interaction effect between groups and blocks was found in each comparison of drinkers (at any level) and never-drinkers. They all gradually switched their preference toward the good decks (E’ and G’) which provided immediate losses but even higher future rewards and away from the bad decks (F’ and H’) which yielded lower immediate punishments but even lower long-term rewards, as reflected by increasingly positive net scores. However, as we mentioned before, on the original version of the IGT, relative to never-drinkers, binge-drinkers had selected significantly more cards from the disadvantageous decks which provided large rewards but even larger unpredictable future punishments instead of the advantageous decks which offered small reward but even smaller later punishments. Thus, these findings indicate that the impaired performance of binge-drinkers on the original version of the IGT is not due to their insensitivity to future consequence but their hypersensitivity to large reward.
Fig. 3.
The variant version of the IGT the net scores ((E’ + G’) – (F’ + H’)) by drinking status (never, ever, in the past 30 days, or binge-drinkers) across five blocks of 20 cards expressed as mean + S.E. Positive net scores reflect advantageous (non-impaired performance) while negative net scores reflect disadvantageous (impaired) performance.
Ever-drinkers seemed to have learned faster than the other three groups on the variant IGT. However, between-within ANOVA tests did not reveal statistically significant differences among groups (ever-drinkers vs. never/past 30-day/binge-drinkers) or an interaction effect between groups and blocks (P > 0.1). Although these results could have been indicative of ever-drinkers having a more explorative style, further studies are needed to address this possibility.
3.3. Working memory and school academic performance
Table 3 shows the mean scores of working memory and school academic performance by drinking involvement (never, ever, in the past 30 days, binge). The independent sample t tests revealed no differences on working memory and school academic performance for drinkers (at any level) compared to never-drinkers (P > 0.1).
Table 3.
Working memory and academic performance scores (mean±S.D.) among drinkers and never drinkers
| Never-drinkers | Ever-drinkers | Past 30-day drinkers | Binge-drinkers | |
|---|---|---|---|---|
| Working memory | 60.64 ± 7.46 | 62.89 ± 5.82 | 61.40 ± 7.00 | 61.14 ± 5.16 |
| School academic performance | 3.46 ± 1.07 | 3.39 ± 1.03 | 3.56 ± 1.16 | 3.14 ± 1.04 |
3.4. Variables predicting binge-drinking
Logistic regressions were performed to predict binge-drinking behavior. Original IGT overall net score, demographic variables, working memory, and academic performance were entered in the model. Results are presented in Table 4. Age produced a significant increase in the predictive power of the model (P < 0.05, OR = 0.37, 95% CI = 0.16, 0.89). Gender, school type, working memory and school academic performance did not predict binge-drinking behavior. After adjusting for demographic variables, working memory and academic performance, IGT was a significant predictor of binge-drinking behavior (P < 0.05, OR = 0.98, 95% CI = 0.95, 0.99). Better IGT performance predicted less binge-drinking.
Table 4.
Summary of logistic regression analysis for variables predicting binge-drinking behavior (N= 207)
| Models | B | S.E. | Exponential (B) | 95% CI for exponential (B) |
|
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Age | −0.99 | 0.44 | 0.37* | 0.16 | 0.89 |
| Gendera | 0.73 | 0.50 | 2.07 | 0.78 | 5.4 |
| School typeb | 1.25 | 0.57 | 3.48 | 0.94 | 10.75 |
| Original IGT net score | −0.02 | 0.01 | 0.98* | 0.95 | 0.99 |
| Working memory | 0.03 | 0.05 | 1.03 | 0.95 | 1.13 |
| School academic performance | −0.06 | 0.25 | 0.94 | 0.05 | 1.51 |
Female as reference group.
Academic school as reference group. IGT, Iowa Gambling Task.
P < 0.05.
4. Discussion
To our knowledge, there has been only one previous study to show that deficits in affective decision-making (as measured by an analogous IGT task) are associated with heavy alcohol use in adolescents (Overman et al., 2004). The current study presents a more detailed analysis than Overman and is in part an extension of that study. By avoiding heavy, prolonged substance abuse, the study provides support for a potential pre-existing neural basis for development of substance abuse in adolescence. This is the first study to apply laboratory-based neuropsychological assessments to the normal adolescent population in China, extending the generalizability of the relationship between affective decision-making and substance use across cultures.
The key finding of this study supports our primary hypothesis that adolescent binge drinkers would show impaired IGT performance, as reflected by significantly lower overall net scores on the original version of the IGT especially in the latter trials, in comparison to never-drinkers. Furthermore, binge-drinkers performed normally on the variant version of the IGT, supporting our secondary hypothesis that hypersensitivity to reward accounts for the observed “myopia” regarding the future. Finally, binge-drinkers showed normal scores on working memory capacity and academic performance, and logistic regression analysis showed that after controlling for the demographic variables, working memory and academic performance, the IGT significantly predicted binge-drinking. Thus, as we expected, the impaired performance on the original version of the IGT cannot be attributed to poor working memory. These findings indicate that decision-making impairment can be separated from general cognitive intelligence impairment, consistent with an OFC/VMPC dysfunction but not a DLPC dysfunction.
In this study, binge-drinkers performed worse in the IGT relative to never-drinkers especially in the latter trials of the original IGT. This response pattern can be understood at least partially in the light of previous research showing that the IGT taps into two decision-making contexts, decisions under ambiguity in the first trials, and decisions under risk in the latter trials (Brand et al., 2007). Thus, these results suggest that binge-drinkers might have developed some awareness or gut feeling about the relative payoffs of good and bad decks in the early trials, but over the set of later blocks did not choose the advantageous decks in a manner similar to non-binge drinkers. Consistent with the original IGT results which suggest that binge-drinker showed impaired decision-making capacity, especially the decisions under risk, adolescent binge-drinkers performed normally on the variant version compared to never-drinkers. Taken together, these results provide support for the hypothesis that the failure of binge-drinkers to decide advantageously is due to hypersensitivity to reward. These results are consistent with many previous studies, which demonstrated the willingness of adolescents to take social, physical, and financial risks to obtain arousing, novel, and rewarding experiences associated with alcohol and other substance use (Donohew et al., 1999; Martin et al., 2002).
To our knowledge, there is only one previous study to investigate affective decision-making relative to adolescent drinking and other substance use behavior with an IGT analogous task (Overman et al., 2004). Although the researchers in that study did not distinguish different levels of drinking as we did, they found that poly-substance use was negatively correlated with performance on the IGT analogous task and individuals preferred selecting cards from decks with infrequent punishments (Decks B and D). Our study captures an earlier stage in progression across abuse trajectories, and provides additional evidence to support the hypothesis that diminished decision-making capacity might be a causal factor in the progression toward habitual and abusive levels of alcohol use. Indeed, our results are consistent with Overman’s report (2004) that people have a preference for decks with infrequent punishment (Decks B and D). However, this tendency could not explain the underperformance of binge drinkers on the original IGT relative to never drinkers. The results from our study suggest that the apparent failure in decision-making of binge-drinkers may stem from their inability to suspend a dominant approach set induced by immediately-rewarded choices, rather than their insensitivity to long-term consequences. In other words, their affective response to the immediate reward is so potent that any negative signal about future consequences becomes relatively ineffective, and perhaps overridden by immediate reward.
This is a behavioral study, and we can only speculate about underlying neural mechanisms underlying hypersensitivity to reward in adolescence from several lines of previous research. Several studies suggest that exaggerated processing of reward in adolescents might be linked to the amygdala-ventral striatum system (Andersen, 2003; Dahl, 2004; Nelson, Leibenluft, McClure, & Pine, 2005); this system is important for automatic and obligatory affective/emotional responses and stimulus-reward (incentive) learning (Bechara, Damasio, & Damasio, 2003; Bechara, 2004a, 2004b; Buchel, Morris, Dolan, & Friston, 1998). Other research demonstrates that the prefrontal cortex may not develop fully until the age of 21 (Giedd, 2004; Gogtay et al., 2004), suggesting that the ability to exercise control over powerful temptations is still developing through adolescence. Therefore, some researchers have proposed that the lag in development between the prefrontal lobe and subcortical regions among adolescents may underlie poor decision-making that predisposes some adolescents to drug use and ultimately, addiction (Chambers, Taylor, & Potenza, 2003; Galvan et al., 2006).
Our finding that working memory was strongly correlated with school academic performance is consistent with previous studies, which indicates that working memory is highly correlated with fluid intelligence (Kane & Engle, 2002), and other high-level cognitive skills such as reading, mathematics, and reasoning (Colom et al., 2004; Engle et al., 1992; Jarrold & Towse, 2006). Compared to never-drinkers, binge-drinkers performed normally on the working memory and scored normally on the school academic performance. In addition, the original IGT significantly predicted the binge-drinking behavior after controlling for the working memory, school academic performance, and other demographic variables. These results suggest that the decision-making impairment among binge drinkers did not result from the type of general cognitive intelligence impairment most likely revealed by tests of working memory (i.e., fluid intelligence). This finding agrees with previous reports of asymmetrical dependent relationship between working memory and decision-making (Bechara & Martin, 2004). That is, working memory is not dependent on the intactness of decision making, while decision-making seems to be influenced by the intactness or impairment of working memory. Therefore, subjects can have deficits in decision-making in the presence of normal working memory. But poor working memory can compromise decision-making. Such an asymmetrical dependent relationship is also supported by several lines of evidence, which suggest that the OFC/VMPC plays a critical role in coupling “cold” cognitive systems dependent on DLPC systems and “hot” affective and emotional systems, such as the mesolimbic reward system, which assigns affective/emotional value to individual experiences associated with reward and punishment (Anderson, Barrash, Bechara, & Tranel, 2006; Beer, John, Scabini, & Knight, 2006; Fellows & Farah, 2005; O’Doherty et al., 2001; Oya et al., 2005).
It is unlikely that the SOPT was too simple a task to detect individual differences because there was no ceiling effect in our study. Moreover, another study has demonstrated that compared to the brain processes assessed by other working memory tasks, the one taxed by an SOPT-similar task develops later in adolescence (Luciana, Conklin, Hooper, & Yarger, 2005). Thus, although considerable evidence shows that both the anterior and medial sectors of the OFC/VMPC and DLPC (especially Brodmann’s Area 10) continue to develop throughout adolescence, and they are among the last brain regions to mature (Fuster, 2002; Giedd, 2004; Gogtay et al., 2004; Paus, 2005; Spear, 2000), our results are consistent with the notion that difficulty with affective regulation in adolescents cannot be explained by their cognitive intelligence (Steinberg, 2005). Previous studies also indicate that in adolescents, developmental improvements in the IGT performance could not be explained by developmental changes in working memory capacity and inductive reasoning (Crone & van der Molen, 2004; Hooper et al., 2004; Overman et al., 2004). Indeed, adolescents are often capable of explaining reward probabilities, despite making disadvantageous decisions (Crone, Jennings et al., 2004; Crone, Somsen et al., 2004). Many aspects of adolescent cognitive functions have been shown to be equivalent to those of adults under laboratory situations, but they show greater deterioration under more real-life, stressful conditions (Steinberg, 2004); adolescents with well-developed decision-making abilities demonstrated under non-emotional conditions seem to have a much more difficult time making a responsible choice under intense emotional arousal (Arnsten & Shansky, 2004). Therefore, our results suggest that binge-drinkers share the same logical competencies of other adolescents, but their actual decision-making ability was relatively compromised.
In the present study, the relationship between affective decision-making and binge-drinking might be affected by other factors such as gender, personality traits, and previous alcohol consumption. In general, several reports have shown that boys outperform girls on the IGT (or modified versions for younger children) in infants (Garon & Moore, 2004), childhood (Crone & van der Molen, 2004), adolescence (Overman, 2004), and adulthood (Reavis & Overman, 2001). In this study, we looked at the IGT results from males and females separately, and we found that males marginally significantly selected more cards from advantageous decks than females in the latter trials. Specifically, compared to males, females marginally significantly selected more cards from the disadvantageous Deck B (the deck with infrequent punishment) (see Appendix A). These results are consistent with previous studies showing that adolescent males chose significantly more advantageous cards in the latter trials of the IGT than adolescent females, and that the reason for the relative female underperformance seems to relate to females’ expressing preference for decks with infrequent punishment, such as Deck B (Overman, 2004). However, the difference in our results does not seem as robust as that reported elsewhere. There could be several reasons for these differences, but the most obvious one in this case might be the difference in culture. Although results from the logistic regression model in our study show that gender did not predict binge-drinking in these Chinese adolescents, the topic of gender differences in decision-making, and especially in terms of susceptibility to substance abuse, is an intriguing topic that warrants intensive research as demonstrated by recent findings (Bolla, Eldreth, Matochik, & Cadet, 2004; Overman et al., 2004; Preston, Buchanan, Stansfield, & Bechara, 2007).
Previous studies showed that performance on the IGT is impaired in young people with high sensation-seeking (Crone, Vendel, & van der Molen, 2003), antisocial personality (Mazas, Finn, & Steinmetz, 2000), and early-onset schizophrenia (Kester et al., 2006). We measured a number of personality traits potentially related to binge-drinking, including sensation-seeking, hostility, aggressiveness, Attention Deficit Hyperactivity Disorder (ADHD), depression, perceived stress, and impulsivity, but we did not find any confounding effects of these variables. The IGT net score did not correlate with any personality traits, and the affective decision-making still significantly predicted binge-drinking behavior after adjusting for these personality traits and demographic variables (see Appendix B).
The prevalence of drinking behaviors such as ever, past 30-day, and binge-drinking in this study was similar to one previous large scale study in China (Xing, Ji, & Zhang, 2006). In this study, several indicators suggest that it is unlikely that the relationship between the original IGT and binge-drinking observed in this study could have resulted from structural or functional neural changes consequent to prior alcohol consumption. First, the results showed that the days drinking in past month and drinking problem did not correlate with the original IGT, working memory, and academic performance. Second, binge drinkers in our sample indicated having drunk just a few days in the past month, and most of them indicated having had none or only one drinking problem in the past year. Moreover, we found that 15 past 30-day drinkers selected the item “I tried to control my drinking (for example: tried to drink only at certain times of the day or in certain places)”, suggesting they had drinking control problems. Although past 30-day drinkers generally performed normally on the original version of the IGT, the participants with control problems showed marginally significant lower net scores on original version than other past 30-day drinkers (mean ± S.D. = 0.80 13.64 vs. 8.53 17.27, respectively). All these findings – suggest ± that a deficit± in affective decision-making might predate and predis-pose some adolescents to binge-style drinking. However, this issue cannot be resolved with this cross-sectional study. A longitudinal approach is necessary.
The notion that poor decision-making might render some adolescents more vulnerable to binge-drinking is consistent with a previous imaging study that investigated the brain structures of high-and low-risk youth prior to the time of any extensive alcohol involvement. It showed that high familial-risk youth had a significantly smaller right amygdala volume but normal hippocampus volume compared to low-risk youth (Hill, 2004). Other studies have revealed that among adolescents with alcohol use disorders there were cognitive functioning problems including working memory impairment and memoryrelated brain structure alteration (Caldwell et al., 2005; De Bellis et al., 2000; Sher, 2006). Together with this study, these previous findings might suggest that whereas adolescent alcohol abuse might lead to some neural function alterations (such as DLPC-hippocampus circuitry related-memory functions), other neural characteristics of high-risk youth (such as OFC/VMPC-amygdala circuitry-related emotions and decisionmaking) may predate alcohol use and may reflect risk factors for, rather than the consequence of, adolescent alcohol abuse. However, further longitudinal research is needed to detail determinants and consequences of adolescent alcohol abuse and to identify potential protective factors in order to reduce the excessive use of alcohol during this critical developmental period.
In conclusion, this study found that binge-drinkers performed poorly on the original version of the IGT but not on the variant version, and they scored normally on working memory and school academic performance. It suggests that poor affective decision-making might predispose adolescents to develop a habit of heavy drinking. Future research should address whether impaired decision-making in high-risk adolescents is linked to genetics, environments, or both potential sources of variability. Furthermore, research should address whether interventions that enhance these high-risk adolescents’ social-emotional capacities might benefit their real-life decision-making, and thus avert potential alcohol and substance abuse (Bechara, Damasio, & Bar-On, 2006). Transdisciplinary research on decision-making integrating neurocognitive and intervention sciences might be a promising way for improving well-being, and reducing adolescents’ risks for substance use and abuse.
Supplementary Material
Acknowledgements
This research was supported by the University of Southern California Pacific Rim Transdisciplinary Tobacco and Alcohol Use Research Center TTAURC funded by the National Institutes of Health (grant #1 P50 CA84735 to C. Anderson Johnson) and the National Institute on Drug Abuse (grant #DA16708 to Antoine Bechara and DA 16094 to Alan Stacy). The authors thank Peggy Gallaher, Steven Cen, Joel Milam, Qian Guo, Kari-Lyn Kobayakawa Sakuma, and Janet Okamoto for their contribution to this project. We also thank Xiaolu Fu, Yong Jia, Qiong Wang, Rong Lu, Jiang Miao, Yi Yuan, and Jingfu Shi for their assistance with project coordination and data collection. Finally, we express our gratitude to the municipal government, Health Bureau, and Education Committee in Chengdu, China for their support.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.neuropsychologia. 2007.09.012.
References
- Andersen SL. Trajectories of brain development: Point of vulnerability or window of opportunity? Neuroscience and Biobehavioral Reviews. 2003;27(1/2):3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Anderson SW, Barrash J, Bechara A, Tranel D. Impairments of emotion and real-world complex behavior following childhood- or adult-onset damage to ventromedial prefrontal cortex. Journal of the International Neuropsychological Society. 2006;12(2):224–235. doi: 10.1017/S1355617706060346. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Shansky RM. Adolescence: Vulnerable period for stress-induced prefrontal cortical function? Introduction to part IV. Annals of the New York Academy of Sciences. 2004;1021:143–147. doi: 10.1196/annals.1308.017. [DOI] [PubMed] [Google Scholar]
- Bechara A. Risky business: Emotion, decision-making, and addiction. Journal of Gambling Studies. 2003;19(1):23–51. doi: 10.1023/a:1021223113233. [DOI] [PubMed] [Google Scholar]
- Bechara A. Disturbances of emotion regulation after focal brain lesions. International Review of Neurobiology. 2004a;62:159–193. doi: 10.1016/S0074-7742(04)62006-X. [DOI] [PubMed] [Google Scholar]
- Bechara A. The role of emotion in decision-making: Evidence from neurological patients with orbitofrontal damage. Brain Cognition. 2004b;55(1):30–40. doi: 10.1016/j.bandc.2003.04.001. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Bar-On R. The anatomy of emotional intelligence and the implications for educating people to be emotionally intelligent. In: Bar-On R, Maree JG, Elias MJ, editors. Education people to be emotionally intelligent. Heinemann Educational Publishers; SA, Johannesburg: 2006. pp. 223–237. [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50(1–3):7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex. 2000;10(3):295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Role of the amygdala in decision-making. Annals of the New York Academy of Sciences. 2003;985:356–369. doi: 10.1111/j.1749-6632.2003.tb07094.x. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. Journal of Neuroscience. 1999;19(13):5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Anderson SW. Dissociation of working memory from decision making within the human prefrontal cortex. Journal of Neuroscience. 1998;18(1):428–437. doi: 10.1523/JNEUROSCI.18-01-00428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Hindes A. Decision-making and addiction (part II): Myopia for the future or hypersensitivity to reward? Neuropsychologia. 2002;40(10):1690–1705. doi: 10.1016/s0028-3932(02)00016-7. [DOI] [PubMed] [Google Scholar]
- Bechara A, Martin EM. Impaired decision making related to working memory deficits in individuals with substance addictions. Neuropsychology. 2004;18(1):152–162. doi: 10.1037/0894-4105.18.1.152. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000;123(Pt 11):2189–2202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- Beer JS, John OP, Scabini D, Knight RT. Orbitofrontal cortex and social behavior: Integrating self-monitoring and emotion-cognition interactions. Journal of Cognitive Neuroscience. 2006;18(6):871–879. doi: 10.1162/jocn.2006.18.6.871. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Sex-related differences in a gambling task and its neurological correlates. Cerebral Cortex. 2004;14(11):1226–1232. doi: 10.1093/cercor/bhh083. [DOI] [PubMed] [Google Scholar]
- Bonomo YA, Bowes G, Coffey C, Carlin JB, Patton GC. Teenage drinking and the onset of alcohol dependence: A cohort study over seven years. Addiction. 2004;99(12):1520–1528. doi: 10.1111/j.1360-0443.2004.00846.x. [DOI] [PubMed] [Google Scholar]
- Brand M, Recknor EC, Grabenhorst F, Bechara A. Decisions under ambiguity and decisions under risk: Correlations with executive functions and comparisons of two different gambling tasks with implicit and explicit rules. Journal of Clinical and Experimental Neuropsychology. 2007;29(1):86–99. doi: 10.1080/13803390500507196. [DOI] [PubMed] [Google Scholar]
- Buchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: An event-related fMRI study. Neuron. 1998;20(5):947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- Caldwell LC, Schweinsburg AD, Nagel BJ, Barlett VC, Brown SA, Tapert SF. Gender and adolescent alcohol use disorders on BOLD (blood oxygen level dependent) response to spatial working memory. Alcohol Alcohol. 2005;40(3):194–200. doi: 10.1093/alcalc/agh134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauffman E, Steinberg L. Im)maturity of judgment in adolescence: Why adolescents may be less culpable than adults. Behavioral Sciences & The Law. 2000;18(6):741–760. doi: 10.1002/bsl.416. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: A critical period of addiction vulnerability. American Journal of Psychiatry. 2003;160(6):1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaytor N, Schmitter-Edgecombe M. Working memory and aging: A cross-sectional and longitudinal analysis using a self-ordered pointing task. Journal of the International Neuropsychological Society. 2004;10(4):489–503. doi: 10.1017/S1355617704104013. [DOI] [PubMed] [Google Scholar]
- Chey J, Lee J, Kim YS, Kwon SM, Shin YM. Spatial working memory span, delayed response and executive function in schizophrenia. Psychiatry Research. 2002;110(3):259–271. doi: 10.1016/s0165-1781(02)00105-1. [DOI] [PubMed] [Google Scholar]
- Colom R, Rebello I, Palacios A, Juan-Espinosa M, Kyllonen PC. Working memory is (almost) perfectly predicted by g. Intelligence. 2004;32:277–296. [Google Scholar]
- Crone EA, Jennings JR, Van der Molen MW. Developmental change in feedback processing as reflected by phasic heart rate changes. Developmental Psychology. 2004;40(6):1228–1238. doi: 10.1037/0012-1649.40.6.1228. [DOI] [PubMed] [Google Scholar]
- Crone EA, Somsen RJ, Van Beek B, Van Der Molen MW. Heart rate and skin conductance analysis of antecendents and consequences of decision making. Psychophysiology. 2004;41(4):531–540. doi: 10.1111/j.1469-8986.2004.00197.x. [DOI] [PubMed] [Google Scholar]
- Crone EA, van der Molen MW. Developmental changes in real life decision making: Performance on a gambling task previously shown to depend on the ventromedial prefrontal cortex. Developmental Neuropsychology. 2004;25(3):251–279. doi: 10.1207/s15326942dn2503_2. [DOI] [PubMed] [Google Scholar]
- Crone EA, Vendel I, van der Molen MW. Decision-making in disinhibited adolescents and adults:insensitivity to future consequences or driven by immediate reward? Personality and Individual Differences. 2003;35:1625–1641. [Google Scholar]
- Dahl RE. The development of affect regulation: Bringing together basic and clinical perspectives. Annals of the New York Academy of Sciences. 2003;1008:183–188. doi: 10.1196/annals.1301.019. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Adolescent brain development: A period of vulnerabilities and opportunities. Keynote address. Annals of the New York Academy of Sciences. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, et al. Hippocampal volume in adolescent-onset alcohol use disorders. American Journal of Psychiatry. 2000;157(5):737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- De Neys W, Schaeken W, d’Ydewalle G. Working memory and counterexample retrieval for causal conditionals. Thinking & Reasoning. 2005a;11:123–150. [Google Scholar]
- De Neys W, Schaeken W, d’Ydewalle G. Working memory and everyday conditional reasoning: Retrieval and inhibition of stored counterexamples. Thinking & Reasoning. 2005b;11:349–381. [Google Scholar]
- Donohew RL, Hoyle RH, Clayton RR, Skinner WF, Colon SE, Rice RE. Sensation seeking and drug use by adolescents and their friends: Models for marijuana and alcohol. Journal of Studies on Alcohol. 1999;60(5):622–631. doi: 10.15288/jsa.1999.60.622. [DOI] [PubMed] [Google Scholar]
- Durston S, Casey BJ. What have we learned about cognitive development from neuroimaging? Neuropsychologia. 2006;44(11):2149–2157. doi: 10.1016/j.neuropsychologia.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Elliott R, Friston KJ, Dolan RJ. Dissociable neural responses in human reward systems. Journal of Neuroscience. 2000;20(16):6159–6165. doi: 10.1523/JNEUROSCI.20-16-06159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle RW, Cantor J, Carullo JJ. Individual differences in working memory and comprehension: A test of four hypotheses. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1992;18:972–992. doi: 10.1037//0278-7393.18.5.972. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, et al. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25(4):1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, McClure EB, Monk CS, Munson S, Eshel N, et al. Choice selection and reward anticipation: An fMRI study. Neuropsychologia. 2004;42(12):1585–1597. doi: 10.1016/j.neuropsychologia.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Faw B. Pre-frontal executive committee for perception, working memory, attention, long-term memory, motor control, and thinking: A tutorial review. Consciousness and Cognition. 2003;12(1):83–139. doi: 10.1016/s1053-8100(02)00030-2. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Different underlying impairments in decision-making following ventromedial and dorsolateral frontal lobe damage in humans. Cerebral Cortex. 2005;15(1):58–63. doi: 10.1093/cercor/bhh108. [DOI] [PubMed] [Google Scholar]
- Finn PR, Mazas CA, Justus AN, Steinmetz J. Early-onset alco-holism with conduct disorder: Go/no go learning deficits, working memory capacity, and personality. Alcoholism-Clinical and Experimental Research. 2002;26(2):186–206. [PubMed] [Google Scholar]
- Fuster JM. Frontal lobe and cognitive development. Journal of Neurocytology. 2002;31(3–5):373–385. doi: 10.1023/a:1024190429920. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. Journal of Neuroscience. 2006;26(25):6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon N, Moore C. Complex decision-making in early childhood. Brain Cognition. 2004;55(1):158–170. doi: 10.1016/S0278-2626(03)00272-0. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley DE, Elsabagh S, File SE. Binge drinking and sex: Effects on mood and cognitive function in healthy young volunteers. Pharmacology Biochemistry and Behavior. 2004;78(3):611–619. doi: 10.1016/j.pbb.2004.04.027. [DOI] [PubMed] [Google Scholar]
- Hill SY. Trajectories of alcohol use and electrophysiological and morphological indices of brain development: Distinguishing causes from consequences. Annals of the New York Academy of Sciences. 2004;1021:245–259. doi: 10.1196/annals.1308.029. [DOI] [PubMed] [Google Scholar]
- Hooper CJ, Luciana M, Conklin HM, Yarger RS. Adolescents’ performance on the Iowa Gambling Task: Implications for the development of decision making and ventromedial prefrontal cortex. Developmental Psychology. 2004;40(6):1148–1158. doi: 10.1037/0012-1649.40.6.1148. [DOI] [PubMed] [Google Scholar]
- Jarrold C, Towse JN. Individual differences in working memory. Neuroscience. 2006;139(1):39–50. doi: 10.1016/j.neuroscience.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Jennison KM. The short-term effects and unintended long-term consequences of binge drinking in college: A 10-year follow-up study. American Journal of Drug and Alcohol Abuse. 2004;30(3):659–684. doi: 10.1081/ada-200032331. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. Working-memory capacity, proactive interference, and divided attention: Limits on long-term memory retrieval. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2000;26(2):336–358. doi: 10.1037//0278-7393.26.2.336. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: An individual-differences perspective. Psychonomic Bulletin & Review. 2002;9(4):637–671. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- Kerr A, Zelazo PD. Development of “hot” executive function: The children’s gambling task. Brain Cognition. 2004;55(1):148–157. doi: 10.1016/S0278-2626(03)00275-6. [DOI] [PubMed] [Google Scholar]
- Kester HM, Sevy S, Yechiam E, Burdick KE, Cervellione KL, Kumra S. Decision-making impairments in adolescents with early-onset schizophrenia. Schizophrenia Research. 2006;85(1–3):113–123. doi: 10.1016/j.schres.2006.02.028. [DOI] [PubMed] [Google Scholar]
- Kolb B, Pellis S, Robinson TE. Plasticity and functions of the orbital frontal cortex. Brain Cognition. 2004;55(1):104–115. doi: 10.1016/S0278-2626(03)00278-1. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: Linking reward to hedonic experience. Nature Reviews Neuroscience. 2005;6(9):691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Luciana M, Conklin HM, Hooper CJ, Yarger RS. The development of nonverbal working memory and executive control processes in adolescents. Child Development. 2005;76(3):697–712. doi: 10.1111/j.1467-8624.2005.00872.x. [DOI] [PubMed] [Google Scholar]
- Martin CA, Kelly TH, Rayens MK, Brogli BR, Brenzel A, Smith WJ, et al. Sensation seeking, puberty, and nicotine, alcohol, and marijuana use in adolescence. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(12):1495–1502. doi: 10.1097/00004583-200212000-00022. [DOI] [PubMed] [Google Scholar]
- May JC, Delgado MR, Dahl RE, Stenger VA, Ryan ND, Fiez JA, et al. Event-related functional magnetic resonance imaging of reward-related brain circuitry in children and adolescents. Biological Psychiatry. 2004;55(4):359–366. doi: 10.1016/j.biopsych.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Mazas CA, Finn PR, Steinmetz JE. Decision-making biases, antisocial personality, and early-onset alcoholism. Alcoholism-Clinical and Experimental Research. 2000;24(7):1036–1040. [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: A neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine. 2005;35(2):163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience. 2001;4(1):95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Overman WH. Sex differences in early childhood, adolescence, and adulthood on cognitive tasks that rely on orbital prefrontal cortex. Brain Cognition. 2004;55(1):134–147. doi: 10.1016/S0278-2626(03)00279-3. [DOI] [PubMed] [Google Scholar]
- Overman WH, Frassrand K, Ansel S, Trawalter S, Bies B, Redmond A. Performance on the IOWA card task by adolescents and adults. Neuropsychologia. 2004;42(13):1838–1851. doi: 10.1016/j.neuropsychologia.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Oya H, Adolphs R, Kawasaki H, Bechara A, Damasio A, Howard MA., III Electrophysiological correlates of reward prediction error recorded in the human prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(23):8351–8356. doi: 10.1073/pnas.0500899102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends in Cognitive Sciences. 2005;9(2):60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Perry W, Heaton RK, Potterat E, Roebuck T, Minassian A, Braff DL. Working memory in schizophrenia: Transient “online” storage versus executive functioning. Schizophrenia Bulletin. 2001;27(1):157–176. doi: 10.1093/oxfordjournals.schbul.a006854. [DOI] [PubMed] [Google Scholar]
- Peterson JB, Pihl RO, Higgins D, Lee A. NeuroCognitive battery. Version 2.0 Deerfield Beach; FL: 2002. [Google Scholar]
- Petrides M. Impairments on nonspatial self-ordered and externally ordered working memory tasks after lesions of the mid-dorsal part of the lateral frontal cortex in the monkey. Journal of Neuroscience. 1995;15(1 Pt 1):359–375. doi: 10.1523/JNEUROSCI.15-01-00359.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Alivisatos B, Meyer E, Evans AC. Functional activation of the human frontal cortex during the performance of verbal working memory tasks. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(3):878–882. doi: 10.1073/pnas.90.3.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Milner B. Deficits on subject-ordered tasks after frontal- and temporal-lobe lesions in man. Neuropsychologia. 1982;20(3):249–262. doi: 10.1016/0028-3932(82)90100-2. [DOI] [PubMed] [Google Scholar]
- Preston SD, Buchanan TW, Stansfield RB, Bechara A. Effects of anticipatory stress on decision making in a gambling task. Behavioral Neuroscience. 2007;121(2):257–263. doi: 10.1037/0735-7044.121.2.257. [DOI] [PubMed] [Google Scholar]
- Pukrop R, Matuschek E, Ruhrmann S, Brockhaus-Dumke A, Tendolkar I, Bertsch A, et al. Dimensions of working memory dysfunction in schizophrenia. Schizophrenia Research. 2003;62(3):259–268. doi: 10.1016/s0920-9964(02)00427-9. [DOI] [PubMed] [Google Scholar]
- Reavis R, Overman WH. Adult sex differences on a decision-making task previously shown to depend on the orbital prefrontal cortex. Behavioral Neuroscience. 2001;115(1):196–206. doi: 10.1037/0735-7044.115.1.196. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, et al. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: Evidence for monoaminergic mechanisms. Neuropsychopharmacology. 1999;20(4):322–339. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- Sher L. Functional magnetic resonance imaging in studies of neurocognitive effects of alcohol use on adolescents and young adults. International Journal of Adolescent Medicine and Health. 2006;18(1):3–7. doi: 10.1515/ijamh.2006.18.1.3. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Risk taking in adolescence: What changes, and why? Annals of the New York Academy of Sciences. 2004;1021:51–58. doi: 10.1196/annals.1308.005. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Cognitive and affective development in adolescence. Trends in Cognitive Sciences. 2005;9(2):69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Thush C, Wiers RW. Explicit and implicit alcohol-related cognitions and the prediction of future drinking in adolescents. Addictive Behaviors. 2007;32(7):1367–1383. doi: 10.1016/j.addbeh.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM, Sowell ER. Mapping brain maturation. Trends in Neurosciences. 2006;29(3):148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull OH, Evans CE, Bunce A, Carzolio B, O’Connor J. Emotion-based learning and central executive resources: An investigation of intuition and the Iowa Gambling Task. Brain Cognition. 2005;57(3):244–247. doi: 10.1016/j.bandc.2004.08.053. [DOI] [PubMed] [Google Scholar]
- Ward H, Shum D, McKinlay L, Baker-Tweney S, Wallace G. Development of prospective memory: Tasks based on the prefrontal-lobe model. Child Neuropsychology. 2005;11(6):527–549. doi: 10.1080/09297040490920186. [DOI] [PubMed] [Google Scholar]
- Xing Y, Ji C, Zhang L. Relationship of binge drinking and other health-compromising behaviors among urban adolescents in China. Journal of Adolescent Health. 2006;39(4):495–500. doi: 10.1016/j.jadohealth.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Zeigler DW, Wang CC, Yoast RA, Dickinson BD, McCaffree MA, Robinowitz CB, et al. The neurocognitive effects of alcohol on adolescents and college students. Preventive Medicine. 2005;40(1):23–32. doi: 10.1016/j.ypmed.2004.04.044. [DOI] [PubMed] [Google Scholar]
- Zetteler JI, Stollery BT, Weinstein AM, Lingford-Hughes AR. Attentional bias for alcohol-related information in adolescents with alcoholdependent parents. Alcohol Alcohol. 2006;41(4):426–430. doi: 10.1093/alcalc/agl026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.