Abstract
Licorice extract has always been recognized as a sweetener and a thirst quencher. Its nutritive value is overrated by many who consume significant amounts and are prone to complications. Glycyrrhetic acid, the active metabolite in licorice, inhibits the enzyme 11-ß-hydroxysteroid dehydrogenase enzyme type 2 with a resultant cortisol-induced mineralocorticoid effect and the tendency towards the elevation of sodium and reduction of potassium levels. This aldosterone-like action is the fundamental basis for understanding its health benefits and the wide spectrum of adverse effects. Herein, we present a comprehensive review of licorice along with the reported complications related to excess intake. Despite its apparent use in a few clinical scenarios, the daily consumption of licorice is never justified because its benefits are minor compared to the adverse outcomes of chronic consumption. The review highlights the importance of investigating the dietary habits and herbal remedies which are being used worldwide on cultural and habitual bases rather than reliable scientific evidence. Licorice is a US Food and Drug Administration (FDA) approved food supplement used in many products without precise regulations to prevent toxicity. Increased awareness among the public is required through TV commercials, newspapers, internet sites, magazines and product labels regarding the upper limit of ingestion and health hazards associated with excess intake. We hope that this review will serve as a warning message that should be transmitted from physicians to patients to avoid excessive licorice intake as well as a message to the FDA to start regulating the use of this substance.
Keywords: Licorice, hyperaldosteronism, pseudo-hyperaldosteronism, hypokalemic myopathy, glycyrrhizin
Introduction
Licorice is a popular sweetener found in many soft drinks, food products, snacks and herbal medicines. The habit of consumption of such a natural beverage is more popular in hot environments, especially during the month of fasting ‘Ramadan’. The traditional belief that licorice is a healthy natural substance without side effects drives its liberal consumption which can occasionally be hazardous. Several characteristics allow the widespread utilization of licorice. Its sweet taste makes it attractive to many manufacturing companies as a sweetener for many products to mask its bitter taste. Its value as a thirst quencher promotes its excess consumption in certain climates. It is also used in several medical indications. Its main constituent, glycyrrhizic acid, mimics mineralocorticoids in its action (sodium reabsorbtion and potassium secretion). The extent of metabolic and acid–base derangement can occasionally be severe enough to cause serious complications. We present a detailed review of this substance and its health hazards illustrated with a case of quadriparesis that followed excessive licorice intake during Ramadan, which is the first reported case after the Egyptian drink ‘erk soos’.
Historical background
Licorice has been present in various forms for a long period of time and has a rich history. It is an old remedy that was used by Egyptians and Assyrians BC, although not in the forms that we know today. In ancient Egypt, licorice was not eaten as strips or ropes of candy but was made into a sweet liquid drink. The extract of the plant called Glycyrrhiza is derived from the ancient Greek term ‘glykos’, meaning sweet, and ‘rhiza’, meaning root. Glycyrrhiza was indulged upon by many prophets and pharaohs. Licorice extract has been utilized in the battlefields and the desert where soldiers and travelers drank it to suppress their thirst sensation on long marches.
The monks first introduced licorice into Pontefract, West Yorkshire, UK in 1562 and George Dunhill, a local chemist, added sugar to it and named it Pontefract cake. Severe cases of hypokalemia, rhabdomyolysis and tetraparesis have been reported due to these cakes. England began using the extract and turned it into licorice candy which then became well known throughout the country. Licorice recipes were brought by the early settlers to America which have been producing and importing licorice products ever since.
Sources of licorice
There are numerous licorice-containing products that are readily available in our everyday use and can be unintentionally consumed by the public in liberal amounts, putting them at risk of complications. Snacks containing licorice include licorice sticks and toffee bars, blackcurrant, Pontefract cakes, torpedos and stimorol chewing gums. Drinks containing licorice include the Egyptian drink ‘erk soos’, Belgian beers, pastis brands and anisettes (Raki, Ouzo, Pernod). Licorice is used by tobacco companies as a flavoring/sweetening agent. Sweet-flavored licorice tobacco twist, traditionally used by miners/sailors for chewing whilst working in ‘no smoking’ environments, is another source of licorice. Although exposure to glycyrrhizic acid via chewing tobacco has been previously reported to cause pseudo-hyperaldosteronism [Blachley and Knochel, 1980], extensive exposure to glycyrrhizic acid is not likely because of pyrolysis [Hoffmann and Hoffmann, 1997]. Licorice extracts are often used as flavoring agents to mask the bitter taste in medicinal preparations. Health products that contain licorice include herbal and licorice-flavored cough mixtures, throat pearls, licorice tea, licorice-flavored diet gum, laxatives (including cascara and compound licorice powder). Licorice extracts have been used for an extended period of time in China and Japan as herbal medicines. In the United States, glycyrrhizin is generally recognized as a safe flavoring agent. De-glycyrrhizinated licorice (DGL) has been manufactured to avoid the side effects of licorice by removing the active compound glycyrrhizin and is available in capsules, lozenges, wafers and liquid. Public awareness of licorice-containing compounds and their potential complications is mandatory to avoid the inadvertent use of such products.
Chemistry
The genus Glycyrrhiza consists of about 30 species of which Glycyrrhiza glabra is generally recognized as licorice because of its sweet taste. G. glabra is a member of the pea family and grows best in subtropical climates in deep, fertile, well drained soils, with full sun, and is harvested in the autumn, 2–3 years after planting. Glycyrrhizin (Figure 1), a triterpenoid compound, accounts for the sweet taste of licorice root and represents a mixture of potassium–calcium–magnesium salts of glycyrrhizic acid. The content of glycyrrhizin in licorice roots varies from 2 to 25%, depending on the particular species. Glycyrrhizin is 50 times sweeter than sucrose (cane sugar). Its sweetness has a slower onset than sugar but persists in the mouth for a longer time. Glycyrrhizic acid is composed of a hydrophilic part, two molecules of glucuronic acid, and a hydrophobic fragment, glycyrrhetic acid [Obolentseva et al. 1999]. Glycyrrhizic acid has an action resembling that of mineralocorticoids. The yellow color of licorice is due to the flavonoid content of the plant, which includes liquiritin, isoliquiritin, isoflavones, glabridin and hispaglabridins. The hispaglabridins A and B have significant antioxidant activity [Vaya et al. 1997], and glabridin and glabrene possess estrogen-like activity [Tamir et al. 2001].
Figure 1.
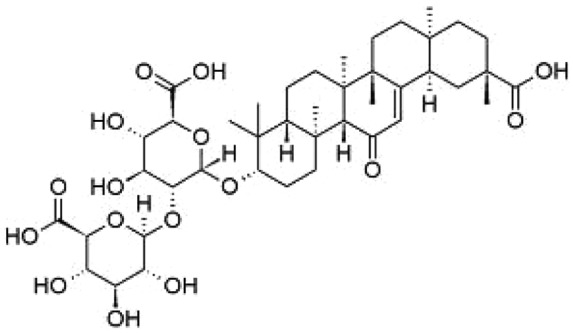
Chemical structure of glycyrrhizin.
Pharmacokinetics
Glycyrrhizin has a poor oral bioavailability and is detected at very low levels after a single oral dose administration. After oral ingestion of licorice in humans, its main constituent, glycyrrhizic acid, is hydrolyzed (pre-systemic hydrolysis) to glycyrrhetic acid by intestinal bacteria possessing a specialized ß-glucuronidase [Hattori et al. 1985]. Glycyrrhetic acid is a 200–1000 times more potent inhibitor of 11-ß-hydroxysteroid dehydrogenase (11-ß-HSD) than glycyrrhizic acid; therefore, its pharmacokinetics is more relevant after oral administration.
Glycyrrhetic acid is then rapidly absorbed and transported via carrier molecules to the liver. In the liver it is metabolized to glucuronide and sulfate conjugates which are transported efficiently and excreted into the bile and are then subjected to entero-hepatic circulation [Ploeger et al. 2000], which may lead to prolonged maintenance of pharmacologically active plasma levels. These conjugates are subsequently hydrolyzed by commensal bacteria [Ploeger et al. 2000, 2001]. The transit rate of gastrointestinal contents through the small and large intestines predominantly determines to what extent glycyrrhetic acid conjugates will be reabsorbed. Therefore in subjects with prolonged gastrointestinal transit times, glycyrrhetic acid might accumulate causing toxicity after repeated intake.
Mechanism of action
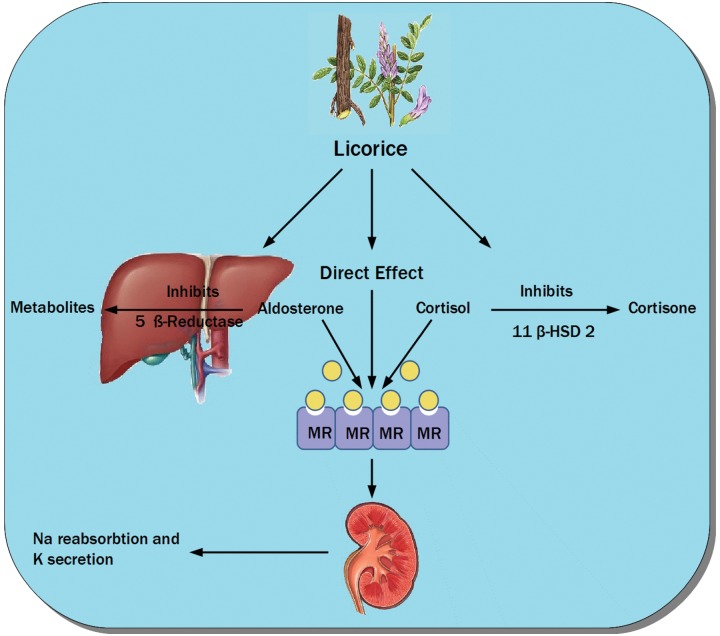
The active metabolites in licorice extract which are glycyrrhizic acid and glycyrrhetic acid can lead to a syndrome known as apparent mineralocorticoid excess [Stewart et al. 1987]. These side effects arise from the inhibition of the enzyme 11-ß-HSD and subsequent increase in the activity of cortisol. This effect is physiologically important because cortisol binds as avidly as aldosterone to the mineralocorticoid receptor (MR) [Funder et al. 1988]. One form of this enzyme 11-ß-HSD type 2 (11-ß-HSD2) is mainly restricted in the kidneys to the aldosterone-sensitive sites in the collecting tubules. Licorice also has a mineralocorticoid-like activity not only by blocking 11-ß-HSD2 but also by directly binding to MR [Calò et al. 2004]. Although initial studies suggested that this was the main mode of action, subsequent studies confirmed that its affinity to the MR is by far less than that of aldosterone and that the main contributing mechanism is through inhibition of 11-ß-HSD2 [Whorwood et al. 1993]. Glycyrrhetic acid also inhibits hepatic metabolism of aldosterone through suppression of 5-ß reductase activity [Latif et al. 1990]. Figure 2 illustrates the mechanisms of action of licorice.
Figure 2.
Demonstration of different mechanisms of action of licorice through inhibition of 11-ß-hydroxysteroid dehydrogenase type 2, 5 ß-reductase (which metabolizes aldosterone) and its direct action on the mineralocorticoid receptors causing sodium reabsorption and potassium secretion. MR, mineralocorticoid receptor; 11-ß-HSD 2, 11-ß-hydroxysteroid dehydrogenase type 2.
Primary and secondary hyperaldosteronism
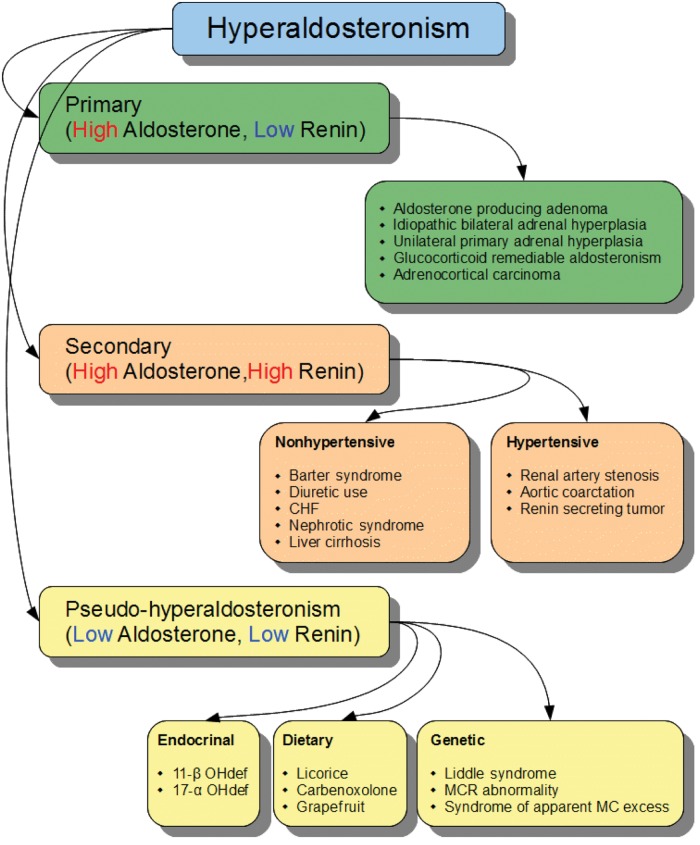
Hyperaldosteronism is classified into primary, secondary and pseudo-hyperaldosteronism. Primary hyperaldosteronism results from autonomous secretion of aldosterone from one or both adrenal glands and is associated with increased plasma aldosterone and low renin activity. In secondary hyperaldosteronism there is secondary activation of the renin–angiotensin system with subsequent increase in the aldosterone levels so the levels of renin and aldosterone are elevated.
Pseudo-hyperaldosteronism
Pseudo-hyperaldosteronism is a condition that clinically mimics hyperaldosteronism with suppression of plasma renin activity and aldosterone levels. Causes of pseudo-hyperaldosteronism can be categorized into dietary, genetic and endocrinal causes. Dietary causes include prolonged overconsumption of licorice, carbenoxolone or grapefruit juice [Palermo et al. 2003] due to an acquired reduction in the activity of 11-ß-HSD. Genetic causes include Liddle’s syndrome due to a mutation of the gene encoding for β and γ subunits of the sodium channel leading to increased activity of this channel and subsequent increased sodium reabsorption [Scheinman et al. 1999]. Other genetic causes include MR abnormalities due to an activating mutation of the MR gene [Armanini et al. 2003] and the syndrome of apparent mineralocorticoid excess (SAME), which is an autosomal recessive disorder resulting from a mutation in 11-ß-HSD2. SAME is characterized by severe juvenile hypertension, low birth weight and failure to thrive in early childhood with extensive target organ damage [Potton et al. 1970]. Endocrinal causes of pseudo-hyperaldosteronism include congenital adrenal hyperplasia (especially due to 17-α hydroxlase and 11-hydroxylase deficiency), leading to accumulation of 11-deoxcorticosterone which has an aldosterone-like action. Figure 3 illustrates the various causes of hyperaldosteronism and the levels of renin and aldosterone in each category.
Figure 3.
Illustration of the various causes of hyperaldosteronism and the levels of renin and aldosterone in each category. 11-β OHdef, 11 β hydroxylase deficiency; 17-α OHdef, 17 α hydroxylase deficiency; CHF, congestive heart failure; MC, mineralocorticoid; MCR, mineralocorticoid receptor.
Diagnosis of overconsumption
Licorice overconsumption should be suspected clinically in patients presenting with otherwise unexplained hypokalemia and muscle weakness. A clue is provided when dietary history reveals excessive licorice intake. Due to its aldosterone-like action, laboratory investigations reveal hypokalemia and metabolic alkalosis. Creatine phosphokinase (CPK) may be elevated in cases with rhabdomyolysis (due to severe hypokalemia) which may be complicated with acute tubular necrosis.
The inhibition of 11-ß-HSD by licorice will cause reduction in the conversion of cortisol to cortisone. Therefore, in conditions causing pseudo-hyperaldosteronism (as licorice excess), the cortisol:cortisone ratio in the peripheral venous plasma is sharply raised. Moreover licorice-induced hypertension is also accompanied by reduction in plasma renin as well as aldosterone level, which is not the case in primary or secondary hyperaldosteronism.
Health benefits of licorice
Understanding the mechanism of action of licorice promoted its therapeutic benefit in several groups of patients. The binding of licorice to the MR explains its utility in patients with Addison’s disease. These effects are due to the affinity of glycyrrhetinic acid for MR in addition to its plasma concentration being more than 5000 fold the concentration of aldosterone [Armanini et al. 1996]. Patients with postural hypotension caused by diabetic autonomic neuropathy have also shown improvement with licorice ingestion [Basso et al. 1994]. Bernardi and colleagues studied the effects of prolonged ingestion of graded doses of licorice on serum potassium level in healthy volunteers [Bernardi et al. 1994]. A significant fall in plasma potassium concentration from 4.3 to 3.5 mmol/liter was noticed, which occurred at the dose of 800 mg or more daily. An important clinical application for this was provided by Farese and colleagues, who demonstrated the use of licorice as an important tool to maintain predialysis potassium levels within a safe limit to decrease the risk of hyperkalemic arrhythmias in patients on chronic dialysis [Farese et al. 2009].
Licorice is one of the most widely prescribed herbs in Chinese medicine. It is used to treat gastric ulcers when administered 20 to 30 minutes before meals through lining the stomach wall. The processed form of licorice (DGL) is not associated with adverse effects and can be used to treat peptic ulcer disease in combination with antacids (this combination has been marketed as Caved-S). However, licorice is rarely used nowadays because of its side effects and the emergence of other more powerful classes of medications for treatment of peptic ulcers. In Japan, glycyrrhizin has been given intravenously for treatment of patients with chronic hepatitis B with improvement in liver functions and occasionally complete recovery. It was suggested that glycyrrhizin is able to suppress the secretion of both hepatitis B surface antigen and its intracellular transport [Sato et al. 1996; Takahara et al. 1994]. In women, licorice has been used in conjunction with spironolactone in the treatment of polycystic ovary syndrome (PCOS) [Armanini et al. 2007]. This estrogenic activity of licorice has been well documented [Oerter et al. 2003].
Other beneficial effects of licorice include its role in bone metabolism which was described by Mattarello and colleagues who demonstrated an increased parathyroid hormone and urinary calcium levels from baseline value in healthy women after 2 months of therapy. The postulated mechanism was the estrogen-like activity of isoflavans which are one of the constituents of licorice roots [Mattarello et al. 2006]. Armanini and colleagues described a reduction in body fat mass without a change in the body mass index in 15 normal weight subjects who consumed 3.5 g/day of licorice for 2 months. This suggested that licorice can reduce fat by inhibiting 11-ß-HSD type 1 at the level of fat cells [Armanini et al. 2003]. It was also demonstrated that licorice can reduce testosterone levels in healthy women due to the block of 17-HSD and 17–20 lyase. It was then concluded that licorice could be considered as an adjuvant therapy of hirsutism and PCOS [Armanini et al. 2004]. Agarwal and colleagues demonstrated the beneficial effect of licorice gargle in reducing the risk of postoperative sore throat [Agarwal et al. 2009]. The anticarcinogenic effects of glycyrrhizin and glycyrrhizinic acid have been thoroughly studied for their effects on mice, rat and human cancer cell lines. Prostate, breast, colon, liver and lung cancer cell lines have also been investigated. Most studies demonstrated a dose-dependent action on cell/tumor proliferation and apoptosis. Suggested mechanisms of action include its antioxidant activity, DNA-protective activity, suppressive action, cyclooxygenase inhibition and phyto-estrogenic and progesterone antagonist activity [Wang and Nixon, 2001; Dong et al. 2007; Lee et al. 2007; Chintharlapalli et al. 2007].
How much is too much?
The main difficulty with licorice dosing lies in its availability in various forms such as candies, beverages, supplements and extracts that contain different amounts of the active components of licorice. In the United States, the manufacture of some dietary supplements, including licorice, is not closely regulated. In 1991, the European Union proposed a provisional figure of 100 mg/day as the upper limit for ingestion of glycyrrhizin (approximately the amount found in 60–70 g licorice) [Murphy et al. 2009]. In April 2003, the Scientific Committee on Food confirmed an upper limit of 100 mg/day [Scientific Committee on Food, 2003]. This was based on data from human volunteer studies. However, the Committee is still of the opinion that an average daily intake for glycyrrhizic acid and ammonium glycyrrhizinate cannot be derived because the new human toxicity studies are too limited (small experimental groups, short duration). The Dutch Nutrition Information Bureau advised against daily glycyrrhizin consumption in excess of 200 mg, assumed to correspond to 150 g of licorice confectionery [Fenwick et al. 1990].
Licorice fluid extracts contain approximately 10–20% glycyrrhizin; typical doses of 2–4 ml deliver 200–800 mg. A review concluded that about 2% of the regular consumers have a daily intake of glycyrrhizinic acid of over 100 mg/day [Maas, 2000]. In 1994, Walker and Edwards demonstrated that a daily oral intake of 1–10 mg of glycyrrhizin, which corresponds to 1–5 g licorice, has been estimated to be a safe dose for most healthy adults [Walker and Edwards, 1994]. Two studies on healthy volunteers were published. In 1994, Bernardi and colleges administered daily doses of 108, 217, 380 and 814 mg glycyrrhizic acid, as ‘licorice pills’ for 4 weeks to four groups of 3 male and 3 female healthy volunteers [Bernardi et al. 1994]. No observed-adverse-effect level (NOAEL) based on the study report was 217 mg/person/day. At higher dose levels, sodium retention and depression of plasma renin and aldosterone levels were observed. Female participants were slightly more sensitive to glycyrrhizinic acid than male participants. Two years later, in the double-blinded randomized placebo-controlled study by Bijlsma and colleagues, four groups of 10 healthy female volunteers received orally 0, 1, 2 or 4 mg of pure glycyrrhizinic acid/kg/day for 8 weeks [Bijlsma et al. 1996]. In this study the NOAEL for glycyrrhizinic acid was 2 mg/kg/day. The European committee considered that the NOAEL obtained in the study by Bijlsma and colleagues is more appropriate because this study comprised a larger group of volunteers (40 volunteers in contrast to 24 volunteers), a longer period of exposure (8 weeks in contrast to 4 weeks) and inclusion of a placebo control group.
Licorice and its derivatives, including ammoniated glycyrrhizin, are generally recognized as safe (GRAS) for use in foods by the US Food and Drug Administration (FDA) (21 CFR 184.1408). This chapter of regulations includes descriptions, specifications and maximum use levels (Table 1) for licorice and licorice derivatives. The FDA assumes that glycyrrhizin levels in foods do not pose a health hazard, provided that these foods are not consumed in excess.
Table 1.
US Food and Drug Administration limitations for the use of licorice and its derivatives in foods (21 CFR 184.1408c) [World Health Organization, 2005].
| Food category | Maximum allowable levels in foods as % glycyrrhizin content | Functional use |
|---|---|---|
| Baked goods | 0.05 | 1, 2 |
| Alcoholic beverages | 0.1 | 1, 2, 3 |
| Nonalcoholic beverages | 0.15 | 1, 2, 3 |
| Chewing gum | 1.1 | 1, 2 |
| Hard candy | 16.0 | 1, 2 |
| Soft candy | 3.1 | 1, 2 |
| Herbs and seasonings | 0.15 | 1, 2 |
| Plant protein products | 0.15 | 1, 2 |
| Vitamin or mineral dietary supplements | 0.5 | 1, 2 |
| All other foods, except sugar substitutes | 0.1 | 1, 2 |
1 = flavor enhancer; 2 = flavoring agent; 3 = surface-active agent.
Factors that increase sensitivity to glycyrrhizin
Susceptibility to glycyrrhizin is influenced by the baseline health status, with some patients developing manifestations of toxicity with intake of smaller amounts than those expected to cause toxicity [Sigurjonsdottir et al. 2003]. These subgroups comprise people with decreased 11-ß-HSD2 activity (the target enzyme of glycyrrhizic acid, for which genetic polymorphisms resulting in reduced basal activity have been described). Prolonged gastrointestinal transit time is another factor that increases sensitivity to glycyrrhizin. The amount of glycyrrhetic acid reabsorbed depends on its transit through the small and large intestines, therefore patients with prolonged gastrointestinal transit times and more prone to toxicity after repeated intake. Other factors include old age, female sex and hypertension.
Licorice toxicity is also potentiated by factors known to predispose to hypokalemia. Common causes include gastrointestinal losses due to diarrhea or renal losses due to diuretic therapy. Diminished intake is seldom the sole cause of potassium depletion since the urinary excretion can be efficiently reduced to less than 15 mEq per day [Singer and Brenner, 2005]. Increased entry into cells as caused by B2 agonists, alkalosis or combined glucose and insulin therapy are other possibilities; however, the resulting hypokalemia is usually mild and transient. Other less common causes of hypokalemia include Cushing syndrome and Conn’s syndrome. Anorexia nervosa is another factor that increases glycyrrhizin sensitivity. Nightingale and colleagues reported a case of licorice-induced myopathy in a patient with anorexia nervosa with a diet comprising very low potassium levels [Nightingale et al. 1981]. Støving and colleagues demonstrated that glycyrrhizin sensitivity is increased in patients with anorexia nervosa suggested by the pronounced hypokalemic response to a relatively low daily dose of licorice [Støving et al. 2011]. Table 2 lists the various factors that can contribute to increased glycyrrhizin sensitivity.
Table 2.
Factors that increase sensitivity to glycyrrhizin.
| Hypokalemia |
| Prolonged gastrointestinal transit time |
| Decreased 11-ß-hydroxysteroid dehydrogenase-2 activity |
| Hypertension |
| Anorexia nervosa |
| Old age |
| Female sex |
Case presentation
A 35-year-old man from Egypt, with no past medical history, presented to the emergency room with progressive weakness that started in his lower extremities and quickly progressed to involve the upper limbs. There was no history of fever, chills, prior diarrhea, upper respiratory tract infection, back pain or trauma. On physical examination, the patient was fully conscious and oriented, had a blood pressure (BP) of 200/110 mmHg and a heart rate of 90/min. Neurological exam revealed bilateral symmetric flaccid paralysis (grade 1/5) of both upper and lower limbs with hypotonia and hyporeflexia. Examination of the sensory system and cranial nerves was unremarkable. Laboratory investigations were pertinent for a potassium level of 1.8 mmol/liter, sodium 144 mmol/liter and a normal CPK, thyroid and liver functions. Electrocardiography revealed a normal sinus rhythm, prominent U waves in the precordial leads and frequent atrial premature beats. A computed tomography scan of the brain was normal.
A provisional diagnosis of hypokalemic myopathy was presumed without a clear precipitating factor. Sodium nitroprusside infusion was initiated for control of BP together with intravenous potassium replacement. Initially, there was no improvement in his neurological status and he remained hypokalemic despite adequate supplementation. On further questioning, the patient admitted to drinking 1 liter daily of licorice, ‘erk soos’, during the whole month of Ramadan. Spironolactone therapy was started and potassium supplements were continued. The potassium level normalized slowly over the next 10 days with a gradual return of his motor function. The patient was discharged after regaining his full muscle power and was advised to avoid the excessive consumption of licorice.
Licorice-related complications
We reviewed all publications up to 2010 pertaining to complications previously described from excess licorice ingestion in any of its present forms. This review is based on data published in scientific journals indexed by the PubMed and Medline databases using the following keywords: ‘licorice, hypokalemic myopathy, licorice induced hyperaldosteronism and pseudo-hyperaldosteronism’. It was evident that most of the published complications are linked to the aldosterone-like action of licorice. The two main categories of complications were licorice-induced hypertension [Mumoli and Cei, 2008; Scali et al. 1990; Holmes et al. 1970; De Klerk et al. 1997] and hypokalemic myopathy [Yaguchi et al. 2008; Maresca et al. 1988; Caradonna et al. 1992; Lin et al. 2003a; Lin et al. 2003b; Gross et al. 1966; Tancevski et al. 2008]. In reports of licorice-induced hypertension, prognosis was favorable with good response after cessation of licorice and starting antihypertensive medications. However, there were a few patients who experienced hypertensive encephalopathy with a trend towards a longer recovery period [Van der Zwan, 1993; Russo et al. 2000; Bramont et al. 1985]. One patient suffered a focal neurological deficit and completely recovered 5 months later [Van der Zwan, 1993], and another patient developed an ischemic stroke [Bramont et al. 1985]. The second main category of complications is hypokalemic myopathy manifesting with flaccid paralysis. This group also had a good prognosis and full recovery was the rule in the majority of cases after cessation of licorice and potassium replacement. Some cases experienced delayed recovery after correction of hypokalemia and a few others exhibited acute renal tubular damage leading to acute renal failure from myoglobinuria [Kasap et al. 2010].
The complication associated with most fatalities is the arrhythmogenic effect of licorice mediated by hypokalemia and subsequent QT prolongation and possible torsade de pointes. The prognosis in the reported cases was poor, with six out of nine cases experiencing cardiac arrest [Campana et al. 2003; Arola, 2003; Böcker and Breithardt, 1991; Crean et al. 2009; Harris, 2000; Eriksson et al. 1999; Montoliu, 1977; Miyamoto et al. 2009; Bannister et al. 1977]. Some reports described a picture of heart failure and acute pulmonary edema which mostly followed a licorice binge [Chamberlain and Abolnik, 1997; Chamberlain, 1970; Hasegawa et al. 1998]. In these cases, recovery was the rule after implementing antifailure measures. A few cases presented with generalized edema which responded well to cessation of licorice and diuretic therapy [Sailler et al. 1993; Crampton, 1961; Francini-Pesenti et al. 2008].
Licorice was previously linked to increased incidence of preterm labor in a study performed on 95 women with preterm deliveries [Strandberg et al. 2001]. The study demonstrated that heavy consumption is associated with a twofold to threefold increase in the risk of preterm (<37 weeks) birth. However, these results were later questioned due to the retrospective collection of data and the possibility of confounding factors that might have biased the results [Hughes et al. 2003]. Several reports demonstrated the occurrence of ocular complications related to licorice ingestion [Hall and Clemett, 2004; Santaella and Fraunfelder, 2007; Dobbins and Saul, 2000; Fraunfelder, 2004]. The underlying pathogenesis involves vasospasm of the optic nerve blood vessels leading to transient monocular or binocular visual loss/aberrations. All patients who experienced transient visual loss/aberrations had resolution of their visual symptoms; the aid of hyperbaric oxygen was required for one patient.
There was another group of rare side effects. A young woman experienced an acute right upper limb embolic ischemia for which embolectomy was performed [Lozano et al. 2000]. Several reports demonstrated the interaction of licorice with drugs and the hepatic microsomal enzyme system. One patient exhibited digoxin toxicity due to hypokalemia induced by licorice intake [Harada et al. 2002]. Licorice root extracts not including glycyrrhizin causes inhibition of P450 and cytochrome P450 3A4 (CYP3A4) systems [Tsukamoto et al. 2005; Kent et al. 2002]. A study demonstrated the potentiation of warfarin effects due to the inhibitory effect of licorice on the hepatic microsomal enzyme system [Heck et al. 2000]. One patient presented with carpal tunnel syndrome with nerve conduction studies revealing bilateral median neuropathies likely attributed to licorice-induced water retention [Tacconi et al. 2009]. Other complications include hypersensitivity to glycyrrhizin [Kuriyama et al. 1975], occupational asthma [Cartier et al. 2002], myoclonus due to licorice-induced metabolic alkalosis [Ishiguchi et al. 2004] and licorice-induced contact dermatitis [Nugmanova and Kalitina, 1979; O’Connell et al. 2008]. Table 3 demonstrates the previously reported licorice-related complications.
Table 3.
Complications related to excess licorice intake.
| Cardiovascular | Hypertension |
| Hypertensive encephalopathy | |
| Cardiac arrhythmias and death due to QT prolongation | |
| Heart failure and pulmonary edema | |
| Generalized edema | |
| Embolic ischemia | |
| Neurological | Hypokalemic myopathy Stroke |
| Rhabdomyolysis | |
| Carpal tunnel syndrome | |
| Licorice-induced myoclonus | |
| Occular deficits | |
| Electrolyte and renal abnormalities | Hypokalemia |
| Metabolic alkalosis | |
| Elevated CPK | |
| Acute tubular necrosis due to myoglobinuria | |
| Allergic reactions | Occupational asthma |
| Contact dermatitis | |
| Drug interaction | Inhibition of the P450 and CYP3A4 systems |
| Potentiation of the effect of warfarin therapy | |
| Digoxin toxicity due to licorice-induced hypokalemia |
CPK, creatine phosphokinase; CYP, cytochrome P450.
Discussion
Licorice is a constituent in many food products and is available in various forms. The public often consume licorice because of a traditional belief in its health benefit with unawareness of the potential hazards of overconsumption. In Egypt, the consumption of erk soos (of which licorice is a main constituent) is a common tradition during Ramadan and especially during the warm summer where it acts as a thirst quencher. The presented case exemplifies one of the common complications of licorice toxicity when a patient without prior medical history presented with severe hypertension, hypokalemia and quadriparesis after prolonged heavy licorice intake.
The side effects of licorice were first described by Revers [Revers, 1948] following its use for the treatment of peptic ulcer disease. Consequently, other adverse effects were reported following exposure through candies or by ingestion of licorice-containing products such as the antituberculosis medication p-aminosalicylic acid [Cayley, 1950; Heard et al. 1950], the antipeptic ulcer medication carbenoxolone sodium [Baron, 1983], the French alcoholic beverage boisson de coco [Mollaret et al. 1960; Jenny et al. 1961], chewing tobacco [Blachley and Knochel, 1980] and some oriental herbal preparations [Sugimoto et al. 1984].
Licorice-induced mineralocorticoid effect can be abated after cessation of intake, adequate potassium replacement and spironolactone therapy. A previous study demonstrated that aldosterone receptor antagonism with either spironolactone or eplerenone normalizes blood pressure, prevents upregulation of vascular endothelin-1, restores nitrous oxide mediated endothelial dysfunction and thus may advance as a novel and specific therapeutic approach in 11-ß-HSD2-deficient hypertension [Thomas et al. 2001]. A considerable period of time is usually required for the reversal of licorice’s mineralocortecoid-like effects as was demonstrated in the presented case. This is attributed to the long half life of glycyrrhetic acid and the long duration required for the renin–angiotensin–aldosterone axis to normalize, which can take up to 6 months [Epstein et al. 1977]. Because of its adverse effect profile, DGL has been manufactured in an attempt to avoid complications from glycyrrhizic acid.
This review creates awareness of the potential hazards of licorice. We emphasize the importance of a thorough and detailed dietary history, including licorice-containing products and herbal medicines, and the need for re-evaluating the traditional habits that promote excessive licorice consumption because of a belief of its health benefits. The FDA should start regulating the use of this substance and create public awareness through the media about its health hazards. We aim to send a warning message that licorice is not just a candy and that serious life-threatening complications can occur with excess use.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Hesham R. Omar, Internal Medicine Department, Mercy Hospital and Medical Center, 2525 South Michigan Avenue, Chicago, IL 60616, USA
Irina Komarova, Internal Medicine Department, Mercy Hospital and Medical Center, Chicago, IL, USA.
Mohamed El-Ghonemi, Critical Care Department, Cairo University, Cairo, Egypt.
Ahmed Fathy, Cardiology Department, National Heart Institute, Cairo, Egypt.
Rania Rashad, Critical Care Department, Cairo University, Cairo, Egypt.
Hany D. Abdelmalak, Internal Medicine Department, Mercy Hospital and Medical Center, Chicago, IL, USA
Muralidhar Reddy Yerramadha, Internal Medicine Department, Mercy Hospital and Medical Center, Chicago, IL, USA.
Yaseen Ali, Internal Medicine Department, Mercy Hospital and Medical Center, Chicago, IL, USA.
Engy Helal, Emergency Department, Elagouza Hospital, Cairo, Egypt.
Enrico M. Camporesi, Department of Surgery/Anesthesiology and Department of Molecular Pharmacology & Physiology, University of South Florida, Tampa, FL, USA
References
- Agarwal A., Gupta D., Yadav G., Goyal P., Singh P., Singh U. (2009) An evaluation of the efficacy of licorice gargle for attenuating postoperative sore throat: a prospective, randomized, single blind study. Anesth Analg 109: 77–81 [DOI] [PubMed] [Google Scholar]
- Armanini D., Calò L., Semplicini A. (2003) Pseudohyperaldosteronism: pathogenetic mechanisms. Crit Rev Clin Lab Sci 40: 295–335 [DOI] [PubMed] [Google Scholar]
- Armanini D., Castello R., Scaroni C., Bonanni G., Faccini G., Pellati D., et al. (2007) Treatment of polycystic ovary syndrome with spironolactone plus licorice. Eur J Obstet Gynecol Reprod Biol 131: 61–67 [DOI] [PubMed] [Google Scholar]
- Armanini D., De Palo C., Mattarello M., Spinella P., Zaccaria M., Ermolao A., et al. (2003) Effect of licorice on the reduction of body fat mass in healthy subjects. J Endocrinol Invest 26: 646–650 [DOI] [PubMed] [Google Scholar]
- Armanini D., Lewicka S., Pratesi C., Scali M., Zennaro M., Zovato S., et al. (1996) Further studies on the mechanism of the mineralocorticoid action of licorice in humans. J Endocrinol Invest 19: 624–629 [DOI] [PubMed] [Google Scholar]
- Armanini D., Mattarello M., Fiore C., Bonanni G., Scaroni C., Sartorato P., et al. (2004) Licorice reduces serum testosterone in healthy women. Steroids 69: 763–766 [DOI] [PubMed] [Google Scholar]
- Arola O. (2003) Black arrhythmias. Duodecim 119: 2145–2147 [PubMed] [Google Scholar]
- Bannister B., Ginsburg R., Shneerson J. (1977) Cardiac arrest due to liquorice induced hypokalaemia. Br Med J 2: 738–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron J. (1983) Side-effects of carbonoxolone. Acta Gastroenterol Belg 46: 469–484 [PubMed] [Google Scholar]
- Basso A., Dalla Paola L., Erle G., Boscaro M., Armanini D. (1994) Licorice ameliorates postural hypotension caused by diabetic autonomic neuropathy. Diabetes Care 17: 1356. [DOI] [PubMed] [Google Scholar]
- Bernardi M., D’Intino P., Trevisani F., Cantelli-Forti G., Raggi M., Turchetto E., et al. (1994) Effects of prolonged ingestion of graded doses of liquorice by healthy volunteers. Life Sci 55: 863–872 [DOI] [PubMed] [Google Scholar]
- Bijlsma J., Van Vloten P., Van Gelderen C., Mensinga T., Mout H., Elvers L., et al. (1996) Study into the effects of different dosages of glycyrrhizin in health female volunteers; in Dutch. RIVM report no. 348801004, RIVM, Bilthoven, The Netherlands
- Blachley J., Knochel J. (1980) Tobacco chewer’s hypokalemia: licorice revisited. N Engl J Med 302: 784–785 [DOI] [PubMed] [Google Scholar]
- Böcker D., Breithardt G. (1991) Induction of arrhythmia by licorice abuse. Z Kardiol 80: 389–391 [PubMed] [Google Scholar]
- Bramont C., Lestradet C., Godart L., Faivre R., Narboni G. (1985) Cerebral vascular accident caused by alcohol-free licorice. Presse Med 14: 746. [PubMed] [Google Scholar]
- Calò L., Zaghetto F., Pagnin E., Davis P., De Mozzi P., Sartorato P., et al. (2004) Effect of aldosterone and glycyrrhetinic acid on the protein expression of PAI-1 and p22(phox) in human mononuclear leukocytes. J Clin Endocrinol Metab 89: 1973–1976 [DOI] [PubMed] [Google Scholar]
- Campana A., Manzo M., Brigante M., Marrazzo N., Melchiorre G. (2003) An unusual cause of cardiac arrest. Ital Heart J Suppl 4: 510–513 [PubMed] [Google Scholar]
- Caradonna P., Gentiloni N., Servidei S., Perrone G., Greco A., Russo M. (1992) Acute myopathy associated with chronic licorice ingestion: reversible loss of myoadenylate deaminase activity. Ultrastruct Pathol 16: 529–535 [DOI] [PubMed] [Google Scholar]
- Cartier A., Malo J., Labrecque M. (2002) Occupational asthma due to liquorice roots. Allergy 57: 863. [DOI] [PubMed] [Google Scholar]
- Cayley F. (1950) Potassium deficiency in p-aminosalicylic acid therapy: cardiac and paralytic effects. Lancet 1: 447–448 [Google Scholar]
- Chamberlain J., Abolnik I. (1997) Pulmonary edema following a licorice binge. West J Med 167: 184–185 [PMC free article] [PubMed] [Google Scholar]
- Chamberlain T. (1970) Licorice poisoning, pseudoaldosteronism and heart failure. JAMA 213: 1343. [PubMed] [Google Scholar]
- Chintharlapalli S., Papineni S., Jutooru I., McAlees A., Safe S. (2007) Structure-dependent activity of glycyrrhetinic acid derivatives as peroxisome proliferator-activated receptor [gamma] agonists in colon cancer cells. Mol Cancer Ther 6: 1588–1598 . [DOI] [PubMed] [Google Scholar]
- Crampton J. (1961) Glycyrrhizinophilia as a cause of edema. Bull Mason Clin 15: 89–92 [PubMed] [Google Scholar]
- Crean A., Abdel-Rahman S., Greenwood J. (2009) A sweet tooth as the root cause of cardiac arrest. Can J Cardiol 25: 357–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Klerk G., Nieuwenhuis M., Beutler J. (1997) Hypokalaemia and hypertension associated with use of liquorice flavoured chewing gum. Br Med J 314: 731–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins K., Saul R. (2000) Transient visual loss after licorice ingestion. J Neuroopthalmol 20: 38–41 [DOI] [PubMed] [Google Scholar]
- Dong S., Inoue A., Zhu Y., Tanji M., Kiyama R. (2007) Activation of rapid signaling pathways and the subsequent transcriptional regulation for the proliferation of breast cancer MCF-7 cells by the treatment with an extract of Glycyrrhiza glabra root. Food Chem Tox 45: 2470–2478 [DOI] [PubMed] [Google Scholar]
- Epstein M., Espiner E., Donald R., Hughes H. (1977) Liquorice toxicity and the renin-angiotensinaldosterone axis in man. Br Med J 1: 209–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson J., Carlberg B., Hillörn V. (1999) Life-threatening ventricular tachycardia due to liquorice-induced hypokalaemia. J Intern Med 245: 307–310 [DOI] [PubMed] [Google Scholar]
- Farese S., Kruse A., Pasch A., Dick B., Frey B., Uehlinger D., et al. (2009) Glycyrrhetinic acid food supplementation lowers serum potassium concentration in chronic hemodialysis patients. Kidney Int 76: 877–884 [DOI] [PubMed] [Google Scholar]
- Fenwick G., Lutomski J., Nieman C. (1990) Liquorice, Glycyrrhiza glabra L. – composition, uses and analysis. Food Chem 38: 119–143 [Google Scholar]
- Francini-Pesenti F., Puato M., Piccoli A., Brocadello F. (2008) Liquorice-induced hypokalaemia and water retention in the absence of hypertension. Phytother Res 22: 563–565 [DOI] [PubMed] [Google Scholar]
- Fraunfelder F. (2004) Ocular side effects from herbal medicines and nutritional supplements. Am J Ophthalmol 138: 639–647 [DOI] [PubMed] [Google Scholar]
- Funder J., Pearce P., Smith R. (1988) Mineralocorticoid action: target tissue specificity is enzyme, not receptor mediated. Science 243: 583–585 [DOI] [PubMed] [Google Scholar]
- Gross E., Dexter J., Roth R. (1966) Hypokalemic myopathy with myoglobinuria associated with licorice ingestion. N Engl J Med 274: 602–606 [DOI] [PubMed] [Google Scholar]
- Hall R., Clemett R. (2004) Central retinal vein occlusion associated with liquorice ingestion. Clin Exp Ophthalmol 32: 341. [DOI] [PubMed] [Google Scholar]
- Harada T., Ohtaki E., Misu K., Sumiyoshi T., Hosoda S. (2002) Congestive heart failure caused by digitalis toxicity in an elderly man taking a licorice-containing Chinese herbal laxative. Cardiology 98: 218. [DOI] [PubMed] [Google Scholar]
- Harris J. (2000) Lethal licorice. Aust Nurs J 7: 1–3 [PubMed] [Google Scholar]
- Hasegawa J., Suyama Y., Kinugawa T., Morisawa T., Kishimoto Y. (1998) Echocardiographic findings of the heart resembling dilated cardiomyopathy during hypokalemic myopathy due to licorice-induced pseudoaldosteronism. Cardiovasc Drugs Ther 12: 599–600 [DOI] [PubMed] [Google Scholar]
- Hattori M., Sakamoto T., Yamagishi T., Sakamoto K., Konishi K., Kobashi K., et al. (1985) Metabolism of glycyrrhizin by human intestinal flora. II. Isolation and characterization of human intestinal bacteria capable of metabolizing glycyrrhizin and related compounds. Chem Pharm Bull (Tokyo) 33: 210–217 [DOI] [PubMed] [Google Scholar]
- Heard K., Campbell A., Hurley J. (1950) Hypokalemia complicating sodium para-aminosalicylate therapy for pulmonary tuberculosis. Med J Australia 2: 606–612 [PubMed] [Google Scholar]
- Heck A., DeWitt B., Lukes A. (2000) Potential interactions between alternative therapies and warfarin. Am J Health Syst Pharm 57: 1221–1227 [PubMed] [Google Scholar]
- Hoffmann D., Hoffmann I. (1997) The changing cigarette, 1950–1995. J Toxicol Environ Health 50: 307–364 [DOI] [PubMed] [Google Scholar]
- Holmes A., Young J., Marrott P., Prentice E. (1970) Pseudohyperaldosteronism induced by habitual ingestion of liquorice. Postgrad Med J 46: 625–629 [PMC free article] [PubMed] [Google Scholar]
- Hughes J., Sellick S., King R., Robbé I. (2003) Re: ‘preterm birth and licorice consumption during pregnancy’. Am J Epidemiol 158: 190–191 [DOI] [PubMed] [Google Scholar]
- Ishiguchi T., Mikita N., Iwata T., Nakata H., Sato H., Higashimoto Y., et al. (2004) Myoclonus and metabolic alkalosis from licorice in antacid. Intern Med 43: 59–62 [DOI] [PubMed] [Google Scholar]
- Jenny M., Muller A., Fabre J., Mach R. (1961) [Hypokaliemie et alcalose par ingestion abusived’extrait de reglisse (Liquorice) et d’eau bicarbonatee]. Schweiz Med Wochenschr 91:869–875 [PubMed] [Google Scholar]
- Kasap B., Soylu A., Cetin B., Camlar S., Türkmen M., Kavukçu S. (2010) Acute kidney injury following hypokalemic rhabdomyolysis: complication of chronic heavy cola consumption in an adolescent boy. Eur J Pediatr 169: 107–111 [DOI] [PubMed] [Google Scholar]
- Kent U., Aviram M., Rosenblat M., Hollenberg P. (2002) The licorice root derived isoflavan glabridin inhibits the activities of human cytochrome P450S 3A4, 2B6 and 2C9. Drug Metab Dispos 30: 709–715 [DOI] [PubMed] [Google Scholar]
- Kuriyama Y., Takano T., Okada F., Nukada T. (1975) Hypersensitivity to glycyrrhizin. A case report. Med J Osaka Univ 26: 75–78 [PubMed] [Google Scholar]
- Latif S., Conca T., Morris D. (1990) The effects of the licorice derivative, glycyrrhetinic acid, on hepatic 3 alpha- and 3 beta-hydroxysteroid dehydrogenases and 5 alpha- and 5 beta-reductase pathways of metabolism of aldosterone in male rats. Steroids 55: 52–58 [DOI] [PubMed] [Google Scholar]
- Lee C., Park K., Lim S., Park J., Chung W. (2007) Effects of the licorice extract against tumor growth and cisplatin-induced toxicity in a mouse xenograft model of colon cancer. Bio Pharm Bull 30: 2191–2195 [DOI] [PubMed] [Google Scholar]
- Lin S., Davids M., Halperin M. (2003a) Hypokalaemia and paralysis. QJM 96: 161–169 [DOI] [PubMed] [Google Scholar]
- Lin S., Yang S., Chau T., Halperin M. (2003b) An unusual cause of hypokalemic paralysis: chronic licorice ingestion. Am J Med Sci 325: 153–156 [DOI] [PubMed] [Google Scholar]
- Lozano P., Flores D., Martínez S., Artigues I., Rimbau E., Gómez F. (2000) Upper limb ischemia induced by chronic licorice ingestion. J Cardiovasc Surg (Torino) 41: 631–632 [PubMed] [Google Scholar]
- Maas P. (2000) Zoethout in levensmiddelen: onderzoek naar het glycyrrhizine gehalte van thee, kruidenmengsels, dranken en drop [Liquorice root in food stuffs: survey of the glycyrrhizin content of tea, herbal mixtures, alcoholic drinks and liquorice] (in Dutch). De Ware(n) Chemicus 30: 65–74 [Google Scholar]
- Maresca M., Calconi G., Amici G., Teodori T., Da Porto A. (1988) Hypopotassemia and rhabdomyolysis. Description of 3 cases of different etiologies. Minerva Med 79: 55–60 [PubMed] [Google Scholar]
- Mattarello M., Benedini S., Fiore C., Camozzi V., Sartorato P., Luisetto G., et al. (2006) Effect of licorice on PTH levels in healthy women. Steroids 71: 403–408 [DOI] [PubMed] [Google Scholar]
- Miyamoto K., Kawai H., Aoyama R., Watanabe H., Suzuki K., Suga N., et al. (2009) Torsades de Pointes induced by a combination of garenoxacin and disopyramide and other cytochrome P450, family 3, subfamily A polypeptide-4-influencing drugs during hypokalemia due to licorice. Clin Exp Nephrol 14: 164–167 [DOI] [PubMed] [Google Scholar]
- Mollaret P., Goulon M., Tournilhac M. (1960) [Quadriplegie avec hypokaliemie et alcalose metabolique secondaire a l’ingestion massive et prolongee d’extrait de reglissie chez unpsychopathe ethylique et potomane]. Bull Mem Soc Med Hop Paris 1: 491–512 [PubMed] [Google Scholar]
- Montoliu J. (1977) Liquorice-induced cardiac arrest. Br Med J 2: 1352–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumoli N., Cei M. (2008) Licorice-induced hypokalemia. Int J Cardiol 124: 42–44 [DOI] [PubMed] [Google Scholar]
- Murphy S., Agger S., Rainey P. (2009) Too much of a good thing: a woman with hypertension and hypokalemia. Clin Chem 55: 2093–2096 [DOI] [PubMed] [Google Scholar]
- Nightingale S., Smith P., Turnbull D. (1981) Anorexia nervosa, liquorice and hypokalaemic myopathy. Postgrad Med J 57: 577–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugmanova M., Kalitina N. (1979) Case of contact dermatitis caused by licorice. Vestn Dermatol Venerol (11): 62–64 [PubMed] [Google Scholar]
- Obolentseva G., Litvinenko V., Ammosov A., Popova T., Sampiev A. (1999) Pharmacological and therapeutic properties of licorice preparations (a review). Pharm Chem J 33: 24–31 [Google Scholar]
- O’Connell R., White I., White J., McFadden J. (2008) Liquorice extract in a cosmetic product causing contact allergy. Contact Dermatitis 59: 52. [DOI] [PubMed] [Google Scholar]
- Oerter Klein K., Janfaza M., Wong J., Chang R. (2003) Estrogen bioactivity in fo-ti and other herbs used for their estrogen-like effects as determined by a recombinant cell bioassay. J Clin Endocrinol Metab 88: 4077–4079 [DOI] [PubMed] [Google Scholar]
- Palermo M., Armanini D., Delitala G. (2003) Grapefruit juice inhibits 11beta-hydroxysteroid dehydrogenase in vivo, in man. Clin Endocrinol (Oxf) 59: 143–144 [DOI] [PubMed] [Google Scholar]
- Ploeger B., Mensinga T., Sips A., Seinen W., Meulenbelt J., DeJongh J. (2001) The pharmacokinetics of glycyrrhizic acid evaluated by physiologically based pharmacokinetic modeling. Drug Metab Rev 33: 125–147 [DOI] [PubMed] [Google Scholar]
- Ploeger B., Meulenbelt J., DeJongh J. (2000) Physiologically based pharmacokinetic modeling of glycyrrhizic acid, a compound subject to presystemic metabolism and enterohepatic cycling. Toxicol Appl Pharmacol 162: 177–188 [DOI] [PubMed] [Google Scholar]
- Potton F., Janin J., Cruaud J. (1970) 2 cases of arterial hypertension due to glycyrrhizin poisoning. Analysis of the syndrome by voluntary poisoning. Lyon Med 223: 721–726 [PubMed] [Google Scholar]
- Revers F. (1948) Behandeling van ulcus ventriculi en ulcus, duodeni mer succus liquiritiae. Nederel Tijdschr v Geneesk 92: 2968–2972 [PubMed] [Google Scholar]
- Russo S., Mastropasqua M., Mosetti M., Persegani C., Paggi A. (2000) Low doses of liquorice can induce hypertension encephalopathy. Am J Nephrol 20: 145–148 [DOI] [PubMed] [Google Scholar]
- Sailler L., Juchet H., Ollier S., Nicodème R., Arlet P. (1993) Generalized edema caused by licorice: a new syndrome. Apropos of 3 cases. Rev Med Interne 14: 984. [DOI] [PubMed] [Google Scholar]
- Santaella R., Fraunfelder F. (2007) Ocular adverse effects associated with systemic medications: recognition and management. Drugs 67: 75–93 [DOI] [PubMed] [Google Scholar]
- Sato H., Goto W., Yamamura J., Kurokawa M., Kageyama S., Takahara T., et al. (1996) Therapeutic basis of glycyrrhizin on chronic hepatitis B. Antiviral Res 30: 171–177 [DOI] [PubMed] [Google Scholar]
- Scali M., Pratesi C., Zennaro M., Zampollo V., Armanini D. (1990) Pseudohyperaldosteronism from liquorice-containing laxatives. J Endocrinol Invest 13: 847–848 [DOI] [PubMed] [Google Scholar]
- Scheinman S., Guay-Woodford L., Thakker R., Warnock D. (1999) Genetic disorders of renal electrolyte transport. N Engl J Med 340: 1177–1187 [DOI] [PubMed] [Google Scholar]
- Scientific Committee on Food (2003) Opinion of the Scientific Committee on Food on Glycyrrhizinic Acid and its Ammonium Salt. Brussels: European Commission Heath and Consumer Protection Directorate General [Google Scholar]
- Sigurjonsdottir H., Manhem K., Axelson M., Wallerstedt S. (2003) Subjects with essential hypertension are more sensitive to the inhibition of 11 beta-HSD by liquorice. J Hum Hypertens 17: 125–131 [DOI] [PubMed] [Google Scholar]
- Singer G., Brenner B. (2005) Fluid and electrolyte disturbances. In: Kasper D., Fauci A., Longo D., Braunwald B., Hauser S., Jameson J. (eds), Harrison’s Principles of Internal Medicine, 16th ed. New York: McGraw-Hill [Google Scholar]
- Stewart P., Wallace A., Valentino R., Burt D., Shackleton C., Edwards C. (1987) Mineralocorticoid activity of liquorice: 11-beta hydroxysteroid dehydrogenase deficiency comes of age. Lancet 2: 821–824 [DOI] [PubMed] [Google Scholar]
- Støving R., Lingqvist L., Bonde R., Andries A., Hansen M., Andersen M., et al. (2011) Is glycyrrhizin sensitivity increased in anorexia nervosa and should licorice be avoided? Case report and review of the literature. Nutrition 27: 855–858 [DOI] [PubMed] [Google Scholar]
- Strandberg T., Järvenpää A., Vanhanen H., McKeigue P. (2001) Birth outcome in relation to licorice consumption during pregnancy. Am J Epidemiol 153: 1085–1088 [DOI] [PubMed] [Google Scholar]
- Sugimoto K., Shionoiri H., Inoue K., Kaneko Y. (1984) A case report of hypokalemic myopathy due to ingestion of large dose of ‘jintan’ (in Japanese). Nippon Naika Gakkai Zasshi 73: 66–70 [PubMed] [Google Scholar]
- Tacconi P., Paribello A., Cannas A., Marrosu M. (2009) Carpal tunnel syndrome triggered by excessive licorice consumption. J Peripher Nerv Syst 14: 64–65 [DOI] [PubMed] [Google Scholar]
- Takahara T., Watanabe A., Shiraki K. (1994) Effects of glycyrrhizin on hepatitis B surface antigen: a biochemical and morphological study. J Hepatol 21: 601–609 [DOI] [PubMed] [Google Scholar]
- Tamir S., Eizenberg M., Somjen D., Izrael S., Vaya J. (2001) Estrogenlike activity of glabrene and other constituents isolated from licorice root. J Steroid Biochem Mol Biol 78: 291–298 [DOI] [PubMed] [Google Scholar]
- Tancevski I., Eller P., Spiegel M., Kirchmair R., Patsch J. (2008) Images in cardiovascular medicine. Malicious licorice. Circulation 117: 299. [DOI] [PubMed] [Google Scholar]
- Thomas Q., Frank R., Sidney S., Thomas F. (2001) Lüscher. Aldosterone receptor antagonism normalizes vascular function in liquorice-induced hypertension. Hypertension 37: 801–805 [DOI] [PubMed] [Google Scholar]
- Tsukamoto S., Aburatani M., Yoshida T., Yamashita Y., El-Beih A., Ohta T. (2005) CYP3A4 inhibitors isolated from licorice. Biol Pharm Bull 28: 2000–2002 [DOI] [PubMed] [Google Scholar]
- Van der Zwan A. (1993) Hypertension encephalopathy after liquorice ingestion. Clin Neurol Neurosurg 95: 35–37 [DOI] [PubMed] [Google Scholar]
- Vaya J., Belinky P., Aviram M. (1997) Antioxidant constituents from licorice roots: isolation, structure elucidation and antioxidative capacity toward LDL oxidation. Free Radic Biol Med 23: 302–313 [DOI] [PubMed] [Google Scholar]
- Walker B., Edwards C. (1994) Licorice-induced hypertension and syndromes of apparent mineralocorticoid excess. Endocrinol Metab Clin North Am 23: 359–377 [PubMed] [Google Scholar]
- Wang Z., Nixon D. (2001) Licorice and cancer. Nutr Cancer 39: 1–11 [DOI] [PubMed] [Google Scholar]
- Whorwood C., Sheppard M., Stewart P. (1993) Licorice inhibits 11 betahydroxysteroid dehydrogenase messenger ribonucleic acid levels and potentiates glucocorticoid hormone action. Endocrinology 132: 2287–2292 [DOI] [PubMed] [Google Scholar]
- World Health Organization (2005) Evaluation of certain food additives. World Health Organ Tech Rep Ser 928: 1–156 [PubMed] [Google Scholar]
- Yaguchi M., Yaguchi H., Sakano M. (2008) A case of hypokalemic myopathy mimicking hemiparesis. Brain Nerve 60: 191–194 [PubMed] [Google Scholar]