Abstract
Normal vision depends on the optimal function of ocular barriers and intact membranes that selectively regulate the environment of ocular tissues. Novel pharmacotherapeutic modalities have aimed to overcome such biological barriers which impede efficient ocular drug delivery. To determine the impact of ocular barriers on research related to ophthalmic drug delivery and targeting, herein we provide a review of the literature on isolated primary or immortalized cell culture models which can be used for evaluation of ocular barriers. In vitro cell cultures are valuable tools which serve investigations on ocular barriers such as corneal and conjunctival epithelium, retinal pigment epithelium and retinal capillary endothelium, and can provide platforms for further investigations. Ocular barrier-based cell culture systems can be simply set up and used for drug delivery and targeting purposes as well as for pathological and toxicological research.
Keywords: Drug Delivery Systems, In Vitro
INTRODUCTION
The cells and tissues of the eye are restrictively regulated to maintain optimal visual function. For such unique specialized function, tight cellular barriers in the anterior and posterior segments of the eye play a key role by selective control of inward and/or outward traverse of fluids and solutes. These barriers also effectively control shuttling of administered drugs; therefore effective drug delivery and targeting is faced by challenges to overcome these barriers. A schematic illustration of main ocular structures and barriers is demonstrated in Fig. 1. The emergence of futuristic medicaments (e.g., gene-based medicines) for treatment of ocular diseases demands effective strategies to enhance drug bioavailability.1,2 Both in vivo animal models and in vitro cell-based models are employed for such investigations. Animal based experiments are important for pharmacological and/or toxicological studies while cell culture models are relevant to mechanistic investigations.2,3
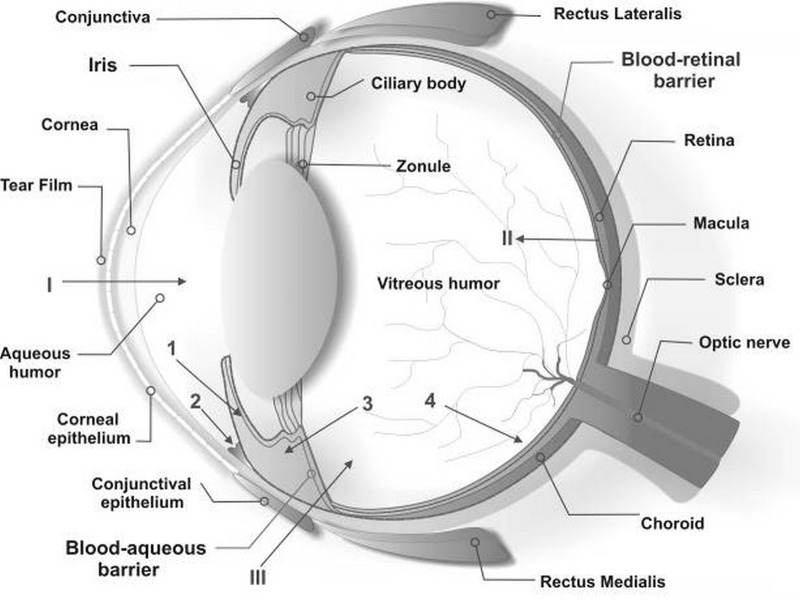
Figure 1.
Schematic illustration of ocular structures and barriers. The primary physiologic obstacle against topically instilled drugs is the tear film. The cornea is the main route for drug transport into the anterior chamber (I). The retinal pigment epithelium and the retinal capillary endothelium are main barriers against systemically administered drugs (II). Intravitreal injection is an invasive strategy to reach the vitreous (III). Administered drugs can be carried out of the anterior chamber by venous blood flow after diffusion across the iris surface (1) or by aqueous humor outflow (2). Drugs may be removed from the vitreous cavity through diffusion into the anterior chamber (3), or by the blood-retinal barrier (4).
Various animal models including rabbits, pigs, dogs, cats, mice, rats and monkeys have been exploited for pharmacokinetic and bioavailability studies. The rabbit model is most commonly used despite morphological and biochemical differences with the human eye such as lower blinking rate, larger corneal and conjunctival surface area, and absence of melanin pigments in the anterior uvea of albino rabbits. Such differences may significantly in-fluence the results of ocular pharmacotherapeutic research.2 Furthermore, animal experiments have been extensively criticized in terms of cost, time and ethical issues.3 To control animal based experiments, many countries have implemented restrictive legislations such as the European Union Acts upon Directive 86/609/EEC rules and regulations.
Such a trend has prompted seeking for alternative in vitro methods to replace animal experiments and has encouraged researchers to recruit cell culture models of ocular barriers which can provide platforms for ophthalmic investigations. These cell-based models are very useful systems for studying ocular barrier functions as well as cellular uptake and transport machineries. These models can be easily set up and used for many cellular and molecular studies such as cellular metabolism and biomarker detections, upon which novel therapeutic modalities (e.g., genome based therapeutics, monoclonal antibodies and nanobodies) can be developed. Cell culture models appear to offer the advantage of a highly defined system, thus resulting in reproducible data. These cell-based systems from human resources can provide very reliable results free from speciesrelated problems. So far, both primary isolated cell culture systems and cell lines have been used for modeling the ocular barriers. The main focus of this review is cell lines since they are commercially available and can be easily set up for various ocular investigations. Of these immortalized human cell lines, we will provide concise overviews on both epithelial and endothelial cell models.
OCULAR BARRIERS AND MEMBRANES
Tear Film
The tear film is the first protective layer of the cornea and conjunctiva; it contains an optimal electrolyte composition, pH and nutrient levels, and a complex mixture of proteins, lipids, and mucin. It consists of three layers: a lipid layer (the outermost layer, 0.1 μm in thickness secreted by meibomian glands), an aqueous layer (the middle layer, 7-10 μm in thickness), and a mucous layer (the innermost layer, 0.2-1.0 μm in thickness). The contents of the tear film are secreted by various glands of the eye and corneal epithelial cells.4 Major tear proteins that display antibacterial/antiviral activities are: lysozyme, secretory immunoglobulin (IgA), lactoferrin, lipocalin, peroxidase and high-molecular weight glycoproteins or "mucins".5
The tear film is able to modulate cellular migration and proliferation during wound healing, normal cellular differentiation, and secretion of electrolytes and water. These functions are accomplished by a wide variety of growth factors, cytokines and biologically active peptides including epidermal growth factor (EGF); hepatocyte growth factor (HGF); transforming growth factor (TGF; Α, Β1 and Β2); basic fibroblast growth factor (bFGF); tumor necrosis factor (TNF-Α) and granulocyte-macrophage colony stimulating factor (GM-CSF). Other substances present in the tear film are interleukin (IL)- 1Α and IL1Β, substance P (Sub P) and endothelin 1.4
Corneal and Non-Corneal Routes of Absorption
Lacrimal drainage and systemic absorption from the conjunctiva can wash away ophthalmic drops which are the most common type of ocular drugs. This results in absorption of a small fraction of the drug.4,6,7 For topical drugs, small lipophilic molecules are normally absorbed through the cornea, while large hydrophilic molecules such as proteins/gene based medicines are absorbed via the conjunctiva and sclera.6,8 Of these routes, the mechanical and chemical barrier functions of the cornea control access of exogenous substances into the eye, thereby protecting intraocular tissues (Fig. 1). The human cornea measures approximately 12 mm in diameter and 520 μm in thickness, and consists of five layers, including the epithelium, basement membrane (Bowman's layer), stroma, Descemet's membrane and endothelium (Fig. 2).
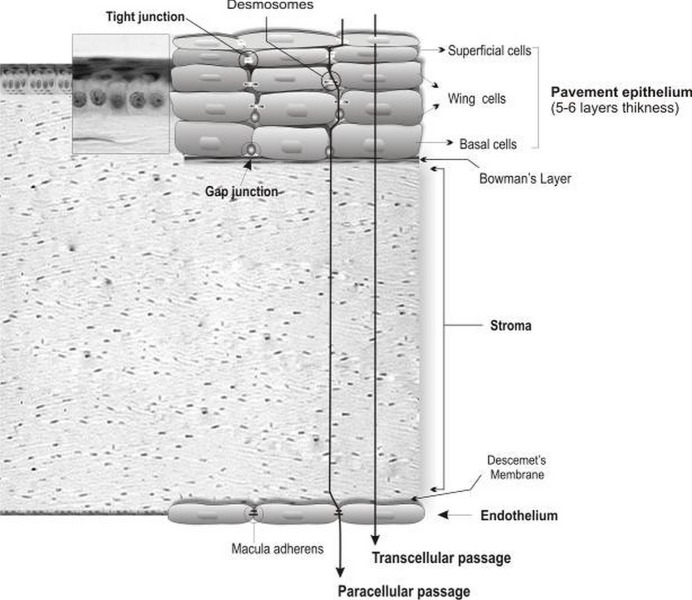
Figure 2.
Corneal cellular organization, the cornea consists of various transport limiting layers. The tightest monolayer is made by outer superficial epithelial cells which display tight junction complexes. The wing and basal cells exhibit gap junctions. The stroma and Descemet’s membrane cover the inner endothelial cells which contain macula adherens and are more permeable.
The human corneal epithelium is a stratified, squamous, non-keratinized epithelium 50 μm in thickness. It is composed of two to three layers of flattened superficial cells, wing cells, and a single layer of columnar basal cells which are separated by a 10-20 nm intercellular spaces and have regular intercommunications. These desmosome-attached cells can communicate via gap junctions through which small molecules traverse. Tight junctions (zonulae occludens) seal the superficial cells, building a diffusion barrier in the surface of the epithelium. Compared to the stroma and endothelium, the corneal epithelium represents a rate-limiting barrier which hinders permeation of hydrophilic drugs and macromolecules. The stroma displays hydrophilic nature due to an abundant content of hydrated collagen, which prevents diffusion of highly lipophilic agents. The corneal endothelial monolayer maintains an effective barrier between the stroma and aqueous humor.9 Active ion and fluid transport mechanisms in the endothelium are responsible for maintaining corneal transparency.10
It has been reported that certain drug properties such as lipophilicity, molecular weight, charge, and degree of ionization can significantly influence its passive permeability across the cornea.11 Of these factors, lipophilicity playsa key role since transcellular permeation of lipophilic drugs through the cornea is fasterand greater as compared to hydrophilic drugs. This route appears to be the main path for absorption of topical drugs. Greater molecular size decreases the rate of paracellular permeation of drugs.12,13 Once in the cornea, the drug can diffuse into the aqueous humor and the anterior segment (Fig. 1). However, local administration of conventional drugs via the corneal route fails to provide adequate concentrations within the vitreous and retina.14,15
The conjunctiva is a mucous membrane consisting of vascularized epithelium (2-3 cell layers thick) and plays an important role as a protective barrier on the ocular surface since tight junctions are present on the apical surface of its cells. In fact, the bulbar conjunctiva represents the first barrier against permeation of topically applied drugs via the non-corneal route, which is the main intraocular route for entry of macromolecules and hydrophilic substances. Due to significant loss of drug through systemic circulation, the conjunctival/scleral pathway appears to be a non-efficient pathresulting in poor bioavailability.8,16
The sclera is about 10 times more permeable than the cornea and half permeable as the conjunctiva. It is poorly vascularized and consists mainly of collagen and mucopolysaccharides, through which drugs can diffuse and enter the posterior segment (uveal tract, retina, choroid, vitreous humor).
Blood-Ocular Barriers
Systemic/intravitreal application is the main route of drug administration for many posterior segment disorders (Fig. 1), by which adequate concentrations of drug can be achieved and maintained in the retina and vitreous. However, certain applications (oral/intravenous routes) may impose unwanted side effects due to use of high doses while on the other hand a very small fraction of the drug reaches ocular tissues due to blood-ocular barriers. Of these biological impediments, the blood-ocular barrier can be overcome using intravitreal injection. However, drawbacks to this method include the risks of endophthalmitis, lens damage, retinal detachment and low compliance. Two blood-ocular barrier systems, the bloodaqueous barrier (BAB) and blood-retinal barrier (BRB), control traverse of solutes and nutrients into inner ocular tissues.
Blood-Aqueous Barrier
Balancing the inflow and outflow of aqueous humor controls intraocular pressure. The BAB is located in the anterior part of the eye (Fig. 1), and is formed by endothelial cells of the blood vessels within the iris as well as the nonpigmented cell layer of the ciliary epithelium. Both of these cell layers contain tight-junction complexes responsible for prevention of nonspecific traverse of solutes into the internal ocular milieu. This function is vital for maintaining transparency of the ocular media and the chemical equilibrium of ocular fluids.17,18 It should be noted that barrier restrictiveness of the BAB is not similar to those of corneal/retinal barrier(s). It has been shown that macromolecules such as horse radish peroxidase (HRP, 40 kDa) fail to pass through iris blood vessels, but fenestrated capillaries of the ciliary body allow its traverse. The less restricted outward movement of substances from the aqueous humor across iris blood vessels into systemic circulation helps maintain transparency of the eye. Due to the architecture and function of the BAB, small lipophilic drugs can enter the uveal blood circulation and are consequently eliminated more rapidly from the anterior chamber. For instance, the clearance rate of pilocarpine is 13.0 μl/min while that of inulin is close to the rate of aqueous humor turnover. It appears that larger and more hydrophilic drugs are merely eliminated by aqueous humor turnover.
Blood-Retinal Barrier
The BRB is located in the posterior part of the eye and is composed of two cell types namely the retinal capillary endothelial (RCE) cells and retinal pigment epithelial (RPE) cells which form the inner and outer BRB, respectively. Retinal cellular architecture and the schematic structure of the blood-retinal barriers are presented in Fig. 3. Specialized transport processes within the RPE together with robust barrier restrictiveness of RPE control the traverse of nutrients/compounds, allowing selective exchange of nutrients between the choroid and retina.7,15 Polarized RPE cells display a predominantly apical localization of Na+,K+ATPase which regulates intracellular Na+ and K+ homeostasis.19,20 The inner BRB covers the lumen of retinal capillaries and protects the retina from circulating molecules in the blood circulation.
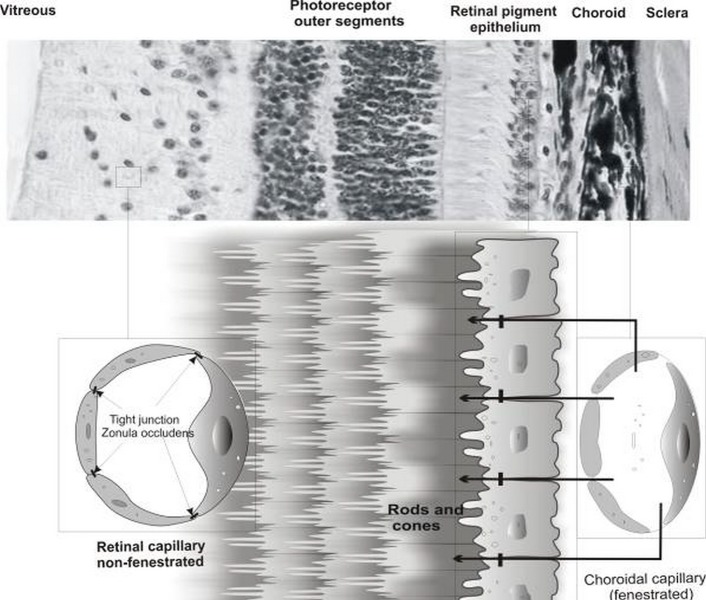
Figure 3.
Retinal cellular architecture, retinal pigment epithelial (RPE) cells and retinal capillary endothelial (RCE) cells represent the outer and inner retinal barriers, respectively. RPE and RCE compose the main organization of the transport limiting layers. The outer layer of the retinal pigment epithelium displays tight barriers due to the presence of tight junctions (zonula occludens). Inner retinal capillary endothelial cells possess tight junctions and are non-fenestrated as opposed to choroidal capillary endothelial cells.
Unlike the fenestrated choroidal capillary endothelial cells, RCE cells possess intercellular tight junctions which are formed by intercellular communications of RCE and glial cells.21 Immuno-staining studies for the tight junction protein occludin reveals a high degree of wellorganized tight junctions in retinal arterioles and capillaries. This, perhaps, highlights the role of astrocytes in formation of tight junctions within RCE cells, similar to their role in formation of the blood-rain barrier (BBB) by endothelial microvasculature.22 In addition, astrocyte-conditioned media supplemented With cAMP inducers can dramatically increasebarrier properties of endothelial cell culture, suggesting that a soluble component may confer barrier properties. The ability of glial cells to induce endothelial barrier properties suggests that disruption of the BRB in ocular diseases could be related to functional changes in glial cells or the retinal vascular endothelium. Because of the functional expression of tight junctions and intercommunication with glial cells (astrocytes and Müller cells), biological characteristics of RCE cells are similar to the BBB which is constituted by brain capillary endothelial cells, pericytes and astrocytes with trans-epithelial electrical resistance (TEER) value of approximately 1500-2000 (Ω.cm2).23–26 Despite these similarities, the density of interendothelial junctions and vesicles are greater in retinal vessels as compared to the brain. Passive diffusion of a vascular tracer has been shown to be significantly higher in the retina than the brain of rats.27
Despite permeation of lipophilic substances across RCE cells, this barrier displays poor permeability for proteins and small hydrophilic compounds.3,10 Satisfactory delivery and efficient pharmacological effect of drugs within the vitreous and retina require systemic or intravitreal drug administration. Systemic application via oral or intravenous administration, however, requires high doses of the drug since blood flow and restriction provided by the BRB allow very small fractions of the drug to reach the posterior chamber; typically only 1-2% of the plasma concentration. Therefore, a large proportion of the drug is disseminated within the entire body leading to unwanted consequences.28 Loss of normal BRB function is a common feature to many retinal degenerative disorders (e.g., in diabetic patients) that are leading causes of visual dysfunction. This necessitates development of therapies to prevent loss of barrier properties or restore them as a high priority in ophthalmology.Despite permeation of lipophilic substances across RCE cells, this barrier displays poor permeability for proteins and small hydrophilic compounds.3,10 Satisfactory delivery and efficient pharmacological effect of drugs within the vitreous and retina require systemic or intravitreal drug administration. Systemic application via oral or intravenous administration, however, requires high doses of the drug since blood flow and restriction provided by the BRB allow very small fractions of the drug to reach the posterior chamber; typically only 1-2% of the plasma concentration. Therefore, a large proportion of the drug is disseminated within the entire body leading to unwanted consequences. 28 Loss of normal BRB function is a common feature to many retinal degenerative disorders (e.g., in diabetic patients) that are leading causes of visual dysfunction. This necessitates development of therapies to prevent loss of barrier properties or restore them as a high priority in ophthalmology.29
Bioelectrical Properties of Ocular Barriers in Primary/Immortalized Cell Cultures
The simplest method to evaluate barrier functions of a cell culture model is to examine its electrophysiological parameters such as TEER. Tables 1–3 represent TEER values for different cell culture models. Paracellular and transcellular permeation characteristics of hydrophilic and lipophilic molecules respectively are other important determinants of barrier functions. Corneal permeability is greater for lipophilic as compared to hydrophilic drugs. The permeation of lipophilic compounds is unlikely to be affected by ocular barrier functions. To determine the dynamic range and selectivity of isolated primary porcine RPE tissue, Steuer et al30 tested various lipophilic and hydrophilic compounds with known in vivo BBB permeability characteristics using liquid chromatography/tandem mass spectrometry and found three major categories of permeability coefficients (Pe): poor permeation of ∼10−7 cm/s, moderate permeation of ∼10−6 cm/s, and pronounced permeation of ∼10−5 cm/s. Obtaining Pe values of 3.17×10−5 cm/s for memantine and 9.06×10−8 cm/s for atenolol, the model provided significant discrimination between lipophilic and hydrophilic compounds. Retinal capillary endothelial cells are also poorly permeable to macromolecules (e.g., proteins) and small hydrophilic compounds, but not to lipophilic compounds.
Table 1.
Bioelectrical properties of selected cell culture models of the corneal epithelial barrier
| Ocular tissue | Cell culture model | TEER (Ω.cm2) | Applications |
|---|---|---|---|
| Corneal epithelium | Primary rabbit cells: cultured onto fibronectin/collagen/laminin coated membrane using SFM for 7-8 days. | ∼ 5000 | Permeability and transport studies62 |
| Immortalized human cells: HCE-T cell line, cultured on collagen-coated membranes using SFM for 6 days | ∼500 | Cell biology, toxicity, ocular irritancy, gene/drug delivery65–67 | |
|
| |||
| Corneal endothelium | Immortalized human corneal endothelial cells: IHCEn cell line, cultivated onto lyophilized human amniotic membrane | – | Positive expression of Na+/K+ ATPase64 |
TEER, trans-epithelial electrical resistance; SFM, serum free media; HCE-T, human corneal epithelial cell; IHCEn, immortalized human corneal endothelial cell.
Table 2.
Bioelectrical properties of selected cell culture models of the Conjunctival epithelial barrier
| Ocular tissue | Cell culture model | TEER (Ω.cm2) | Applications |
|---|---|---|---|
| Conjunctival epithelium | Primary rabbit cells: cultured on collagen-coated membrane using SFM for 8-10 days | ∼ 1900 | Permeability and transport studies70 |
| Primary bovine cells: cultured on collagen-coated membrane, 10% serum medium for 9-11 days | ∼5600 | Cytotoxicity screening, cytokeratin expression71,72 | |
| Immortalized rat cells: CJ4.1A and CJ4.3C cell lines, cultured in 10% serum medium for 4 days | ∼600–800 | Investigation of antigen translocation across a mucosal barrier69 |
TEER, trans-epithelial electrical resistance; SFM, serum free media.
Table 3.
Bioelectrical properties of selected cell culture models of the blood-retinal barriers
| Ocular tissue | Cell culture model | TEER (Ω.cm2) | Applications |
|---|---|---|---|
| Retinal pigment epithelium | Primary isolated bovine cells: co-culture with endothelial cells for 14 days | ∼200 | Effect of endothelial cells on barrier function of the RPE83 |
| Primary isolated rat cells: cultured onto laminin coated filters using SFM for 5-7 days | ∼200 | Influence of serum on tight junction formation84 | |
| Immortalized human cells: ARPE-19 cell line, cultured onto collagen-coated membrane, 10% serum medium for 9-11 days | ∼100 | Characterization of ARPE-19 as a human RPE cell line forming polarized epithelial monolayers85 | |
|
| |||
| Retinal capillary endothelium | Primary isolated bovine retinal capillary endothelial cells: cultured onto polycarbonate filters (coated with gelatin, laminin, fibronectin, and collagen) | ∼ 150 | Establishment of retinal capillary endothelial cell model86 |
| Immortalized rat retinal capillary endothelial cells: TRiBRB cell line | ∼30 | Functional expression of cell membrane transporters87,88 | |
TEER, trans-epithelial electrical resistance; SFM, serum free media; ARPE, a human retinal pigment epithelial cell line; TRiBRB, immortalized rat retinal endothelial cell line.
The vitreous body occupies a volume of about 4.5 ml and is the largest single structure in the eye, contributing to 80% of total ocular volume. It supports the retina, and is probably essential for preservation of crystalline lens clarity. The vitreous body is a gel containing more than 99% water, stabilized by collagen fibrils, glycosaminoglycans and proteoglycans. Intact vitreous acts as a barrier against bulk movement of solutes. High concentrations of antioxidants, such as ascorbic acid, can therefore accumulate in the vitreous, and this might protect the lens against oxidative damage.31 The ability of the vitreous to prevent bulk movement of solutes depends on the degree of liquefaction of the gel. Although it is difficult to assess the degree of vitreous liquefaction by ophthalmic imaging techniques, diffusion within the vitreous of a tracer substance such as fluorescein can be illustrated in vivo by vitreous fluorophotometry in human.32
Direct intravitreal injection entails the obvious advantage of being able to achieve immediate therapeutic concentrations in the eye while largely avoiding systemic exposure. Nevertheless, drugs are rapidly eliminated from the vitreous, typically by first-order kinetics.33 Thus, repeated injections are needed to retain therapeutic concentrations in the eye, which are associated with risks of endophthalmitis, cataract formation and retinal detachment. To sustain sufficient intraocular drug levels after intravitreal injection, prolonged delivery of drugs were shown in liposomal systems whose small unilamellar vesicles (SUVs) and large unilamellar vesicles (LUVs) possess half-lives of approximately 10 and 20 days, respectively.34,35 It appears that both anterior and posterior routes are involved in elimination of drugs from the vitreous, where active transport machineries and/or passive diffusion are responsible for such function. The anterior route involves drainage into the anterior chamber followed by clearance via bulk aqueous flow, while the posterior route involves active or passive permeation across the retina and RPE followed by systemic dissipation.3 Following intravitreal drug administration, high drug lipophilicity or the presence of an active transport mechanism leads to rapid transport across the retina into systemic circulation. Therefore, longer vitreous half-life can be observed when drug passage through the BRB is not possible and the drug has to diffuse into the anterior chamber first to be removed either by aqueous flow or by diffusion across the iris. For instance, gentamicin and penicillin are removed from the vitreous via the anterior chamber and by crossing the retina at a rate of 0.035 h−1 and 0.18 h−1, respectively. This difference clearly highlights the impact of these elimination routes.
OCULAR MEMBRANE TRANSPORT MACHINERIES
Cell membranes impose a barrier to free movement of molecules through the plasma membrane lipid bilayer. A solute, based on its molecular properties, is shuttled across cell membranes by passive/active transport, carriermediated transport, receptor-mediated transport (endocytosis and transcytosis).36 Most ocular tissues, such as corneal epithelial and endothelial cells, display Na+/H−; exchanger, Na+;/HCO3− symporter, and Cl−/HCO3− exchanger which are involved in the regulation of intracellular pH.37,38 The Na+/H+ exchanger is present in the basolateral membranes of both epithelial and endothelial cells, while the Na+/HCO3− transporter is predominantly localized to the basolateral aspect of the corneal endothelium and only faintly expressed in the corneal epithelium. This implies that apical and basolateral membrane distribution of these channel transporters serves cellular needs and physiologic functions.39,40 Active transporters use energy to transport solutes against a concentration gradient; the energy is derived directly by the carrier from hydrolysis of ATP, or indirectly from the energy stored in ion gradients such as proton or more commonly sodium. Coupling of ion transport with that of the solute can be of symport or antiport types.36 Further investigation is required to elucidate functional expression, transport directionality, membrane distribution and exchange potentials of these transporters.
Influx and Efflux Transporters
Influx and efflux transport machineries are functional in major membranous barriers including the cornea, conjunctiva, iris, ciliary body, and retina.41 Uni- or bi-directional influx transporters such as monocarboxylate transporters (MCTs), glucose transporters (e.g., Glut1), amino acid transporters (LAT1 and LAT2) and peptide transporters (Pept) supply essential nutrients.36 For example, the LAT1 transporter in brain capillary endothelial cells functions bi-directionally with greater efflux activity,24 however its functional directionality in corneal and retinal barriers remains to be determined.
Among the ATP-binding cassette (ABC) superfamily, the P-glycoprotein (P-gp) and multidrug resistance associated proteins (MRPs) play a key role in uni-directional efflux of substances. Both human and rabbit corneal epithelium were shown to significantly express P-gp42 and MRPs.43 Similarly, such "efflux pumps" have been identified in different ocular tissues such as retinal capillary endothelial cells,44 RPE cells, non-pigmented ciliary epithelium, 45 conjunctival epithelial cells,46 and iris and ciliary endothelial cells.46 Based on current knowledge about the functional expression of P-gp in ocular tissues, it is expected to offer modifications in drug delivery strategies which may increase ocular bioavailability and provide more efficient treatment for ocular disorders.
Endocytosis and Transcytosis
Specialized receptors exist within ocular barriers which control passage of xenobiotics. Endocytosis pathways via clathrin coated or caveolae (non/smooth coated) vesicles account for ocular receptor mediated transport. Expression of clathrin and the integral protein of the caveolae domain, caveolin-1, has been reported in ocular tissues.47–51 Using cultured human retinal pigment epithelial (ARPE-19) cells and in a mouse model, Mo et al52 showed involvement of caveolae mediated endocytosis pathways in uptake of albumin nanoparticles containing encapsulating Cu, Zn superoxide dismutase gene. However, Qaddoumi et al53 reported that endocytosis of poly(lactic co glycolic acid) (PLGA) nanoparticles in primary cultured rabbit conjunctival epithelial cells occurs mostly independently of clathrin- and caveolin-1-mediated pathways despite mRNA and protein expression of clathrin. In contrast, albumin transport in rabbit lens epithelial cells has revealed a transcellular transport mechanism employing both clathrin and caveolae mediated transcytotic pathways. More investigations are required to resolve the ambiguity of endocytic pathways for macromolecular delivery within the eye.
ANIMAL VERSUS IN VITRO CELL CULTURE MODELS
Considering the dilemmas of animal models for development of strategies to overcome ocular barriers for targeted ocular drug delivery, use of cell culture models seems crucial.3 Applications of animal experiments include pharmacokinetic, pharmacodynamic and toxicity studies. For example, the "Draize Test" is an acute toxicity test devised in 1944 by Food and Drug Administration (FDA) toxicologist John H Draize to assess the impact of 0.5 ml or 0.5 g of a test substance on an animal's eye or skin for 4 hours.54 Of different animals used for ocular drug delivery examinations, the rabbit model is most commonly used. Nevertheless, the rabbit eye shows distinct differences from the human eye including less frequent blinking which may significantly decrease precorneal drainage of topically applied solutions, and larger corneal and conjunctival surface area.55 As a result, ocular bioavailability of topically applied drugs in the rabbit is less influenced by non-productive absorption through the conjunctiva.3 Although animal experimentation is considered an essential part of ocular drugs advancement, they have been largely criticized from ethical and economical viewpoints. For example, model animals must be sacrificed at each time point in ocular pharmacokinetic studies and a large number of animals must be exploited for a single study. In the European Union, tightly restricted regulations (Council Directive 86/609/EEC) have been implemented for protection of animals in experimental/scientificuse in order to minimize such investigations.3
CELL BASED IN VITRO MODELS
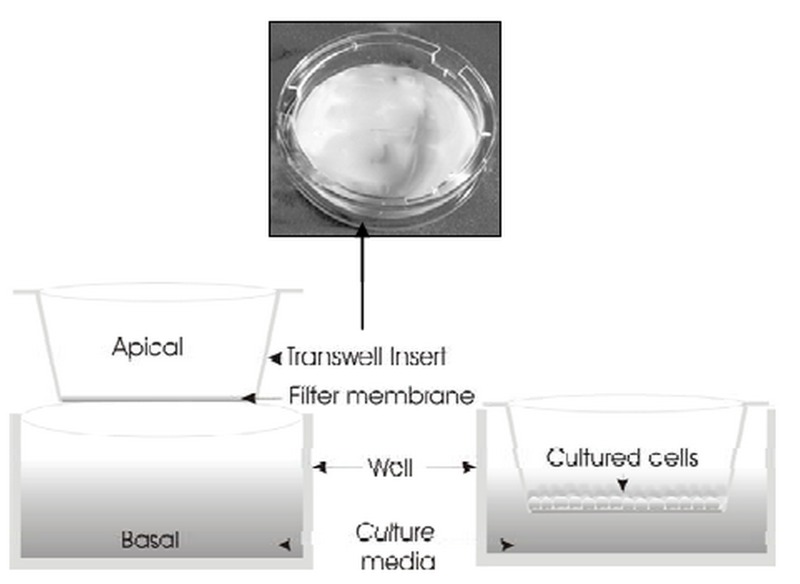
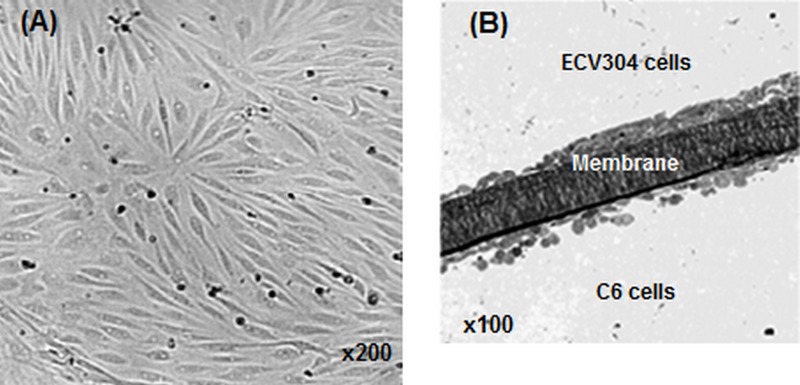
Restrictions associated with animal models have prompted researchers to pursue in vitro cell-based models including primary and immortalized cell culture models with particular emphasis on models serving blood-eye barriers. Fig. 4 depicts a schematic presentation of an in vitro cell culture model. The main challenge associated with in vitro cultivation of any cell type serving an epithelial or endothelial barrier model appears to be establishment of proper growth conditions, upon which cultured cells can form a monolayer/multilayer of cells displaying tight junctions which confer restrictive barrier functions.36 In fact, the closer the culture conditions are to the natural environment, the more closely the resulting cells will mimic in vivo tissue characteristics.3 Implementation of permeable support systems such as the "Transwell® insert" has proven to be a valuable tool for assessment of bioelectrical and/or permeability properties of cultured cells (Fig. 4). These membrane based systems allow cells to grow in a polarized fashion. Furthermore, it is possible to grow cells in coculture systems, which allow determination of the effect of other cell types (e.g., astrocytes or pericytes) on endothelial cells (Fig. 5).23,25,56 Light microscopic images of brain capillary endothelial cells and side-by-side coculture of ECV304 and C6 cells are shown in Fig. 5.
Figure 4.
Schematic representation of the in vitro cell culture model. Transwell® insert filters are widely used for assessment of bioelectrical properties of target cells and screening for drug permeability and targeting.
Figure 5.
Light microscopic images of brain capillary endothelial cells. A) Porcine brain capillary endothelial cells. B) Side-by-side coculture of ECV304 and C6 cells on top and underneath the Transwell® insert filter membrane.
Handling cultured cells on filter membranes(polycarbonate or polyester) is easy; furthermore cellular functions (e.g., permeability and carrier/receptor mediated transport) can be simply investigated since independent access is possible to apical and basolateral aspects of cells grown on such membranes. Using such systems, we have studied the influence of astrocytes on endothelial cells b.End3.24 To mimic the extracellular matrix, cell culture filters can be coated with collagen (type I), laminin, fibronectin or their mixture. Such coating has been reported to facilitate cell attachment, proliferation and differentiation. Cultivation media in cell culture models usually contain fetal bovine serum (FBS) in concentrations of 2–20% and various growth supplements such as epidermal growth factor (EGF) and hydrocortisone. Varying concentrations of FBS may influence cell proliferation and differentiation; however, cells grown in serum free media (SFM) have been shown to exhibit better barrier functions.57–61. Table 1 lists selected in vitro cellbased models for ocular barriers.
Cell Culture Models for Corneal Epithelium and Endothelium
The corneal epithelium represents the ratelimiting barrier for transcorneal permeation. As shown in Fig. 2, the uppermost epithelial cell layers provide over 60% of total corneal resistance since they contain tight junctions. Various corneal epithelial cell culture models (both isolated primary and immortalized cells) have been widely used for toxicity testing, and transcorneal permeation investigations (Table 1). For example, isolated bovine primary corneal epithelial cells cultured onto fibronectin/collagen/laminin coated membrane with serum-free medium (SFM) resulted in TEER value of 5000 Ω.cm2.62 While, TEER values of isolated rabbit corneas (epithelium–troma–ndothelium) were determined to be 3200–500 Ω.cm2. Of immortalized corneal epithelial cell lines established from rabbit, rat, hamster and humans, immortalized human cell lines (e.g.,human corneal epithelial [HCE], HCE-T and tet human papilloma virus [HPV] 16-E6/E7 transduced HCE cell lines) with TEER values of 400-500 Ω.cm2 are widely used in cell culture models.3 Of these models, simian virus (SV) 40 immortalized human corneal epithelial cells (HCE-T) have been widely used for in vitro models of human corneal epithelial cells. Recently, this cell line was assessed for genomic aberrations and cellular heterogeneity using array-based comparative genomic hybridization (CGH) analysis. Using the array CGH analysis, the genomic content of HCE-T cells appeared to be different from normal healthy genome; furthermore results of cellular functional assays such as real-time polymerase chain reaction (RT-PCR) and chromosomal fluorescent in situ hybridization (cFISH) strongly indicated that HCE-T cells consisted of a significant number of heterogeneous cell populations. 63 This clearly indicates that HCE-T cells have altered genomic content, are composed of heterogeneous cell populations and thus cannot represent normal human corneal epithelium.
To establish an immortalized human corneal endothelial cell line (IHCEn) by transducing HPV 16 E6/E7 oncogenes, primary human corneal endothelial cells (PHCEn) were infected with HPV using a retroviral vector; transformed cells were clonally selected and cultivated on lyophilized human amniotic membrane (LAM). Growth properties and characteristics of IHCEn were then compared with that of PHCEn. Immunohistochemical staining and immunofluorescence examinations revealed that IHCEn can be considered a successful in vitro model of human corneal endothelial cells.64
Cell Culture Models for Conjunctival Epithelium
Primary cell culture models of conjunctival epithelium are usually obtained from rabbits and cows (Table 2), which were shown to provide TEER values of 1000-2000 and 5000 Ω.cm2 respectively, while TEER of excised rabbit conjunctiva was 1300 Ω.cm2.3 To characterize a new nontransfected spontaneously immortalized epithelial cell line from normal human conjunctiva (IOBA-NHC), outgrowing cells from explanted conjunctival tissue were successively passaged and preliminarily characterized at passage 3 to assess epithelial origin. These cells were then further characterized (i.e., at passages 15 to 20, 40, 60, and 100) by analyzing various properties (e.g., viability, plating efficiency, colony forming efficiency and colony size, and Ki-67 protein expression), epithelial marker expression (e.g., cytokeratins, desmoplakins, EGF receptor), and expression of conjunctival differentiation markers (i.e., mucin gene expression). IOBA-NHC cells demonstrated high proliferative ability in vitro and typical epithelial morphology. Furthermore, cytokeratins, mannose, and sialic acid residues were immunologically detected; it therefore appears that this cell line can be a useful experimental tool in the field of ocular surface cell biology.68
Recently, in order to develop a conjunctival epithelial cell line for investigation of antigen translocation across a mucosal barrier, conjunctival epithelial cells from Fischer 344 rats were immortalized with pSV3(neo) resulting in two cell lines, i.e., CJ4.1A and CJ4.3C. The cell lines were in culture for over 60 passages with population doubling time of ∼22 hours and TEER value of 600-800 Ω.cm2 after attaining total confluency (i.e., 3-4 days) and expressing tight junction molecules. Morphological and functional characterization indicated that these cell lines may serve as a useful experimental conjunctival epithelial cell line to assess strategies for enhancing transepithelial antigen uptake.69
Cell Culture Models for Blood-Retinal Barriers
Numerous cell culture models have been set up as models for the inner and outer BRB (Table 3). However, establishment of an appropriate in vitro model for such barriers remains a challenge.
Retinal Pigmented Epithelial Barrier
Many primary cell culture models of the RPE (obtained from frog, rat, chick, cow and human RPE cells) have been used for various purposes of which, two primary cells (i.e., human and bovine) are most commonly used (Table 3).
Of immortalized cell lines, the rat RPE-J cell line was produced by infection of rat RPE cells with a temperature sensitive SV40 virus followed by isolation of epithelial clones.73 This cell line displayed a highly differentiated phenotype in culture, but the polarity of Na+,K+-ATPase and the neural cell adhesion molecule (N-CAM) differed from the in vivo localization. Nabi et al73 showed that, under defined growth conditions, RPE-J cells were able to form a tight cell monolayer with a TEER value of 350-400 Ω.cm2, which also displayed circumferential expression of the tight-junction protein, ZO-1. The RPE-J cells were demonstrated to be of RPE origin due to expression of the rat RPE marker, RET-PE2. This cell line has been used in many investigations.74–77 Mora et al75 examined the expression and distribution of caveolae and caveolins in the RPE, which play key roles in retinal support, visual cycle, and act as the main barrier between blood and retina. They proved the presence of caveolae (caveolin-1 and -2) in both apical and basolateral domains of the plasma membrane of all RPE-J cells. Since all other epithelial cells (e.g., liver, kidney, thyroid, and intestinal) assemble caveolae only at the basolateral side, the bipolar distribution of caveolae in the RPE is striking and may reflect specialized roles in signaling and trafficking which are important for visual function.
Davis et al78 cloned a spontaneously arising cell line (i.e., D407) from a primary culture of human RPE. These cells were shown to possess most metabolic and morphologic characteristics of RPE cells in vivo (e.g., epithelial cobblestonemorphology, expression of typical keratins, and synthesis of retina specific CRALBP protein), but the model lacked certain enzymatic activities. This cell line has been exploited in various studies.79–82 For instance, Mannermaa et al79 investigated the expression of efflux proteins in two secondary (ARPE-19, D407) and two primary (HRPEpiC and bovine) RPE cell lines and showed that similar protein efflux profiles were shared between ARPE-19 and primary RPE cells, whereas the D407 cell line was notably different. MRP1, MRP4 and MRP5 were identified in all human RPE cell lines but MRP6 was not expressed in any cell line. D407 cells expressed MRP2 and BCRP, which were absent in other cell lines. This clearly implies that such differences in expression of efflux transporters should be taken into consideration when these cell lines are used for particular drug delivery studies.
Another human RPE cell line (i.e., ARPE-19) was established and characterized by Dunn et al85 in 1996. The cell line was characterized by its morphology, the expression of retina specific markers (CRALP and RPE65), and its barrier properties. Despite the presence of tight-junction complexes, these cells displayed poor TEER values of ∼100 Ω.m2. Various attempts have been made to alter the culture conditions of ARPE-19 to more accurately reproduce in situ RPE phenotype, such as cocultivation with immortalized astrocytes or with C6 glioma cells.89 The ARPE-19 cell line failed to respond to co-culture or conditioned medium, perhaps due to its heterogeneous nature.
Retinal Capillary Endothelial Barrier
Many pathologic conditions of the eye (e.g., diabetic retinopathy) are believed to be associated with breakdown of the inner blood-etinal barrier. Therefore, development of novel treatment strategies demands cell based in vitro models to investigate transport machineries of this ocular barrier. This can be helpful for identification of factors associated with such diseases. The second passage of primary isolated bovine retinal capillary endothelial cells (BRCECs) cultured on polycarbonate filters (coated with gelatin, laminin, fibronectin, and collagen) resulted in TEER values of ∼150Ω.cm2.86 Recently, a conditionally immortalized rat retinal capillary endothelial cell line (TRiBRB) was developed from a transgenic rat harboring the temperature sensitive SV40 T antigen gene.87 When grown on permeable membrane, TR-iBRB cells expressed functional P-gp and GLUT1, however they showed very low TEER values (30 Ω.cm2).90 TR-iBRB cells possess endothelial markers, such as von Willebrand factor (vWF) and a scavenger receptor for uptake of acetylated low densitylipoprotein (Ac-LDL). They also express vascular endothelial growth factor (VEGF) receptor-2 (KDR/Flk-1), which may play a critical role in binding to VEGF and possibly in development of neovascularization in diabetic retinopathy. These cells are also used for identifying transporters and studying their regulation under pathological conditions.88 Although, at present, transport machineries of the inner BRB (e.g., the amino acid transporters) remain largely unknown, preliminary examinations have resulted in expression of some amino acid transporters such as system L "LAT-1" that is also expressed in the bloodbrain barrier.24 Retinal endothelial cells are surrounded by retinal pericytes and Müller cells. Although retinal microvascular biology/function may be due to possible intercommunications between endothelial cells and the two other types of cells, signal transduction mechanisms for tight junction regulation of endothelial cells demands further investigation in particular for disorders such as diabetic retinopathy.
SUMMARY
Development of in vitro cell culture models for studying ocular barriers undoubtedly provides a platform to investigate the impact of nutrient/pharmaceutical trafficking on ophthalmic diseases. This can also provide grounds for detection of cell surface biomarkers for drug delivery and targeting, resulting in novel therapeutic modalities when safe delivery of agents to the posterior segment is necessary. Inner BRB transport machineries, in particular endocytosis and transcytosis, can help advance the use of systemically administered medicines for ophthalmic diseases. Current knowledge of inner BRB transport appears to be very limited as compared to that of the BBB. The scientific community has begun to change the face of this field by establishing inner BRB cell lines which allow identification of transporters and their regulation under physiological and pathological conditions. Due to the tedious task of isolating of different ocular tissues, immortalized cell lines appear to offer obvious advantages over primary cell lines. Setting up immortalized cells is an easy task and the characteristics of these cultures are deemed to remain stable over a large number of passages. However, as compared to primary cells, immortalized cell lines may show abnormal characteristics in gene expression or biological functions. 79 In contrast, isolated primary cultures are likely to reflect in vivo cell morphology and function more accurately, but nevertheless these cells stop growing after a few passages in culture. In the case of human primary cells, due to limited availability of human donor eyes, it would be very difficult to obtain several isolates. All these issues have prompted researchers to exploit immortalized cell lines. But it should be stressed that no perfect cell lines are yet available to serve both drug delivery/targeting investigations on one hand, and biologic studies of ocular membranes and barriers on the other. This domain of science has promoted investigations to establish new cell lines; the next step for achieving the desired goals appears to be utilization of stem cell approaches91 and omics based technologies (e.g., genomics, proteomics, metabolomics).
REFERENCES
- 1.Barar J, Javadzadeh AR, Omidi Y. Ocular novel drug delivery: impacts of membranes and barriers. Expert Opin Drug Deliv. 2008;5:567–581. doi: 10.1517/17425247.5.5.567. [DOI] [PubMed] [Google Scholar]
- 2.Urtti A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv Drug Deliv Rev. 2006;58:1131–1135. doi: 10.1016/j.addr.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 3.Hornof M, Toropainen E, Urtti A. Cell culture models of the ocular barriers. Eur J Pharm Biopharm. 2005;60:207–225. doi: 10.1016/j.ejpb.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Dartt DA, Hodges RR, Zoukhri D. Tear and Their Secretion. In: Fischbarg J, editor. The biology of the eye. New York: Academic Press; 2006. pp. 21–82. [Google Scholar]
- 5.Fullard RJ, Tucker D. Tear protein composition and the effects of stimulus. Adv Exp Med Biol. 1994;350:309–314. doi: 10.1007/978-1-4615-2417-5_52. [DOI] [PubMed] [Google Scholar]
- 6.Macha S, Mitra AK. Overview of ocular drug delivery. In: Mitra AK, editor. Ophthalmic drug delivery systems. 2nd ed. New York: Marcel Dekker; 2003. pp. 1–12. [Google Scholar]
- 7.Mitra AK, Anand BS, Duvvuri S. Drug delivery to the eye. In: Fischbarg J, editor. The biology of the eye. New York: Academic Press; 2006. pp. 307–351. [Google Scholar]
- 8.Ahmed I. The noncorneal route in ocular drug delivery. In: Mitra AK, editor. Ophthalmic drug delivery systems. 2nd ed. New York: Marcel Dekker; 2003. pp. 335–363. [Google Scholar]
- 9.Huang HS, Schoenwald RD, Lach JL. Corneal penetration behavior of beta-blocking agents II:Assessment of barrier contributions. J Pharm Sci. 1983;72:1272–1279. doi: 10.1002/jps.2600721109. [DOI] [PubMed] [Google Scholar]
- 10.Sunkara G, Kompella UB. Membrane transport processes in the eye. In: Mitra AK, editor. Ophthalmic drug delivery systems. 2nd ed. New York: Marcel Dekker; 2003. pp. 13–58. [Google Scholar]
- 11.Schoenwald RD, Huang HS. Corneal penetration behavior of beta-blocking agents I: physiochemical factors. J Pharm Sci. 1983;72:1266–1272. doi: 10.1002/jps.2600721108. [DOI] [PubMed] [Google Scholar]
- 12.Huang AJ, Tseng SC, Kenyon KR. Paracellular permeability of corneal and conjunctival epithelia. Invest Ophthalmol Vis Sci. 1989;30:684–689. [PubMed] [Google Scholar]
- 13.Hamalainen KM, Kananen K, Auriola S, Kontturi K, Urtti A. Characterization of paracellular and aqueous penetration routes in cornea, conjunctiva, and sclera. Invest Ophthalmol Vis Sci. 1997;38:627–634. [PubMed] [Google Scholar]
- 14.Janoria KG, Gunda S, Boddu SH, Mitra AK. Novel approaches to retinal drug delivery. Expert Opin Drug Deliv. 2007;4:371–388. doi: 10.1517/17425247.4.4.371. [DOI] [PubMed] [Google Scholar]
- 15.Duvvuri S, Majumdar S, Mitra AK. Drug delivery to the retina: challenges and opportunities. Expert Opin Biol Ther. 2003;3:45–56. doi: 10.1517/14712598.3.1.45. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed I, Patton TF. Importance of the noncorneal absorption route in topical ophthalmic drug delivery. Invest Ophthalmol Vis Sci. 1985;26:584–587. [PubMed] [Google Scholar]
- 17.Freddo TF. Shifting the paradigm of the bloodaqueous barrier. Exp Eye Res. 2001;73:581–592. doi: 10.1006/exer.2001.1056. [DOI] [PubMed] [Google Scholar]
- 18.Cunha-Vaz JG. The blood-ocular barriers: past, present, and future. Doc Ophthalmol. 1997;93:149–157. doi: 10.1007/BF02569055. [DOI] [PubMed] [Google Scholar]
- 19.Lin H, Kenyon E, Miller SS. Na-dependent pHi regulatory mechanisms in native human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1992;33:3528–3538. [PubMed] [Google Scholar]
- 20.Quinn RH, Miller SS. Ion transport mechanisms in native human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1992;33:3513–3527. [PubMed] [Google Scholar]
- 21.Gardner TW, Antonetti DA, Barber AJ, Lieth E, Tarbell JA. (Penn State Retina Research Group). The molecular structure and function of the inner blood-retinal barrier. Doc Ophthalmol. 1999;97:229–237. doi: 10.1023/a:1002140812979. [DOI] [PubMed] [Google Scholar]
- 22.Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987;325:253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- 23.Barar J, Omidi Y. Bioelectrical and permeability properties of brain microvasculature endothelial cells: Effects of tight junction modulators. J Biol Sci. 2008;8:556–562. [Google Scholar]
- 24.Omidi Y, Barar J, Ahmadian S, Heidari HR, Gumbleton M. Characterization and astrocytic modulation of system L transporters in brain microvasculature endothelial cells. Cell Biochem Funct. 2008;26:381–391. doi: 10.1002/cbf.1455. [DOI] [PubMed] [Google Scholar]
- 25.Omidi Y, Campbell L, Barar J, Connell D, Akhtar S, Gumbleton M. Evaluation of the immortalised mouse brain capillary endothelial cell line, b.End3, as an in vitro blood-brain barrier model for drug uptake and transport studies. Brain Res. 2003;990:95–112. doi: 10.1016/s0006-8993(03)03443-7. [DOI] [PubMed] [Google Scholar]
- 26.Smith M, Omidi Y, Gumbleton M. Primary porcine brain microvascular endothelial cells: biochemical and functional characterisation as a model for drug transport and targeting. J Drug Target. 2007;15:253–268. doi: 10.1080/10611860701288539. [DOI] [PubMed] [Google Scholar]
- 27.Stewart PA, Tuor UI. Blood-eye barriers in the rat: correlation of ultrastructure with function. J Comp Neurol. 1994;340:566–576. doi: 10.1002/cne.903400409. [DOI] [PubMed] [Google Scholar]
- 28.Selvin BL. Systemic effects of topical ophthalmic medications. South Med J. 1983;76:349–358. doi: 10.1097/00007611-198303000-00020. [DOI] [PubMed] [Google Scholar]
- 29.Antonetti DA, Gardner TW, Barber AJ. Blood-retinal barrier. In: Jaffe GJ, Ashton P, Pearson PA, editors. Intraocular drug delivery. New York: Taylor & Francis; 2006. pp. 27–39. [Google Scholar]
- 30.Steuer H, Jaworski A, Stoll D, Schlosshauer B. In vitro model of the outer blood-retina barrier. Brain Res Protoc. 2004;13:26–36. doi: 10.1016/j.brainresprot.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Lund-Andersen H, Sebag J, Sander B, La Cour M. The Vitreous. In: Fischbarg J, editor. The biology of the eye. New York: Academic Press; 2006. pp. 181–194. [Google Scholar]
- 32.Ghate D, Brooks W, McCarey BE, Edelhauser HF. Pharmacokinetics of intraocular drug delivery by periocular injections using ocular fluorophotometry. Invest Ophthalmol Vis Sci. 2007;48:2230–2237. doi: 10.1167/iovs.06-0954. [DOI] [PubMed] [Google Scholar]
- 33.Ashton P. Retinal drug delivery. In: Jaffe GJ, Ashton P, Pearson PA, editors. Intraocular drug delivery. New York: Taylor & Francis; 2006. pp. 1–25. [Google Scholar]
- 34.Guidetti B, Azema J, Malet-Martino M, Martino R. Delivery systems for the treatment of proliferative vitreoretinopathy: materials, devices and colloidal carriers. Curr Drug Deliv. 2008;5:7–19. doi: 10.2174/156720108783331050. [DOI] [PubMed] [Google Scholar]
- 35.Yasukawa T, Ogura Y, Tabata Y, Kimura H, Wiedemann P, Honda Y. Drug delivery systems for vitreoretinal diseases. Prog Retin Eye Res. 2004;23:253–281. doi: 10.1016/j.preteyeres.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Omidi Y, Gumbleton M. Biological membranes and barriers. In: Mahato RI, editor. Biomaterials for delivery and targeting of proteins and nucleic acids. New York: CRC Press; 2005. pp. 232–274. [Google Scholar]
- 37.Jentsch TJ, Matthes H, Keller SK, Wiederholt M. Anion dependence of electrical effects of bicarbonate and sodium on cultured bovine corneal endothelial cells. Pflugers Arch. 1985;403:175–185. doi: 10.1007/BF00584097. [DOI] [PubMed] [Google Scholar]
- 38.Jentsch TJ, Keller SK, Wiederholt M. Ion transport mechanisms in cultured bovine corneal endothelial cells. Curr Eye Res. 1985;4:361–369. doi: 10.3109/02713688509025149. [DOI] [PubMed] [Google Scholar]
- 39.Gao J, Sun X, Yatsula V, Wymore RS, Mathias RT. Isoform-specific function and distribution of Na/K pumps in the frog lens epithelium. J Membr Biol. 2000;178:89–101. doi: 10.1007/s002320010017. [DOI] [PubMed] [Google Scholar]
- 40.Sun XC, Bonanno JA, Jelamskii S, Xie Q. Expression and localization of Na(+)-HCO(3)(−) cotransporter in bovine corneal endothelium. Am J Physiol Cell Physiol. 2000;279:C1648–C1655. doi: 10.1152/ajpcell.2000.279.5.C1648. [DOI] [PubMed] [Google Scholar]
- 41.Mannermaa E, Vellonen KS, Urtti A. Drugtransport in corneal epithelium and blood-retina barrier: emerging role of transporters in ocular pharmacokinetics. Adv Drug Deliv Rev. 2006;58:1136–1163. doi: 10.1016/j.addr.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 42.Dey S, Patel J, Anand BS, Jain-Vakkalagadda B, Kaliki P, Pal D, et al. Molecular evidence and functional expression of P-glycoprotein (MDR1) in human and rabbit cornea and corneal epithelial cell lines. Invest Ophthalmol Vis Sci. 2003;44:2909–2918. doi: 10.1167/iovs.02-1142. [DOI] [PubMed] [Google Scholar]
- 43.Karla PK, Pal D, Quinn T, Mitra AK. Molecular evidence and functional expression of a novel drug efflux pump (ABCC2) in human corneal epithelium and rabbit cornea and its role in ocular drug efflux. Int J Pharm. 2007;336:12–21. doi: 10.1016/j.ijpharm.2006.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holash JA, Stewart PA. The relationship of astrocyte-like cells to the vessels that contribute to the blood-ocular barriers. Brain Res. 1993;629:218–224. doi: 10.1016/0006-8993(93)91323-k. [DOI] [PubMed] [Google Scholar]
- 45.Wu J, Zhang JJ, Koppel H, Jacob TJ. P-glycoprotein regulates a volume-activated chloride current in bovine non-pigmented ciliary epithelial cells. J Physiol. 1996;491(Pt 3):743–755. doi: 10.1113/jphysiol.1996.sp021254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saha P, Yang JJ, Lee VH. Existence of a pglycoprotein drug efflux pump in cultured rabbit conjunctival epithelial cells. Invest Ophthalmol Vis Sci. 1998;39:1221–1226. [PubMed] [Google Scholar]
- 47.Lo WK, Zhou CJ, Reddan J. Identification of caveolae and their signature proteins caveolin 1 and 2 in the lens. Exp Eye Res. 2004;79:487–498. doi: 10.1016/j.exer.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 48.Bridges CC, El-Sherbeny A, Roon P, Ola MS, Kekuda R, Ganapathy V, et al. A comparison of caveolae and caveolin-1 to folate receptor alpha in retina and retinal pigment epithelium. Histochem J. 2001;33:149–158. doi: 10.1023/a:1017991925821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lo WK, Mills A, Zhang W, Zhu H. Polarized distribution of coated pits and coated vesicles in the rat lens: an electron microscopy and WGA-HRP tracer study. Curr Eye Res. 1991;10:1151–1163. doi: 10.3109/02713689109024133. [DOI] [PubMed] [Google Scholar]
- 50.Hunt RC, Dewey A, Davis AA. Transferrin receptors on the surfaces of retinal pigment epithelial cells are associated with the cytoskeleton. J Cell Sci. 1989;92(Pt 4):655–666. doi: 10.1242/jcs.92.4.655. [DOI] [PubMed] [Google Scholar]
- 51.Sabah JR, Schultz BD, Brown ZW, Nguyen AT, Reddan J, Takemoto LJ. Transcytotic passage of albumin through lens epithelial cells. Invest Ophthalmol Vis Sci. 2007;48:1237–1244. doi: 10.1167/iovs.06-0620. [DOI] [PubMed] [Google Scholar]
- 52.Mo Y, Barnett ME, Takemoto D, Davidson H, Kompella UB. Human serum albumin nanoparticles for efficient delivery of Cu, Zn superoxide dismutase gene. Mol Vis. 2007;13:746–757. [PMC free article] [PubMed] [Google Scholar]
- 53.Qaddoumi MG, Gukasyan HJ, Davda J, Labhasetwar V, Kim KJ, Lee VH. Clathrin and caveolin-1 expression in primary pigmented rabbit conjunctival epithelial cells: role in PLGA nanoparticle endocytosis. Mol Vis. 2003;9:559–568. [PubMed] [Google Scholar]
- 54.Parascandola J. The development of the Draize test for eye toxicity. Pharm Hist. 1991;33:111–117. [PubMed] [Google Scholar]
- 55.Urtti A, Salminen L. Animal pharmacokinetic studies. In: Mitra AK, editor. Ophthalmic drug delivery systems. New York: Marcel Dekker; 1998. pp. 121–136. [Google Scholar]
- 56.Barar J, Gumbleton M, Asadi M, Omidi Y. Barrier functionality and transport machineries of human ECV304 cells. Med Sci Monit. 2009;16 in press. [PubMed] [Google Scholar]
- 57.Toropainen E, Ranta VP, Talvitie A, Suhonen P, Urtti A. Culture model of human corneal epithelium for prediction of ocular drug absorption. Invest Ophthalmol Vis Sci. 2001;42:2942–2948. [PubMed] [Google Scholar]
- 58.Hakvoort A, Haselbach M, Wegener J, Hoheisel D, Galla HJ. The polarity of choroid plexus epithelial cells in vitro is improved in serum-free medium. J Neurochem. 1998;71:1141–1150. doi: 10.1046/j.1471-4159.1998.71031141.x. [DOI] [PubMed] [Google Scholar]
- 59.Hoheisel D, Nitz T, Franke H, Wegener J, Hakvoort A, Tilling T, et al. Hydrocortisone reinforces the blood-brain barrier properties in a serum free cell culture system. Biochem Biophys Res Commun. 1998;244:312–316. doi: 10.1006/bbrc.1997.8051. [DOI] [PubMed] [Google Scholar]
- 60.Ainscough SL, Barnard Z, Upton Z, Harkin DG. Vitronectin supports migratory responses of corneal epithelial cells to substrate bound IGF-I and HGF, and facilitates serum-free cultivation. Exp Eye Res. 2006;83:1505–1514. doi: 10.1016/j.exer.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 61.Ang LP, Cheng ZY, Beuerman RW, Teoh SH, Zhu X, Tan DT. The development of a serum-free derived bioengineered conjunctival epithelial equivalent using an ultrathin poly(epsiloncaprolactone) membrane substrate. Invest Ophthalmol Vis Sci. 2006;47:105–112. doi: 10.1167/iovs.05-0512. [DOI] [PubMed] [Google Scholar]
- 62.Chang JE, Basu SK, Lee VH. Air-interface condition promotes the formation of tight corneal epithelial cell layers for drug transport studies. Pharm Res. 2000;17:670–676. doi: 10.1023/a:1007569929765. [DOI] [PubMed] [Google Scholar]
- 63.Yamasaki K, Kawasaki S, Young RD, Fukuoka H, Tanioka H, Nakatsukasa M, et al. Genomic aberrations and cellular heterogeneity in SV40- immortalized human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2009;50:604–613. doi: 10.1167/iovs.08-2239. [DOI] [PubMed] [Google Scholar]
- 64.Kim HJ, Ryu YH, Ahn JI, Park JK, Kim JC. Characterization of immortalized human corneal endothelial cell line using HPV 16 E6/E7 on lyophilized human amniotic membrane. Korean J Ophthalmol. 2006;20:47–54. doi: 10.3341/kjo.2006.20.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kruszewski FH, Walker TL, DiPasquale LC. Evaluation of a human corneal epithelial cell line as an in vitro model for assessing ocular irritation. Fundam Appl Toxicol. 1997;36:130–140. [PubMed] [Google Scholar]
- 66.Reichl S. Cell culture models of the human cornea - a comparative evaluation of their usefulness to determine ocular drug absorption in-vitro. J Pharm Pharmacol. 2008;60:299–307. doi: 10.1211/jpp.60.3.0004. [DOI] [PubMed] [Google Scholar]
- 67.Becker U, Ehrhardt C, Schneider M, Muys L, Gross D, Eschmann K, et al. A comparative evaluation of corneal epithelial cell cultures for assessing ocular permeability. Altern Lab Anim. 2008;36:33–44. doi: 10.1177/026119290803600106. [DOI] [PubMed] [Google Scholar]
- 68.Diebold Y, Calonge M, Enriquez de Salamanca A, Callejo S, Corrales RM, Saez V, et al. Characterization of a spontaneously immortalized cell line (IOBA-NHC) from normal human conjunctiva. Invest Ophthalmol Vis Sci. 2003;44:4263–4274. doi: 10.1167/iovs.03-0560. [DOI] [PubMed] [Google Scholar]
- 69.O’Sullivan NL, Baylor AE 3rd, Montgomery PC. Development of immortalized rat conjunctival epithelial cell lines: an in vitro model to examine transepithelial antigen delivery. Exp Eye Res. 2007;84:323–331. doi: 10.1016/j.exer.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saha P, Kim KJ, Lee VH. A primary culture model of rabbit conjunctival epithelial cells exhibiting tight barrier properties. Curr Eye Res. 1996;15:1163–1169. doi: 10.3109/02713689608995151. [DOI] [PubMed] [Google Scholar]
- 71.Civiale C, Paladino G, Marino C, Trombetta F, Pulvirenti T, Enea V. Multilayer primary epithelial cell culture from bovine conjunctiva as a model for in vitro toxicity tests. Ophthalmic Res. 2003;35:126–136. doi: 10.1159/000070047. [DOI] [PubMed] [Google Scholar]
- 72.Paladino G, Marino C, La Terra Mule S, Civiale C, Rusciano D, Enea V. Cytokeratin expression in primary epithelial cell culture from bovine conjunctiva. Tissue Cell. 2004;36:323–332. doi: 10.1016/j.tice.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 73.Nabi IR, Mathews AP, Cohen-Gould L, Gundersen D, Rodriguez-Boulan E. Immortalization of polarized rat retinal pigment epithelium. J Cell Sci. 1993;104(Pt 1):37–49. doi: 10.1242/jcs.104.1.37. [DOI] [PubMed] [Google Scholar]
- 74.Cashman SM, Sadowski SL, Morris DJ, Frederick J, Kumar-Singh R. Intercellular trafficking of adenovirus-delivered HSV VP22 from the retinal pigment epithelium to the photoreceptors-- implications for gene therapy. Mol Ther. 2002;6:813–823. doi: 10.1006/mthe.2002.0806. [DOI] [PubMed] [Google Scholar]
- 75.Mora RC, Bonilha VL, Shin BC, Hu J, Cohen-Gould L, Bok D, et al. Bipolar assembly of caveolae in retinal pigment epithelium. Am J Physiol Cell Physiol. 2006;290:C832–C843. doi: 10.1152/ajpcell.00405.2005. [DOI] [PubMed] [Google Scholar]
- 76.Rosenthal R, Bakall B, Kinnick T, Peachey N, Wimmers S, Wadelius C, et al. Expression of bestrophin-1, the product of the VMD2 gene, modulates voltage-dependent Ca2+ channels in retinal pigment epithelial cells. FASEB J. 2006;20:178–180. doi: 10.1096/fj.05-4495fje. [DOI] [PubMed] [Google Scholar]
- 77.West KA, Yan L, Miyagi M, Crabb JS, Marmorstein AD, Marmorstein L, et al. Proteome survey of proliferating and differentiating rat RPE-J cells. Exp Eye Res. 2001;73:479–491. doi: 10.1006/exer.2001.1058. [DOI] [PubMed] [Google Scholar]
- 78.Davis AA, Bernstein PS, Bok D, Turner J, Nachtigal M, Hunt RC. A human retinal pigment epithelial cell line that retains epithelial characteristics after prolonged culture. Invest Ophthalmol Vis Sci. 1995;36:955–964. [PubMed] [Google Scholar]
- 79.Mannermaa E, Vellonen KS, Ryhanen T, Kokkonen K, Ranta VP, Kaarniranta K, et al. Efflux protein expression in human retinal pigment epithelium cell lines. Pharm Res. 2009;26:1785–1791. doi: 10.1007/s11095-009-9890-6. [DOI] [PubMed] [Google Scholar]
- 80.Geisen P, McColm JR, King BM, Hartnett ME. Characterization of barrier properties and inducible VEGF expression of several types of retinal pigment epithelium in medium-term culture. Curr Eye Res. 2006;31:739–748. doi: 10.1080/02713680600837408. [DOI] [PubMed] [Google Scholar]
- 81.Tuovinen L, Ruhanen E, Kinnarinen T, Ronkko S, Pelkonen J, Urtti A, et al. Starch acetate microparticles for drug delivery into retinal pigment epithelium-in vitro study. J Control Release. 2004;98:407–413. doi: 10.1016/j.jconrel.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 82.Pitkanen L, Ruponen M, Nieminen J, Urtti A. Vitreous is a barrier in nonviral gene transfer by cationic lipids and polymers. Pharm Res. 2003;20:576–583. doi: 10.1023/a:1023238530504. [DOI] [PubMed] [Google Scholar]
- 83.Hartnett ME, Lappas A, Darland D, McColm JR, Lovejoy S, D’Amore PA. Retinal pigment epithelium and endothelial cell interaction causes retinal pigment epithelial barrier dysfunction via a soluble VEGF-dependent mechanism. Exp Eye Res. 2003;77:593–599. doi: 10.1016/s0014-4835(03)00189-1. [DOI] [PubMed] [Google Scholar]
- 84.Chang CW, Ye L, Defoe DM, Caldwell RB. Serum inhibits tight junction formation in cultured pigment epithelial cells. Invest Ophthalmol Vis Sci. 1997;38:1082–1093. [PubMed] [Google Scholar]
- 85.Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res. 1996;62:155–169. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- 86.Gillies MC, Su T, Naidoo D. Electrical resistance and macromolecular permeability of retinal capillary endothelial cells in vitro. Curr Eye Res. 1995;14:435–442. doi: 10.3109/02713689509003753. [DOI] [PubMed] [Google Scholar]
- 87.Hosoya K, Tomi M, Ohtsuki S, Takanaga H, Ueda M, Yanai N, et al. Conditionally immortalized retinal capillary endothelial cell lines (TR-iBRB) expressing differentiated endothelial cell functions derived from a transgenic rat. Exp Eye Res. 2001;72:163–172. doi: 10.1006/exer.2000.0941. [DOI] [PubMed] [Google Scholar]
- 88.Hosoya K, Tomi M. Advances in the cell biology of transport via the inner blood-retinal barrier: establishment of cell lines and transport functions. Biol Pharm Bull. 2005;28:1–8. doi: 10.1248/bpb.28.1. [DOI] [PubMed] [Google Scholar]
- 89.Constable PA, Lawrenson JG. Glial cell factors and the outer blood retinal barrier. Ophthalmic Physiol Opt. 2009;29:557–564. doi: 10.1111/j.1475-1313.2009.00671.x. [DOI] [PubMed] [Google Scholar]
- 90.Shen J, Cross ST, Tang-Liu DD, Welty DF. Evaluation of an immortalized retinal endothelial cell line as an in vitro model for drug transport studies across the blood-retinal barrier. Pharm Res. 2003;20:1357–1363. doi: 10.1023/a:1025789606885. [DOI] [PubMed] [Google Scholar]
- 91.Akrami H, Soheili ZS, Khalooghi K, Ahmadieh H, Rezaie-Kanavi M, Samiei S, et al. Retinal Pigment Epithelium culture; a potential source of retinal stem cells. J Ophthalmic Vis Res. 2009;4:134–141. [PMC free article] [PubMed] [Google Scholar]