Abstract
Patients with type 2 diabetes mellitus (T2DM) frequently require multiple therapies to effectively control hyperglycemia, and many new agents for glucose control have been developed over the past few decades. Linagliptin is a recently approved oral antidiabetic drug that acts by inhibiting the enzyme dipeptidyl peptidase-4 (DPP-4). Unlike other DPP-4 inhibitors, linagliptin is excreted chiefly via the enterohepatic system, and can be used without dose adjustment in patients with renal or hepatic impairment. Linagliptin was approved by the US Food and Drug Administration based on a large development program, including four pivotal trials in patients with T2DM, assessing the efficacy and safety of linagliptin when used as monotherapy or in combination with other oral antidiabetes drugs. Linagliptin was associated with significant improvements in glycosylated hemoglobin, fasting plasma glucose and postprandial glucose, and more patients receiving linagliptin showed meaningful improvements and achieved targets for glycosylated hemoglobin. Linagliptin was well tolerated, with an adverse event profile similar to that of placebo, and low rates of hypoglycemic events. Taken together, the pivotal trials confirm linagliptin is effective and safe in patients with T2DM: the convenience of oral dosing with no requirement for dose adjustment in patients with renal or hepatic impairment make linagliptin a valuable option when considering therapies for patients with T2DM.
Keywords: DPP-4 inhibitor, linagliptin, oral antidiabetic drug, Tradjenta, Trajenta, type 2 diabetes
Introduction
Type 2 diabetes mellitus (T2DM) is a progressive disease, and it occurs with increasing prevalence in the elderly and those with other comorbidities. Blood glucose control presents a challenge that is magnified by these co-existing problems. To achieve glycemic targets, many patients need more than one antidiabetic drug, and additional medications are often required as glucose control deteriorates [Nathan et al. 2009]. Consequently, the development of new antidiabetic drugs that can help meet this challenge has been an area of intensive research.
The dipeptidyl peptidase-4 (DPP-4) inhibitors are one of the recently developed therapeutic classes for treatment of hyperglycemia in T2DM. The various agents in the class have differing chemical structures, but all act by inhibiting the DPP-4 enzyme, thus prolonging the life of incretin hormones, which in turn raise insulin levels and suppress glucagon secretion in a glucose-dependent manner. As a class, DPP-4 inhibitors have been shown to provide significant improvements in glycosylated hemoglobin (HbA1c), and to have a good safety profile. In addition, their glucose-dependent mechanism of action is associated with a low rate of hypoglycemic events [Richter et al. 2008].
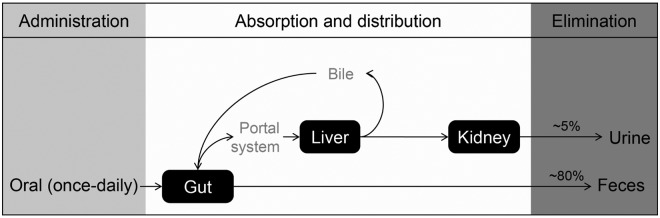
High-throughput screening using an assay to detect inhibition of DPP-4 led to the discovery of linagliptin, a xanthine-based molecule with a high selectivity for DPP-4. The pharmacokinetics and pharmacodynamics of linagliptin have been reviewed in detail elsewhere [Scheen, 2011; Toth, 2011]. Of note, unlike other DPP-4 inhibitors, which are predominantly excreted via the kidneys, linagliptin is mainly excreted unchanged via the enterohepatic system (Figure 1) [Blech et al. 2010; Heise et al. 2009]. Based on pharmacokinetic studies, no dose adjustment is needed for patients with renal or hepatic impairment [Boehringer Ingelheim Pharmaceuticals Inc., 2011; Sloan et al. 2011]. Early studies showed linagliptin is suitable for once-daily dosing, with similar reductions in HbA1c to those seen with other DPP-4 inhibitors and without clinically significant pharmacokinetic interactions when co-administered with other medications [Forst et al. 2010; Graefe-Mody et al. 2010, 2011].
Figure 1.
Excretion of linagliptin. Approximately 90% of linagliptin is excreted unchanged, indicating that metabolism is a minor elimination pathway.
On the basis of the early studies, an extensive clinical trial program was undertaken to assess the efficacy and safety of linagliptin. Of these, four pivotal trials, trials designed to meet US Food and Drug Administration (FDA) criteria for assessing efficacy and safety of a drug before it is approved for use in patients in the US, have been reported over the past year or so. The positive results of these trials led to FDA approval of linagliptin to improve glycemic control in patients with T2DM in May 2011.
Overview of linagliptin pivotal trials
Since patients in clinical practice may well be receiving more than one antidiabetic medication, the efficacy of linagliptin was assessed alone (as monotherapy) and in combination with other commonly used antidiabetic agents (metformin, a sulfonylurea or a thiazolidinedione). Table 1 summarizes the design of the studies [Del Prato et al. 2011; Gomis et al. 2011; Owens et al. 2011; Taskinen et al. 2011]. All four were multicenter trials with a randomized, double-blind, placebo-controlled design, and a treatment phase of 6 months (24 weeks), considered the optimum balance between robust assessment of efficacy and the risk for patients exposed to placebo [US Food and Drug Administration, 2008]. All of the study designs incorporated a rescue therapy option for any patient whose glucose control was poor.
Table 1.
Linagliptin pivotal clinical trials: summary of study design.
| Monotherapy | Add-on to metformin | Add-on to metformin plus sulfonylurea | Combination with pioglitazone | |
|---|---|---|---|---|
| Reference | Del Prato et al. [2011] | Taskinen et al. [2011] | Owens et al. [2011] | Gomis et al. [2011] |
| NCT ID | NCT00621140 | NCT00601250 | NCT00602472 | NCT00641043 |
| Patients | Adults Type 2 diabetes BMI ≤40 kg/m2 | Adults Type 2 diabetes BMI ≤40 kg/m2 | Adults Type 2 diabetes BMI ≤40 kg/m2 | Adults Type 2 diabetes BMI ≤40 kg/m2 |
| Prior treatment | Treatment-naïve or treated with one other OAD (except TZDs) | Metformin as monotherapy or with one other OAD | Stable regimen of metformin and a sulfonylurea | Treatment-naïve or treated with any OAD |
| Baseline HbA1c | 7.0–10.0% (after washout of OAD) | 7.0–10.0% (after washout of OADs other than metformin) | 7.0–10.0% (no washout needed) | 7.5–11.0% (after washout of OAD) |
| Study drug | Linagliptin 5 mg | Linagliptin 5 mg | Linagliptin 5 mg | Linagliptin 5 mg + pioglitazone 30 mg |
| Comparator | Placebo | Placebo | Placebo | Placebo + pioglitazone 30 mg |
| Background therapy | None | Metformin | Metformin + sulfonylurea | None |
| Treatment period | 24 weeks | 24 weeks | 24 weeks | 24 weeks |
| Patients, n (linagliptin/comparator)1 | 503 (336/167) | 701 (523/177) | 1058 (792/263) | 389 (259/130) |
| Primary outcome measure2 | Change from baseline in HbA1c | Change from baseline in HbA1c | Change from baseline in HbA1c | Change from baseline in HbA1c |
NCT ID, ClinicalTrials.gov identification number; BMI, body mass index; OAD, oral antidiabetic drug; TZD, thiazolidinedione; HbA1c, glycosylated hemoglobin.
Adults are aged ≥18 and ≤80 years.
Number of patients is the treated set: all randomized patients who received ≥1 dose of study drug.
Change from baseline in HbA1c is adjusted for baseline HbA1c and previous antidiabetic medication.
The nature of clinical trials can alter patients’ behavior, such as diet, exercise or compliance with medication, which may in turn impact blood glucose control. To counteract this, all trials included a 2-week open-label placebo run-in to allow patients to acclimatize to trial conditions. All patients were provided with standard diet and exercise counseling as well as equipment for home blood glucose monitoring at the start of the run-in.
All four trials recruited adults with T2DM and a body mass index ≤40 kg/m2, and the primary outcome measure was mean change in HbA1c level from baseline at 24 weeks, which is a standard measure of efficacy in the testing of investigational agents for T2DM [Nathan et al. 2009; US Food and Drug Administration, 2008]. The number of patients achieving target reductions in HbA1c and fasting plasma glucose (FPG) levels were assessed as secondary endpoints in all trials, while postprandial glucose (PPG) was assessed in a subgroup of patients in two of the trials [Del Prato et al. 2011; Gomis et al. 2011; Owens et al. 2011; Taskinen et al. 2011].
Linagliptin monotherapy
To study the efficacy and safety of linagliptin monotherapy, adults with T2DM who were treatment-naïve or who had been treated with one oral antidiabetic drug (OAD; except a thiazolidinedione because of the long washout needed for these medications) were recruited [Del Prato et al. 2011]. Treatment-naïve patients were eligible if they had screening HbA1c levels between 7.0% and 10.0%, while previously treated patients were eligible with screening HbA1c levels between 6.5% and 9.0%, and between 7.0% and 10.0% after a 4-week washout of their other OAD. Patients who were treatment-naïve entered directly into the 2-week open-label placebo run-in after screening; previously treated patients entered the run-in after their washout.
In total, 503 patients were randomized in a 2:1 ratio to linagliptin 5 mg/day (n = 336) or placebo (n = 167) for 24 weeks [Del Prato et al. 2011]. Just over half the study patients were women, the mean age was 55.7 years and most patients were either White or Asian. Baseline characteristics were well balanced across the two randomized study groups.
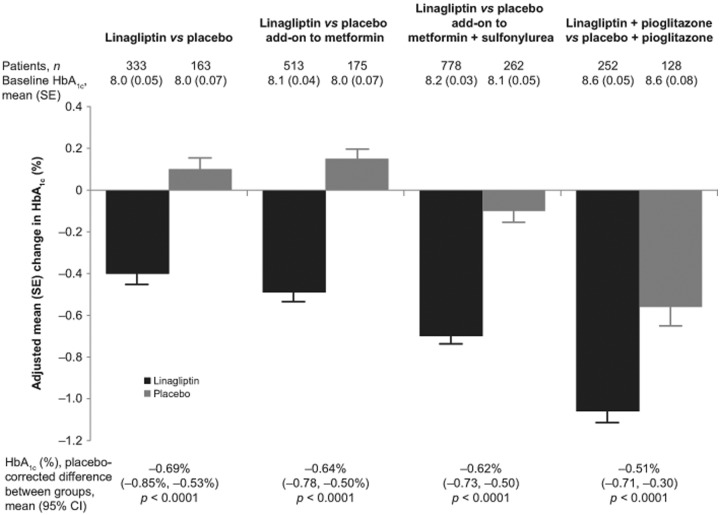
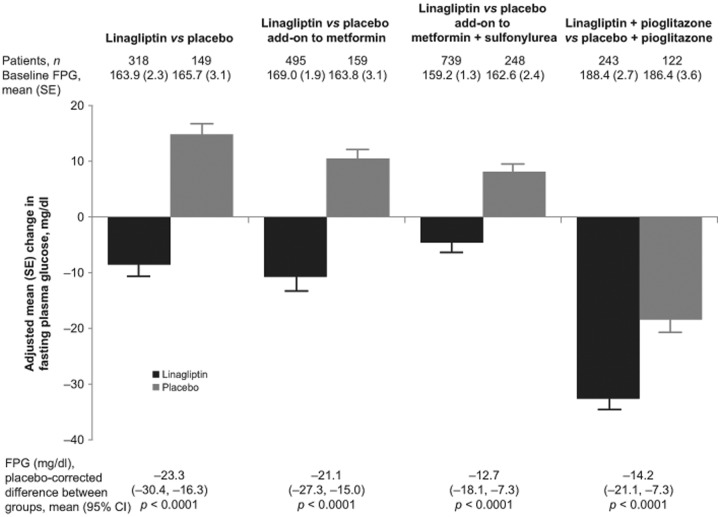
All patients with a baseline and on-treatment measurement of HbA1c or FPG were included in the relevant analyses. Changes in HbA1c between baseline and week 24 significantly favored linagliptin over placebo, with a difference between groups of −0.69% (95% confidence interval [CI]: −0.85% to −0.53%; p < 0.0001; Figure 2). Similarly, the linagliptin group had a significantly greater change in FPG, with a difference between groups of −23.3 mg/dl (95% CI: −30.4 to −16.3 mg/dl; p < 0.0001; Figure 3). Patients in the linagliptin group were also significantly more likely to achieve HbA1c <7.0% (by week 24, 25.2% of patients in the linagliptin group with baseline HbA1c ≥7.0% achieved this target compared with 11.6% in the placebo group; odds ratio [OR]: 2.9, p = 0.0006; Table 2). In addition, patients receiving linagliptin were significantly more likely to achieve an HbA1c reduction ≥0.5%, with 47.1% of the linagliptin group and 19.0% of the placebo group achieving an HbA1c reduction ≥0.5% at 24 weeks (OR: 4.2, p < 0.0001; Table 2).
Figure 2.
Glycosylated hemoglobin (HbA1c): change from baseline with linagliptin or placebo. Number of patients is the full analysis set (all patients with a baseline and at least one on-treatment HbA1c value). All studies were of 24 weeks’ duration. Mean change in HbA1c from baseline to week 24 was adjusted for baseline HbA1c and previous oral antidiabetic drug treatment. The treatment difference (linagliptin minus placebo) was highly significant for all studies (p < 0.0001). SE, standard error; CI, confidence interval.
Figure 3.
Fasting plasma glucose (FPG): change from baseline with linagliptin or placebo. Number of patients is the full analysis set (all patients with a baseline and at least one on-treatment FPG value). All studies were of 24 weeks’ duration. Mean change in FPG from baseline to week 24 was adjusted for baseline HbA1c, FPG and previous oral antidiabetic drug treatment. Difference (linagliptin minus placebo) was highly significant for all studies (p < 0.0001). To convert glucose from milligrams per deciliter (mg/dl) to millimoles per liter (mmol/l), multiply by 0.05551.
FPG, fasting plasma glucose; SE, standard error; CI, confidence interval.
Table 2.
Goal attainment by week 24.
| Monotherapy [Del Prato et al. 2011] |
Add-on to metformin [Taskinen et al. 2011] |
Add-on to metformin plus sulfonylurea [Owens et al. 2011] |
Combination with pioglitazone [Gomis et al. 2011] |
|||||
|---|---|---|---|---|---|---|---|---|
| Linagliptin | Placebo | Linagliptin | Placebo | Linagliptin | Placebo | Linagliptin | Placebo | |
| Achieve HbA1c <7.0% (in patients with baseline HbA1c ≥7.0%) | ||||||||
| Patients, % | 25.2% | 11.6% | 26.2% | 9.2% | 29.2% | 8.1% | 42.9% | 30.5% |
| Odds ratio, linagliptin versus placebo (p-value) |
2.9 (p = 0.0006) | 4.4 (p = 0.0001) | 5.5 (p < 0.0001) | 2.1 (p = 0.005) | ||||
| Achieve HbA1c reduction ≥0.5% | ||||||||
| Patients, % | 47.1% | 19.0% | 49.7% | 21.7% | 58.2% | 30.2% | 75.0% | 50.8% |
| Odds ratio, linagliptin versus placebo (p-value) |
4.2 (p < 0.0001) | 3.8 (p < 0.0001) | 3.4 (p < 0.0001) | 3.8 (p < 0.0001) | ||||
HbA1c, glycosylated hemoglobin.
To assess the effect of linagliptin on PPG, a meal tolerance test was performed 30 minutes after study drug dosing in a subset of patients (67 patients in the linagliptin group and 24 in the placebo group). After 24 weeks, the adjusted mean change from baseline in the linagliptin group was −33.5 mg/dl (standard error [SE], 6.2) compared with an increase of 24.9 mg/dl (SE, 10.3) in the placebo group. The difference between groups significantly favored linagliptin, with a placebo-corrected mean change of −58.4 mg/dl (95% CI: −82.3 to −34.4; p < 0.0001).
Linagliptin combination therapy
Add-on combination therapy with metformin
Metformin is the OAD widely considered the first-line treatment for patients with T2DM, especially those who are overweight or obese, and this study was undertaken to assess linagliptin as add-on therapy to metformin [Taskinen et al. 2011]. To be eligible for the study, patients had to have insufficient glycemic control while on metformin (≥1500 mg/day or their maximum tolerated dose, with dose unchanged for at least 12 weeks prior to randomization) and not more than one other OAD (with dose unchanged for at least 10 weeks prior to informed consent). Patients had a washout of any OAD other than metformin; metformin was continued at the patient’s usual dose throughout the trial. Patients on metformin alone were required to have screening HbA1c levels of 7.0–10.0%. Patients also receiving another OAD were required to have screening HbA1c levels of 6.5–9.0%; after screening, they stopped the OAD for a 4-week washout, and were required to have postwashout HbA1c levels of 7.0–10.0%. All patients then underwent a 2-week open-label placebo run-in before being randomized in a 3:1 ratio to linagliptin or placebo in addition to their metformin for 24 weeks.
In total, 701 patients were randomized (524 to linagliptin 5 mg/day and 177 to placebo) [Taskinen et al. 2011]. The mean age was 56.5 years, 54% of patients were men, and most patients were White or Asian. Approximately two- thirds of patients had been previously treated with metformin alone, and one-third had received metformin in combination with another OAD.
After 24 weeks of treatment, patients receiving linagliptin in addition to metformin had significantly greater changes in HbA1c (Figure 2) and FPG (Figure 3) than patients receiving placebo plus metformin. For HbA1c, the difference between study groups was −0.64% (95% CI: −0.78 to −0.50%; p < 0.0001); for FPG it was −21.1 mg/dl (95% CI: −27.4 to −15.0 mg/dl; p < 0.0001).
Patients in the linagliptin plus metformin group were also significantly more likely to achieve HbA1c <7.0%, with 26% and 9% of those with baseline HbA1c ≥7.0% achieving this target in the linagliptin and placebo groups (OR: 4.4; p = 0.0001). Similarly, an HbA1c reduction ≥0.5% was seen in 50% of the linagliptin group compared with 22% of the placebo group (OR: 3.8; p < 0.0001; Table 2).
PPG was also assessed in this study, with data available for a subset of 78 patients in the linagliptin group and 21 in the placebo group. The adjusted mean change from baseline to week 24 showed significantly better control in the linagliptin group, with a placebo-corrected mean change of −67.1 mg/dl (95% CI: −94.7 to −39.6; p < 0.0001).
Add-on combination therapy with metformin and a sulfonylurea
Sulfonylureas were the first available OADs, and until the 1990s were considered first-line therapy for most patients with T2DM. Since that time, metformin has generally replaced them as the first-line choice because of issues with weight gain and the increased risk of hypoglycemia with sulfonylureas resulting from the non-glucose-dependent manner by which they increase insulin secretion from the pancreas. Given the fact that many patients will need combination therapy to effectively treat blood glucose, sulfonylureas are still widely used, often added on to metformin; yet a significant number of patients will have insufficient glycemic control even with combined therapy [Nathan et al. 2009]. Since linagliptin has a mechanism of action complementary to those of metformin and sulfonylureas, a third study was undertaken to examine the efficacy and safety of linagliptin 5 mg/day when added to metformin plus sulfonylurea.
The study recruited patients whose glycemia was insufficiently controlled with metformin plus a sulfonylurea [Owens et al. 2011]. Patients with T2DM were eligible if their HbA1c levels were between 7.0% and 10.0%, despite 1500 mg/day metformin (or their maximum tolerated dose, if lower) and the maximum tolerated dose of sulfonylurea, with their regimen stable for at least 10 weeks.
No washout was needed, as patients on other OADs were not eligible; therefore, all patients entered directly into the 2-week placebo run-in. After the run-in, patients were randomized in a 3:1 ratio to linagliptin 5 mg/day or placebo for 24 weeks, in addition to their established metformin plus sulfonylurea regimen, which was continued at unchanged doses.
Of 1058 patients randomized, 3 did not receive study drug, leaving 1055 patients included in the analysis (792 in the linagliptin arm and 263 in the placebo arm). Baseline and demographic data were similar between the two groups. About half the patients in each arm were women, the overall mean age was 58 years, and the majority of patients were White or Asian. Almost all patients (98%) had had diabetes for more than a year, as would be expected given that patients were already stable on two OADs, and nearly three-quarters had been diagnosed for more than 5 years.
After 24 weeks of double-blind treatment, patients receiving add-on linagliptin had significantly greater changes in HbA1c (with a placebo-corrected adjusted mean change of −0.62%; 95% CI: −0.73% to −0.50%; p < 0.0001; Figure 2). Changes in FPG were also significantly greater in the linagliptin group (placebo-corrected adjusted mean change: −12.7 mg/dl; 95% CI: −18.1 to −7.3 mg/dl; p < 0.0001; Figure 3). Among patients with a baseline HbA1c ≥7.0%, significantly more patients in the linagliptin group achieved HbA1c <7.0% by week 24 (29.2% versus 8.1%; OR: 5.5; p < 0.0001), and significantly more patients in the linagliptin group achieved a reduction in HbA1c of ≥0.5% (58.2% versus 30.2%; OR: 3.4; p < 0.0001; Table 2). PPG was not assessed in this study.
Initial combination therapy with pioglitazone
Pioglitazone is an OAD in the thiazolidinedione class, a group of agents that decrease insulin resistance by selectively stimulating the nuclear receptor peroxisome proliferator-activated receptor gamma (PPAR-γ), making fat, muscle and liver cells more sensitive to insulin. Pioglitazone has been available for more than 10 years, and has been widely used in patients with T2DM. The fourth pivotal linagliptin clinical trial was undertaken to assess the safety of linagliptin 5 mg/day as initial therapy in combination with pioglitazone.
Treatment-naïve patients were eligible if screening HbA1c levels were between 7.5% and 11.0%; patients receiving treatment with any other OAD were eligible if HbA1c levels were between 7.0% and 9.5% at screening, and between 7.5% and 11.0% after a 4-week washout of all OADs. After the washout, all patients underwent a 2-week open-label placebo run-in, and were then randomized in a 2:1 ratio to either an initial combination of pioglitazone 30 mg/day and linagliptin 5 mg/day or pioglitazone 30 mg/day and placebo for 24 weeks [Gomis et al. 2011].
In total, 389 patients were randomized, 259 patients to linagliptin plus pioglitazone, and 130 to placebo plus pioglitazone. Overall, 61% of the patients were men, most patients were White or Asian, and the mean age was 57.5 years. The two study groups were well balanced for baseline characteristics, although the body mass index appeared slightly lower in the linagliptin plus pioglitazone group than in the placebo plus pioglitazone group (mean ± standard deviation: 28.7 ± 4.8 kg/m2 versus 29.7 ± 4.8 kg/m2).
Adjusted mean changes in HbA1c and FPG for linagliptin plus pioglitazone were significantly greater than with placebo plus pioglitazone (Figures 2 and 3). After 24 weeks of treatment, the difference between the linagliptin plus pioglitazone and placebo plus pioglitazone groups in the adjusted mean HbA1c was −0.51% (95% CI: −0.71% to −0.30%; p < 0.0001), and the difference in FPG was −14.2 mg/dl (95% CI: −21.1 to −7.3 mg/dl; p < 0.0001). Among patients with HbA1c ≥7.0% at baseline, 42.9% of patients in the linagliptin plus pioglitazone group and 30.5% in the placebo plus pioglitazone group achieved the target of HbA1c <7.0% (OR: 2.1; p = 0.005). Patients in the linagliptin group were also significantly more likely than those in the placebo group to have an HbA1c reduction of ≥0.5% at 24 weeks, seen in 75.0% and 50.8% of patients, respectively (OR: 3.8; p < 0.0001; Table 2). PPG was not assessed in this study.
Weight
Being overweight or obese is associated with an increased risk of T2DM, and weight management is an important part of diabetes care [American Diabetes Association, 2011; Must et al. 1999]. In clinical trials, thiazolidinediones, sulfonylureas and glinides have been associated with weight gain, whereas metformin, glucagon-like peptide-1 analogs, alpha-glucosidase inhibitors and DPP-4 inhibitors have been associated with stable weight or weight loss [Phung et al. 2010].
To examine the effect of linagliptin on patients’ weight in the linagliptin pivotal trials, weight was measured when the patient visited the clinic for screening, at randomization (after the placebo run-in and the washout, if applicable) and at 24 weeks [Del Prato et al. 2011; Gomis et al. 2011; Owens et al. 2011; Taskinen et al. 2011]. All of the trials excluded severely obese patients (body mass index >40 kg/m2), but patients were, on average, overweight at baseline. When linagliptin was used as monotherapy, as add-on to metformin, or as add on to the combination of metformin plus sulfonylurea, neither the placebo or linagliptin groups had significant changes from baseline to week 24 in body weight [Del Prato et al. 2011; Owens et al. 2011; Taskinen et al. 2011]. When patients were randomized to linagliptin plus pioglitazone or placebo plus pioglitazone, mean weight increased in both groups [Gomis et al. 2011]. This is perhaps not surprising given that pioglitazone has been associated with weight increases; however, the adjusted mean change was larger in the linagliptin plus pioglitazone group than in the placebo plus pioglitazone group (difference between groups: 1.1 kg; 95% CI: 0.2–2.0; p = 0.014). The significance is as yet unclear, especially as the mean weight of the linagliptin plus pioglitazone group was lower than that of the placebo plus pioglitazone group at both baseline (78.3 versus 82.7 kg) and at week 24 (80.8 versus 84.0 kg) [Gomis et al. 2011].
Rescue medication
As with all well-designed studies for T2DM, rescue medication was used as a safety net for any patient who had inadequate blood glucose control. All patients were trained in home blood glucose monitoring and provided with equipment at the beginning of the study. During the washout and open-label placebo run-in periods, patients were asked to monitor blood glucose daily before breakfast and at any time they felt symptoms of hyperglycemia or hypoglycemia. During the randomized, double-blind period of the trials, daily testing was no longer required, but patients could perform tests if they had symptoms. If the patient did measure high blood glucose levels, they were confirmed with a second measurement on a different day, with at least one measurement at the study site, before rescue therapy was started. During the trials, a significantly higher proportion of patients in the placebo group received rescue medication than in the linagliptin group (Table 3). Patients receiving rescue medication were included in the primary efficacy analysis, using their HbA1c measurement prior to the start of rescue therapy. When analyses were repeated, including only patients who received treatment per protocol, results were consistent with the primary analysis, with significantly greater reductions in HbA1c in the linagliptin groups [Del Prato et al. 2011; Owens et al. 2011].
Table 3.
Rescue medication.
| Monotherapy [Del Prato et al. 2011] |
Add-on to metformin [Taskinen et al. 2011] |
Add-on to metformin plus sulfonylurea [Owens et al. 2011] |
Combination with pioglitazone [Gomis et al. 2011] |
|||||
|---|---|---|---|---|---|---|---|---|
| Linagliptin | Placebo | Linagliptin | Placebo | Linagliptin | Placebo | Linagliptin | Placebo | |
| Rescue therapy medication | Metformin | Sulfonylurea1 | Pioglitazone2 | Metformin3 | ||||
| Rescue therapy requirement | Weeks 1–12: confirmed fasting glucose >240 mg/dl Weeks 13–24: confirmed fasting glucose >200 mg/dl |
Weeks 1–12: confirmed fasting glucose >240 mg/dl Weeks 13–24: confirmed fasting glucose >200 mg/dl or >400 mg/dl in a randomly performed measurement |
Weeks 1–12: confirmed fasting glucose >240 mg/dl Weeks 13–24: confirmed fasting glucose >200 mg/dl or >400 mg/dl in a randomly performed measurement |
Weeks 1–12: confirmed fasting glucose >240 mg/dl Weeks 13–24: confirmed fasting glucose >200 mg/dl or >400 mg/dl in a randomly performed measurement |
||||
| Required rescue therapy, % | 10.2% | 20.9% | 8% | 19% | 5.4% | 13.0% | 7.9% | 14.1% |
| Odds ratio, linagliptin versus placebo (p-value) |
0.3 (p = 0.0002) | 0.28 (p = 0.0001) | 0.36 (p < 0.0001) | 0.45 (p = 0.035) | ||||
‘Confirmed’ indicates that the patient had a minimum of two measurements, on different days, with at least one measurement taken at the investigational site.
Typically glimepiride.
In Canada only, insulin could also be used.
Another oral antidiabetic drug could be used if metformin was not tolerated or was contraindicated.
Safety and tolerability
All patients who were randomized and received at least one dose of study drug were included in analyses of safety. Whether as monotherapy or in combination with other OADs, linagliptin was well tolerated, with a similar safety profile to placebo, and few patients discontinuing due to adverse events (Table 4). In patients treated with linagliptin, no adverse events occurred in at least 5% of patients and at a rate twofold or more than in patients treated with placebo [Del Prato et al. 2011; Gomis et al. 2011; Owens et al. 2011; Taskinen et al. 2011]. Nasopharyngitis, which has been associated with other DPP-4 inhibitors [Willemen et al. 2011], was reported in 5.8% of linagliptin patients versus 5.5% of placebo patients in a pooled analysis of placebo-controlled trials that included the four pivotal trials [Boehringer Ingelheim Pharmaceuticals Inc., 2011]. In the three pivotal trials in which nasopharyngitis was reported, it was seen at similar rates in the linagliptin and placebo groups (monotherapy: 3.9% versus 4.2%; add-on to metformin: 5.2% versus 5.1%; add-on to metformin plus sulfonylurea: 5.2% versus 4.6%, for the linagliptin and placebo groups, respectively).
Table 4.
Summary of safety outcomes across the linagliptin pivotal trials.
| Monotherapy [Del Prato et al. 2011] |
Add-on to metformin [Taskinen et al. 2011] |
Add-on to metformin plus sulfonylurea [Owens et al. 2011] |
Combination with pioglitazone [Gomis et al. 2011] |
|||||
|---|---|---|---|---|---|---|---|---|
| Linagliptin | Placebo | Linagliptin | Placebo | Linagliptin | Placebo | Linagliptin | Placebo | |
| Safety population, n | 336 | 167 | 523 | 177 | 792 | 263 | 259 | 130 |
| Any AE | 52.4 | 58.7 | 52.8 | 55.4 | 66.3 | 59.7 | 52.5 | 53.1 |
| Any drug-related AE | 5.1 | 3.6 | 6.9 | 10.7 | 17.9 | 11.4 | 6.2 | 4.6 |
| Serious AE | 3.0 | 4.2 | 3.4 | 2.3 | 3.8 | 3.2 | 3.1 | 2.3 |
| AE leading to discontinuation | 1.2 | 2.4 | 1.5 | 1.7 | 2.9 | 1.9 | 1.5 | 4.6 |
| Hypoglycemic event | 0.3 | 0.6 | 0.6 | 2.8 | 14.8 | 22.7 | 1.2 | 0 |
| Hypoglycemic event of severe intensity/ requiring third-party assistance |
0 | 0 | 0 | 0 | 2.7 | 4.8 | 0 | 0 |
AE, adverse event.
All values are percentages unless indicated: because patients were not randomized in a 1:1 ratio, percentages of patients experiencing ≥1 AE in each category have been provided.
Cough, hyperlipidemia and weight increase were the only adverse events reported in at least 2% of patients treated with linagliptin and at a rate at least twofold greater than with placebo. Cough was reported in 2.4% of patients in the linagliptin group and 1.1% of patients in the placebo group when linagliptin was used as add-on to metformin and sulfonylurea [Owens et al. 2011]. When linagliptin was used in combination with pioglitazone, hyperlipidemia was reported in 2.7% and 0.8%, and weight increase in 2.3% and 0.8% of the linagliptin and placebo groups, respectively [Boehringer Ingelheim Pharmaceuticals Inc., 2011].
Hypoglycemia is an outcome of special interest with therapies for T2DM, since the aim of therapy is to reduce blood glucose, but excessive reductions will lead to hypoglycemia. Because DPP-4 inhibitors such as linagliptin work in a glucose-dependent manner, they have not been associated with hypoglycemia [Richter et al. 2008]; nonetheless, investigators in the linagliptin pivotal studies were asked to closely monitor symptoms that could be attributed to hypoglycemia. As recommended by the American Diabetes Association, hypoglycemic events were classified as asymptomatic (not accompanied by typical symptoms of hypoglycemia but with a measured plasma glucose concentration ≤70 mg/dl) or symptomatic events. Symptomatic events could be further classified by plasma glucose levels and whether the patient needed the assistance of another person to actively administer carbohydrate, glucagon or other resuscitative actions [Workgroup on Hypoglycemia and American Diabetes Association, 2005].
During the trials, linagliptin was indeed associated with a low risk of hypoglycemia, whether used as monotherapy, or with metformin or pioglitazone (Table 4) [Del Prato et al. 2011; Gomis et al. 2011; Taskinen et al. 2011]. When linagliptin was added to a stable regimen of metformin plus sulfonylurea, hypoglycemia was reported more frequently in the linagliptin group (Table 4). This increased incidence has been seen with other DPP-4 inhibitors in combination with metformin plus sulfonylurea; therefore, lowering the dose of sulfonylurea may be required to reduce the risk of hypoglycemia when adding linagliptin in combination with an insulin secretagogue [Boehringer Ingelheim Pharmaceuticals Inc., 2011].
Other areas of safety of particular interest to clinicians are cardiovascular risk and renal function. To date, among the pivotal trials, none have reported clinically meaningful changes in blood pressure or pulse rate [Del Prato et al. 2011; Gomis et al. 2011; Owens et al. 2011; Taskinen et al. 2011]. An analysis of vital signs and relevant biomarkers across 12 placebo-controlled trials, including the four pivotal trials, has reported top-line results, with no clinically meaningful changes in vital signs (blood pressure and pulse rate) seen in patients treated with linagliptin [Boehringer Ingelheim Pharmaceuticals Inc., 2011]. Laboratory analyses also showed similar changes in patients treated with linagliptin or placebo: increases in uric acid were the only change occurring at least 1% more often in patients receiving linagliptin than in those receiving placebo (1.3% in the placebo group, 2.7% in the linagliptin group) [Boehringer Ingelheim Pharmaceuticals Inc., 2011].
For lipid profiles, more detailed information has been reported for only two studies, and the publications do not report the patients’ use of lipid-lowering medication, leaving the results difficult to interpret from a clinical perspective. When linagliptin was added to a stable regimen of metformin and sulfonylurea, mean changes from baseline to 24 weeks were similar for the linagliptin and placebo groups for total cholesterol, high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) [Owens et al. 2011]. At baseline, both groups had mean triglyceride values above the normal reference range. Mean values for the two groups remained above the reference range at 24 weeks, with the mean triglyceride level in the linagliptin group remaining stable, and the triglyceride level in the placebo group decreasing by 12 mg/dl. Similarly, the patients randomized to combination linagliptin plus pioglitazone or placebo plus pioglitazone had mean values for total cholesterol, HDL-C and LDL-C within the normal reference range at baseline and end of treatment [Gomis et al. 2011]. At baseline, mean triglyceride values were above the normal reference range for both groups (linagliptin plus pioglitazone: 228 mg/dl; placebo plus pioglitazone: 236 mg/dl). At 24 weeks, both groups had a mean decrease in triglycerides, although the mean value in the placebo group remained above the normal reference range (linagliptin plus pioglitazone: −35 mg/dl decrease to 193 mg/dl; placebo plus pioglitazone: −18 mg/dl decrease to 219 mg/dl).
The biomarker analyses provide reassurance regarding the cardiovascular safety of linagliptin; however, the impact on cardiovascular events can only be definitively studied by looking at clinical outcomes. The clinical trial program for linagliptin includes studies looking at various patient groups with T2DM—at the time of writing, 32 studies of linagliptin were registered on the US National Institutes of Health clinical trial registry (see ClinicalTrials.gov). This included a 2-year study comparing linagliptin with a sulfonylurea that captured cardiovascular events as a safety outcome [Gallwitz et al. 2011]. Adults with T2DM receiving ongoing stable metformin (≥1500 mg/day for ≥10 weeks) were randomized to double-blind linagliptin 5 mg/day (n = 764) or glimepiride 1−4 mg/day (n = 755), and prospectively followed for prespecified cardiovascular events (cardiovascular death, nonfatal myocardial infarction, nonfatal stroke and hospitalized unstable angina). The two groups had similar HbA1c outcomes; despite this, the linagliptin group showed a significant reduction in the rate of cardiovascular events (relative risk: 0.46; 95% CI: 0.23–0.91; p = 0.02). These results have been further supported by a meta-analysis of 8 randomized, double-blind controlled trials of linagliptin, including a total of 5239 patients. In all eight trials, cardiovascular events were prospectively adjudicated by a blinded, independent expert committee. The risk of cardiovascular events (a composite of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke and hospitalized unstable angina) was significantly reduced with linagliptin versus comparator (hazard ratio: 0.34; 95% CI: 0.16–0.70; p < 0.05) [Johansen et al. 2011]. Taken together, these results suggest a good cardiovascular safety profile for linagliptin, and this is currently being prospectively tested in the CAROLINA study (CARdiOvascular Outcome Study of the DPP-4 Inhibitor LINAgliptin [ClinicalTrials.gov identifier: NCT01243424]), a large trial with a planned recruitment of 6000 patients. The trial will compare linagliptin with glimepiride, with cardiovascular outcomes as the primary outcome measure [Boehringer Ingelheim Pharmaceuticals Inc., 2010; Rosenstock et al. 2011].
Conclusion
The efficacy and safety of linagliptin 5 mg once daily for improving glycemic control in adults with T2DM has been demonstrated in these four randomized, double-blind, placebo-controlled, pivotal trials. When used in combination with other commonly used therapies for T2DM or when used as monotherapy, linagliptin provided clinically meaningful reductions in FPG and HbA1c, significantly increasing the odds of achieving HbA1c targets. In addition, linagliptin was well tolerated, with a similar safety profile to placebo and low rates of hypoglycemic events. Other than when used in combination with pioglitazone, patients receiving linagliptin did not gain weight or have adverse changes in cardiovascular biomarkers. Based on this evidence, linagliptin appears to provide a useful treatment option for patients with T2DM.
Acknowledgments
Writing and editorial assistance was provided by Geraldine Thompson of Envision Scientific Solutions, which was contracted by Boehringer Ingelheim Pharmaceuticals, Inc for these services. The author meets criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE), was fully responsible for all content and editorial decisions, and was involved at all stages of manuscript development. The author received no compensation related to the development of the manuscript.
Blood glucose levels have been provided in the standard US units (mg/dl). To convert to millimoles per liter (mmol/l), multiply by 0.05551.
Footnotes
Funding: This work was supported by Boehringer Ingelheim Pharmaceuticals, Inc.
Conflict of interest statement: Janet McGill has received consultancy and speaker fees from Boehringer Ingelheim.
References
- American Diabetes Association (2011) Standards of medical care in diabetes - 2011. Diabetes Care 34(Suppl. 1): S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blech S., Ludwig-Schwellinger E., Graefe-Mody E.U., Withopf B., Wagner K. (2010) The metabolism and disposition of the oral dipeptidyl peptidase-4 inhibitor, linagliptin, in humans. Drug Metab Dispos 38: 667–678 [DOI] [PubMed] [Google Scholar]
- Boehringer Ingelheim Pharmaceuticals Inc (2010) CAROLINA, Cardiovascular outcome study of linagliptin versus glimepiride in patients with type 2 diabetes. Available at: http://clinicaltrials.gov/ct2/show/NCT01243424?term=BI1356&recr=Open&rank=5
- Boehringer Ingelheim Pharmaceuticals Inc (2011) Tradjenta (linagliptin) prescribing information. Available at: http://bidocs.boehringer-ingelheim.com/BIWebAccess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing+Information/PIs/Tradjenta/Tradjenta.pdf
- Del Prato S., Barnett A.H., Huisman H., Neubacher D., Woerle H.J., Dugi K.A. (2011) Effect of linagliptin monotherapy on glycaemic control and markers of beta-cell function in patients with inadequately controlled type 2 diabetes: a randomized controlled trial. Diabetes Obes Metab 13: 258–267 [DOI] [PubMed] [Google Scholar]
- Forst T., Uhlig-Laske B., Ring A., Graefe-Mody U., Friedrich C., Herbach K., et al. (2010) Linagliptin (BI 1356), a potent and selective DPP-4 inhibitor, is safe and efficacious in combination with metformin in patients with inadequately controlled Type 2 diabetes. Diabet Med 27: 1409–1419 [DOI] [PubMed] [Google Scholar]
- Gallwitz B., Uhlig-Laske B., Battacharaya S., Patel S., Woerle H.-J. (2011) Linagliptin has similar efficacy to glimepiride but improved cardiovascular safety over 2 years in patients with type 2 diabetes inadequately controlled on metformin (Poster 39-LB). In: Proceedings of the 71st Annual Scientific Session of the American Diabetes Association (ADA), Vol. 60 (Suppl. 1), 24–28 June 2011, San Diego, CA [Google Scholar]
- Gomis R., Espadero R.M., Jones R., Woerle H.J., Dugi K.A. (2011) Efficacy and safety of initial combination therapy with linagliptin and pioglitazone in patients with inadequately controlled type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab 13: 653–661 [DOI] [PubMed] [Google Scholar]
- Graefe-Mody E.U., Jungnik A., Ring A., Woerle H.J., Dugi K.A. (2010) Evaluation of the pharmacokinetic interaction between the dipeptidyl peptidase-4 inhibitor linagliptin and pioglitazone in healthy volunteers. Int J Clin Pharmacol Ther 48: 652–661 [DOI] [PubMed] [Google Scholar]
- Graefe-Mody U., Rose P., Ring A., Zander K., Iovino M., Woerle H.J. (2011) Assessment of the pharmacokinetic interaction between the novel DPP-4 inhibitor linagliptin and a sulfonylurea, glyburide, in healthy subjects. Drug Metab Pharmacokinet 26: 123–129 [DOI] [PubMed] [Google Scholar]
- Heise T., Graefe-Mody E.U., Huttner S., Ring A., Trommeshauser D., Dugi K.A. (2009) Pharmacokinetics, pharmacodynamics and tolerability of multiple oral doses of linagliptin, a dipeptidyl peptidase-4 inhibitor in male type 2 diabetes patients. Diabetes Obes Metab 11: 786–794 [DOI] [PubMed] [Google Scholar]
- Johansen O.-E., Neubacher D., Von Eynatten M., Patel S., Woerle H.-J. (2011) Cardiovascular risk with linagliptin in patients with type 2 diabetes: a pre-specified, prospective, and adjudicated meta-analysis from a large phase III program (Abstract 0030-LB). In: Proceedings of the 71st Annual Scientific Session of the American Diabetes Association (ADA), Vol. 60 (Suppl. 1), 24–28 June 2011, San Diego, CA [Google Scholar]
- Must A., Spadano J., Coakley E.H., Field A.E., Colditz G., Dietz W.H. (1999) The disease burden associated with overweight and obesity. JAMA 282: 1523–1529 [DOI] [PubMed] [Google Scholar]
- Nathan D.M., Buse J.B., Davidson M.B., Ferrannini E., Holman R.R., Sherwin R., et al. (2009) Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 32: 193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens D.R., Swallow R., Dugi K.A., Woerle H.J. (2011) Efficacy and safety of linagliptin in persons with type 2 diabetes inadequately controlled by a combination of metformin and sulphonylurea: a 24-week randomized study. Diabet Med 28: 1352–1361 [DOI] [PubMed] [Google Scholar]
- Phung O.J., Scholle J.M., Talwar M., Coleman C.I. (2010) Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA 303: 1410–1418 [DOI] [PubMed] [Google Scholar]
- Richter B., Bandeira-Echtler E., Bergerhoff K., Lerch C. (2008) Emerging role of dipeptidyl peptidase-4 inhibitors in the management of type 2 diabetes. Vasc Health Risk Manag 4: 753–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstock J., Marx N., Kahn S.E., Zinman B., Kastelein J.J., Lachin J.M.et al. (2011) Rationale and design of the CAROLINA trial: An active comparator CARdiOvascular outcome study of the DPP-4 inhibitor LINAgliptin in patients with type 2 diabetes at high cardiovascular risk (Abstract 1103-P). In: Proceedings of the 71st Annual Scientific Session of the American Diabetes Association (ADA), Vol. 60 (Suppl. 1), 24–28 June 2011, San Diego, CA [Google Scholar]
- Scheen A.J. (2011) Linagliptin for the treatment of type 2 diabetes (pharmacokinetic evaluation). Expert Opin Drug Metab Toxicol 7: 1561–1576 [DOI] [PubMed] [Google Scholar]
- Sloan L., Newman J., Sauce C., von Eynatten M., Patel S., Woerle H.-J. (2011) Safety and efficacy of linagliptin in type 2 diabetes patients with severe renal impairment. In: Proceedings of the 71st Annual Scientific Session of the American Diabetes Association (ADA), Vol. 60 (Suppl. 1), 24–28 June 2011, San Diego, CA [Google Scholar]
- Taskinen M.R., Rosenstock J., Tamminen I., Kubiak R., Patel S., Dugi K.A., et al. (2011) Safety and efficacy of linagliptin as add-on therapy to metformin in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab 13: 65–74 [DOI] [PubMed] [Google Scholar]
- Toth P.P. (2011) Linagliptin: a new DPP-4 inhibitor for the treatment of type 2 diabetes mellitus. Postgrad Med 123: 46–53 [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration (2008) Guidance for Industry. Diabetes Mellitus: Developing Drugs and Therapeutic Biologics for Treatment and Prevention. DRAFT GUIDANCE. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071624.pdf
- Willemen M.J., Mantel-Teeuwisse A.K., Straus S.M., Meyboom R.H., Egberts T.C., Leufkens H.G. (2011) Use of dipeptidyl peptidase-4 inhibitors and the reporting of infections: a disproportionality analysis in the World Health Organization VigiBase. Diabetes Care 34: 369–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workgroup on Hypoglycemia and American Diabetes Association (2005) Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care 28: 1245–1249 [DOI] [PubMed] [Google Scholar]