Abstract
Cyclic neutropenia occurs in humans and gray collie dogs, is characterized by recurrent neutropenia, and is treated by repeated injections of recombinant granulocyte colony-stimulating factor (rG-CSF). As dose escalation of lentivirus may be clinically necessary, we monitored the outcome of four sequential intramuscular injections of G-CSF-lentivirus (3×107 IU/kg body weight) to a normal dog and a gray collie. In the normal dog absolute neutrophil counts were significantly increased after each dose of virus, with mean levels of 27.75±3.00, 31.50±1.40, 35.05±1.68, and 43.88±2.94×103 cells/μl, respectively (p<0.001), and elevated neutrophil counts of 31.18±7.81×103 cells/μl were maintained for more than 6 years with no adverse effects. A gray collie dog with a mean count of 1.94±1.48×103 cells/μl received G-CSF-lentivirus and we observed sustained elevations in neutrophil levels for more than 5 months with a mean of 26.00±11.00×103 cells/μl, significantly increased over the pretreatment level (p<0.001). After the second and third virus administrations mean neutrophil counts of 15.80±6.14 and 11.52±4.90×103 cells/μl were significantly reduced compared with cell counts after the first virus administration (p<0.001). However, after the fourth virus administration mean neutrophil counts of 15.21±4.50×103 cells/μl were significantly increased compared with the previous administration (p<0.05). Throughout the nearly 3 years of virus administrations the dog gained weight, was healthy, and showed neutrophil counts significantly higher than pretreatment levels (p<0.001). These studies suggest that patients with cyclic and other neutropenias may be treated with escalating doses of G-CSF-lentivirus to obtain a desired therapeutic neutrophil count.
Yanay and colleagues demonstrate that four sequential intramuscular injections of lentivirus-encoded granulocyte colony-stimulating factor (G-CSF) lead to an increase in absolute neutrophil counts in dogs. This sustained elevation in neutrophil levels has been maintained for more than 6 years with no adverse effects. These results suggest that patients with cyclic and other neutropenias may be treated by this approach.
Introduction
Cyclic neutropenia, or cyclic hematopoiesis, is a rare disease that occurs both in humans and gray collie dogs. In collie dogs, cyclic neutropenia is inherited as an autosomal recessive disease (Dale et al., 1972; Jones and Lange, 1983) associated with a mutation in the adaptor protein complex-3 (AP3) β subunit. In these dogs recurrent severe neutropenia leads to bacterial infections and shortened life expectancy (Lothrop et al., 1988; Hammond et al., 1990). Current treatment includes antibiotics for infections and daily administration of recombinant human granulocyte colony-stimulating factor (G-CSF) to prevent neutropenia and infections (Morstyn et al., 2001; Lyman, 2005). In humans, the disease is usually caused by mutations in ELANE, the gene for neutrophil elastase, and daily subcutaneous administration of recombinant human G-CSF reduces the severity of the recurrent neutropenia and thereby greatly ameliorates the disease (Morstyn et al., 2001). G-CSF delivery by gene therapy using lentiviral vectors is an alternative approach to the treatment of cyclic neutropenia and other diseases causing severe chronic neutropenia. Lentiviral vectors derived from human immunodeficiency virus type 1 (HIV-1) possess the important attribute of transducing nondividing cells (Enssle et al., 2010; Matrai et al., 2010), and this property has been shown to permit high-level in vivo transduction of muscle tissue and sustained transgene expression in rats (Seppen et al., 2001; Barry et al., 2005; Brzezinski et al., 2007) and affected gray collie dogs (Yanay et al., 2003, 2006). Studies of lentivirus-transduced canine bone marrow cells have also shown that transgene expression can be sustained for more than 5 years after transplantation, supporting the use of such vectors for gene therapy studies in humans (Enssle et al., 2010). Also important, studies in patients infected with HIV-1, using lentiviral vectors, have not shown evidence of insertional mutagenesis (Levine et al., 2006; Wang et al., 2009). However, one study using a lentiviral vector for gene therapy of human β-thalassemia showed that most of the therapeutic benefit in one adult patient resulted from a dominant, myeloid-biased cell clone, in which the integrated vector caused transcriptional activation of HMGA2 in erythroid cells (Cavazzana-Calvo et al., 2010).
Thus far it has not been shown that repeated administration of G-CSF-expressing lentivirus can be used to increase blood neutrophil counts to target levels, a protocol that may be required for clinical applications. To address this we treated both a normal dog and a gray dog with cyclic hematopoiesis with four sequential doses of G-CSF-lentivirus administered intramuscularly and monitored blood neutrophils and other hematopoietic cells.
Materials and Methods
Construction, packaging, and titering of vectors
The expression plasmid pRRL-cPPT-CMV-cGCSF-PRE-SIN was constructed by inserting the canine G-CSF cDNA (Osborne et al., 1993) into the multiple cloning site of pRRL-cPPT-CMV-X-PRE-SIN (Barry et al., 2001). In brief, lentivirus was generated by calcium phosphate cotransfection of pRRL-cPPT-CMV-cGCSF-PRE-SIN expression plasmid and three packaging plasmids, the HIV gag/pol packaging construct, a rev expression plasmid, and the vesicular stomatitis virus protein G (VSV-G) expression plasmid (Soneoka et al., 1995), into 293T cells, as previously described (Seppen et al., 2000; Barry et al., 2001). Viral supernatant (700 ml) was passed through a 0.2-μm (pore size) filter and concentrated by centrifugation for 17 hr at 6100 rpm in a Sorvall HS-4 rotor with a relative centrifugal force (RCF) of 7129×g. The virus pellet was resuspended in 5 ml of phosphate-buffered saline (PBS) and centrifuged for 2 hr at 35,000 rpm in a TL100 tabletop ultracentrifuge with an RCF of 55,000×g. The final pellet was resuspended in 400 μl of Tris-buffered saline and stored at −80°C. Lentiviruses encoding canine G-CSF were assayed for virus p24 Gag content and expressed as infectious units per milliliter by comparison with enhanced green fluorescent protein (eGFP) viral titer determined by flow cytometry (Seppen et al., 2000; Barry et al., 2001; Yanay et al., 2003). Replication-competent virus was assayed by screening the supernatant of serially passaged transduced 293T cells for p24 Gag protein, using a specific ELISA (Barry et al., 2000). Using this assay, all virus samples were replication negative (data not shown).
Quantitative PCR of dog kidney and liver to detect PRE sequences
Kidney and liver from a normal dog and kidney and a representative sample of abnormal liver from the gray collie were tested. Quantitative PCR was performed on an Mx3005P multiplex QPCR system (Stratagene, La Jolla, CA) with samples loaded in triplicate, using ∼100 ng of genomic DNA. Quantitative PCR was run in a 10-μl reaction using SYBR green PCR master mix (Applied Biosystems, Foster City, CA) (5 μl of 2× master mix and a 400 nM concentration of each primer) with PCR cycling conditions of 95°C for 10 min, and 40 cycles of 95°C for 15 sec, 60°C for 1 min. After each assay, a dissociation curve was run to confirm the specificity of all PCR amplicons. Primers were from Integrated DNA Technologies (Coralville, IA) and designed with Applied Biosystems Primer Express 2.0 software. The primers used for the human hepatitis B virus posttranscriptional regulatory element (PRE) were hPRE-496F (GCCAAGTGTTTGCTGACGC) and hPRE-576R (GCCAAGTGTTTGCTGACGC). Plasmid DNA containing the PRE was used to generate a standard curve as 1:4 serial dilutions. The standard curve showed a reaction efficiency of 98.3% (R2=0.999). Resulting Ct values were converted to copies, normalized to total genomic DNA, and expressed as the average of triplicate samples±1 standard deviation. Genomic DNA was isolated with Gentra Puregene reagents according to the manufacturer's instructions (Qiagen, Valencia, CA). Genomic DNA and plasmid DNA (used to convert to copy number) were quantitated on the Mx3005P multiplex QPCR system in triplicate wells with a PicoGreen DNA quantitation kit (Molecular Probes, Eugene, OR), using standards supplied by the manufacturer.
Virus-neutralizing assay
Serum was collected from dogs before and after injection with G-CSF-lentivirus and serially diluted (≥1:10) with PBS. One microliter of pRRL-cPPT-CMV-cGCSF-PRE-SIN virus (6×108 IU/ml) was added to 1 ml of test serum, incubated at room temperature for 1 hr, filtered through a 0.2-μm (pore size) syringe filter, and added with DEAE-dextran, at a final concentration of 10 μg/ml, to a 100-mm dish containing 104 293T cells. The next day the medium was changed and 3 days later the medium was collected and passed through a 0.2-μm (pore size) syringe filter, and the G-CSF concentration was measured in a murine NFS-60 cell proliferation assay (Lejnieks et al., 1996; Yanay et al., 2003). Recombinant canine G-CSF (rcG-CSF) was used as a positive control.
Dog care, virus administration, and blood drawing
Procedures involving animals were approved by the Animal Care and Use Committee of the University of Washington (Seattle, WA). Sugar, a gray collie dog, was recognized at birth by her abnormal coat color and was generously donated to our institute at 7 weeks of age by Paul and Michelle Tennis (Brandon, WI). Buster, a normal tricolor hound dog, was supplied by Marshall BioResources (North Rose, NY). To estimate the production of endogenous G-CSF production after injection of the lentiviral constructs, recombinant canine G-CSF (provided as a gift by Amgen, Thousand Oaks, CA) was administered subcutaneously at three dose levels, and blood neutrophils were determined daily. The doses were as follows: 2 μg/kg for 6 days over an 8-day period in the normal dog and 13 days at 0.5 μg/kg, 17 days at 1.0 μg/kg, and 22 days at 2 μg/kg in the gray collie. Sugar, the gray collie, also received subcutaneous rcG-CSF at 2.5 μg/kg on days 662 to 666 after the fourth virus administration to determine whether it was possible to increase neutrophil production above the response from lentivirus administration. Before lentivirus administration the muscle injection sites on the left and right thighs were shaved and treated with iodine solution. The normal dog weighed 9 kg and received 0.45 ml of a stock virus solution that had a titer of 2.1×108 IU/ml, for a dose of 107 IU/kg. Virus was administered slowly with a 1-ml insulin syringe, and 50 μl per injection was delivered equally to left and right thigh muscles and site swabbed with iodine after injections. Anticoagulated blood samples (0.5 ml) were obtained serially from a peripheral vein and total white blood cell (WBC), platelet, and hematocrit values were determined with an automated blood cell counter (Coulter, Hialeah, FL). In some cases differential WBC counts were performed manually (Lejnieks et al., 1996).
Results
Normal dog
No treatment
Buster, a male tricolor hound, entered our institution at 7 weeks of age. Blood cell counts were monitored serially over a period of 18 days. Mean neutrophil and WBC counts were 3.28±0.16×103 and 8.50±0.46×103 cells/μl, respectively (Table 1), within the normal range for a healthy dog (Yanay et al., 2006; Welles et al., 2009). Values for lymphocytes, platelets, and hematocrit were also all in the normal range (Table 1). However, monocytes were 1.73±0.70×103 cells/μl, slightly above the normal range of 0.70–1.40×103 cells/μl previously reported (Welles et al., 2009).
Table 1.
Normal Dog Blood Cell Counts
| Treatment | Neutrophils (×103/μl) | WBC (×103/μl) | Lymphocytes (×103/μl) | Platelets (×103/μl) | Monocytes (×103/μl) | Hematocrit (%) |
|---|---|---|---|---|---|---|
| None (n=5) | 3.28±0.16 | 8.50±0.46 | 3.37±0.35 | 339.0±19.1 | 1.73±0.17 | 30.54±0.59 |
| rcG-CSF (n=6) | 25.70±1.38a | 38.25±1.14a | 7.33±0.55a | 389.8±30.7b | 5.23±0.91a | 31.72±0.97a |
| First virus (n=12) | 27.75±3.00c | 38.31±2.34c | 6.17±0.56a | 311.6±56.0b | 4.27±1.12c | 33.48±1.09b |
| Second virus (n=10) | 31.50±1.40a | 43.74±1.13a | 7.31±1.11b | 330.6±37.cc | 4.89±1.08c | 35.64±1.41a |
| Third virus (n=27) | 35.05±1.68a | 48.13±1.54a | 7.42±0.96c | 257.9±32.1a | 5.59±1.17c | 38.65±0.65a |
| Fourth virus (n=17) | 43.88±2.94a | 58.88±2.44a | 8.45±1.97b | 179.2±37.5a | 7.22±1.59a | 40.95±1.27a |
| Adoption (n=5) | 31.18±7.81a | 38.50±12.86a | 1.83±1.33b | 206.8±49.2a | 4.45±3.19c | 48.84±3.34a |
WBC, white blood cell count; rcG-CSF, recombinant canine granulocyte colony-stimulating factor.
Note: Data are expressed as means±SD. Recombinant cG-CSF was administered subcutaneously at 2 μg/kg. Student t tests were done between no-treatment and rcG-CSF and between each consecutive virus administration.
p<0.001.
p<0.05.
p>0.01.
Recombinant canine G-CSF
To determine Buster's response to G-CSF we administered six doses of recombinant canine G-CSF (2 μg/kg) subcutaneously over an 8-day period and monitored blood cell counts. Mean neutrophil and WBC counts were 25.70±1.38×103 and 38.25±1.14×103 cells/μl, respectively, significantly elevated over the counts before treatment (p<0.001; Table 1 and Fig. 1A). Monocyte levels were 5.23±0.91×103 cells/μl, lymphocyte counts were 7.33±0.55×103 cells/μl, and the mean hematocrit was 31.72±0.97%, levels that were all significantly elevated over pretreatment values (p<0.001; Table 1 and Fig. 1B–D). Platelet counts were 389.80±30.70×103 cells/μl and were elevated over the untreated control value of 339.00±19.10×103 cells/μl (p<0.05; Table 1 and Fig. 1E).
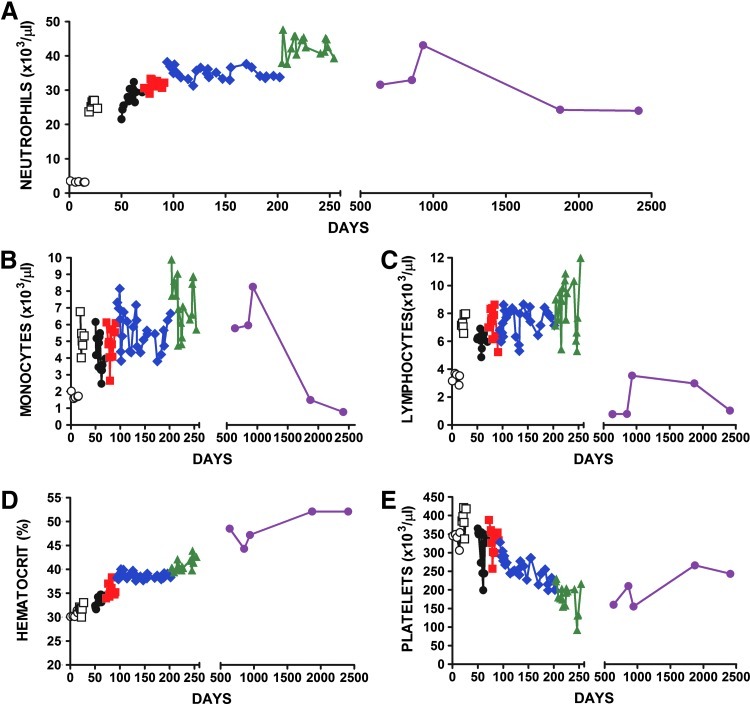
FIG. 1.
Normal dog serial blood cell counts. Serial blood cell counts of a normal dog before and after treatment with recombinant canine G-CSF and G-CSF-lentivirus. Pretreatment values (open circles). Recombinant canine G-CSF administered subcutaneously at 2.0 μg/kg (open squares). Four sequential doses of G-CSF-lentivirus at 3×107 infectious units per kilogram body weight were administered intramuscularly: first virus (solid black circles) second virus (solid red squares), third virus (solid blue diamonds), and fourth virus (solid green triangles). Blood cell counts after adoption from the university (solid purple circles). Shown are absolute neutrophil counts (A), monocytes (B), lymphocytes (C), hematocrit (D), and platelets (E). Color images available online at www.liebertpub.com/hum
G-CSF-lentivirus administration
Having shown that Buster's neutrophil production was increased by recombinant cG-CSF, we administered G-CSF-lentivirus intramuscularly at a dose of 107 IU/kg. At the time of the first virus administration, Buster weighed 9 kg and received a total of 9×107 IU of virus divided equally to the right and left thighs. Buster showed no ill effects in response to the virus injections; no fever, no apparent stiffness or soreness. Neutrophil counts over a 29-day period were increased and showed a mean count of 27.75±3.00×103 cells/μl, which was significantly elevated over the pretreatment value of 3.280±0.16×103 cells/μl (p<0.001; Table 1 and Fig. 1A) and comparable to the levels observed after administration of G-CSF at 2 μg/kg/day. Monocyte levels were not significantly changed from levels recorded during rcG-CSF administration (p>0.1; Table 1 and Fig. 1B). The mean lymphocyte count was 6.17±0.56×103 cells/μl, which was reduced from values recorded during rcG-CSF administration (p<0.001) and was significantly elevated over the pretreatment value (Table 1 and Fig. 1C). The mean hematocrit increased to 33.48±1.09% (Table 1 and Fig. 1D), a change that was probably age related in this young dog (Faldyna et al., 2001). Platelet numbers were significantly decreased from values recorded during recombinant G-CSF administration (p<0.05; Table 1 and Fig. 1E).
After the next three virus injections mean neutrophil counts of 31.50±1.40, 35.05±1.68, and 43.88±2.94×103 cells/μl were recorded, respectively, levels that were all significantly elevated over the preceding neutrophil numbers (p<0.001; Table 1 and Fig. 1A). For each of these doses Buster was monitored for 21, 111, and 52 days, respectively, and showed no adverse effects. As anticipated, WBCs were also significantly elevated after serial virus administration (Table 1). Monocyte numbers were not different after the second and third virus delivery but were significantly increased after the final virus administration (p<0.001; Table 1 and Fig. 1B). We also observed wide excursions in monocyte numbers after the fourth virus administration. Lymphocyte counts were elevated after the second and fourth virus administrations (p<0.05) but not after the third (Table 1 and Fig. 1C). Although red blood cells (percent hematocrit) increased after the second, third, and fourth virus administrations (p<0.001; Table 1 and Fig. 1D) the values were all within a normal range of 39.10±9.23% (Welles et al., 2009) and were probably age-related increases (Faldyna et al., 2001). Platelet numbers were not significantly different after the first and second virus administrations, but they were significantly decreased after the third and fourth virus deliveries (p<0.001; Table 1 and Fig. 1E). In our previous studies of single administration of G-CSF lentivirus we also observed decreases in platelet numbers (Yanay et al., 2003, 2006). Buster did not show any adverse effects in response to the repeated virus administrations, and was healthy and gained weight throughout the study period, increasing from 9 to 15 kg from the first to fourth virus administration.
After the four virus administrations Buster was adopted from our institution and we continued to monitor his blood cell production over the next 68 months (Table 1 and Fig. 1). Over the time of adoption we recorded a mean absolute neutrophil count of 31.18±7.81×103 cells/μl (n=5) that was significantly elevated over the pretreatment value of 3.28±0.16×103 cells/μl and was similar to his mean neutrophil production of 31.50±1.68×103 cells/μl after the second virus delivery (Table 1 and Fig. 1A). In the adoption period the mean monocyte number was 4.45±3.19×103 cells/μl, which was increased over the no-treatment period but was not significantly different (p>0.1; Table 1 and Fig. 1B). The mean lymphocyte level over the adoption period was 1.83±1.33×103 cells/μl, which was reduced from the pretreatment value of 3.37±0.35×103 cells/μl (p<0.05; Table 1 and Fig. 1C). However, these changes may be age related as it has been shown that lymphocyte counts in beagle dogs decreased from 5.02±0.92×103 cells/μl at 2 months of age to 2.76±0.94×103 cells/μl at >5 years of age (Faldyna et al., 2001). Hematocrit values increased over this period to 48.84±3.34% from the pretreatment level of 30.54±0.59% (p<0.001; Table 1 and Fig. 1D). Increased hematocrit levels were recorded at all time points and probably reflect age-related increases in red cell production (Faldyna et al., 2001). The mean platelet count was 206.80±49.24×103 cells/μl, which was significantly reduced from the level of 339.00±19.10×103 cells/μl observed before treatment (p<0.001; Table 1 and Fig. 1E).
To investigate the potential for neutralizing antibody production, serial dilutions of serum (≥1:10) mixed with G-CSF-lentivirus were used to transduce 293T cells and conditioned medium was tested for bioactive G-CSF. None of the sera inactivated virus (data not shown), indicating the lack of virus-neutralizing antibody. These data are consistent with the sustained neutrophil production that we observed in Buster.
Grey collie dog
No treatment
Sugar, a female dog gray collie, was recognized as an affected dog at birth because of her silver gray coat color. She was weaned at 7 weeks of age and admitted to our institute. Serial blood cell counts showed that neutrophils were cycling with minimal and maximal neutrophils of 0 and 4.94×103 cells/μl, respectively, and the overall mean was 1.94±1.48×103 cells/μl (n=14) (Table 2 and Fig. 2A). The WBC count showed essentially the same regular periodicity as neutrophils but with greater changes in amplitude. We observed minimal and maximal WBC counts of 3.7×103 and 10.5×103 cells/μl, respectively, with a mean of 7.32±2.25×103 cells/μl (n=14) (Table 2). Monocyte counts during the period of no treatment were cycling with a mean monocyte number of 1.00±0.40×103 cells/μl (Table 2 and Fig. 2B) and minimal and maximal values of 0.24×103 and 1.73×103 cells/μl, respectively. Lymphocyte counts also cycled and showed minimal and maximal counts of 2.63×103 and 6.04×103 cells/μl, respectively, with a mean value of 4.21±1.17×103 cells/μl (Table 2 and Fig. 2C). During this period of no treatment the hematocrit was essentially constant with a mean of 29.64±1.57% (Table 2 and Fig. 2D). Platelet counts did not show cycling and had a mean of 481.1±32.1×103 cells/μl (Table 2 and Fig. 2E).
Table 2.
Gray Collie Dog Blood Cell Counts
| Treatment | Neutrophils (×103/μl) | WBC (×103/μl) | Lymphocytes (×103/μl) | Platelets (×103/μl) | Monocytes (×103/μl) | Hematocrit (%) |
|---|---|---|---|---|---|---|
| None (n=14) | 1.94±1.48 | 7.32±2.25 | 4.21±1.17 | 481.1±32.1 | 1.00±0.40 | 29.64±1.57 |
| 0.5 rcG-CSF (n=10) | 7.13±4.79a | 19.76±6.20a | 10.22±1.95a | 419.7±25.2a | 2.38±0.61a | 34.80±2.51a |
| 1.0 rcG-CSF (n=14) | 11.21±5.16b | 23.77±4.53c | 10.00±1.95c | 414.5±27.3c | 2.96±1.54c | 33.72±0.90c |
| 2.0 rcG-CSF(n=13) | 15.85±6.14b | 26.01±5.55c | 8.34±2.76c | 260.3±91.4a | 3.50±1.06c | 33.73±1.73c |
| First virus (n=64) | 26.00±11.00a | 38.95±13.10a | 4.18±2.05c | 321.1±84.8a | 7.50±2.55a | 37.71±3.18a |
| Second virus (n=55) | 15.80±6.14a | 25.83±6.80a | 2.63±1.18a | 333.8±91.7c | 5.97±1.43a | 42.21±3.04a |
| Third virus (n=23) | 11.52±4.90b | 19.66±5.74a | 1.96±1.04a | 304.8±47.9c | 5.39±1.31c | 43.33±1.42c |
| Fourth virus (n=52) | 15.21±4.50b | 24.63±5.24a | 2.21±1.02c | 277.5±50.2b | 6.39±1.53b | 41.73±2.87b |
WBC, white blood cell count; rcG-CSF, recombinant canine granulocyte colony-stimulating factor.
Note: Data are expressed as means±SD. Fourth virus mean less four values for G-SCF at 2.5 μg/kg. Student t tests were done between no-treatment and consecutive rcG-CSF and between each consecutive virus administration. First virus compared with no treatment.
p<0.001.
p<0.05.
p>0.01.
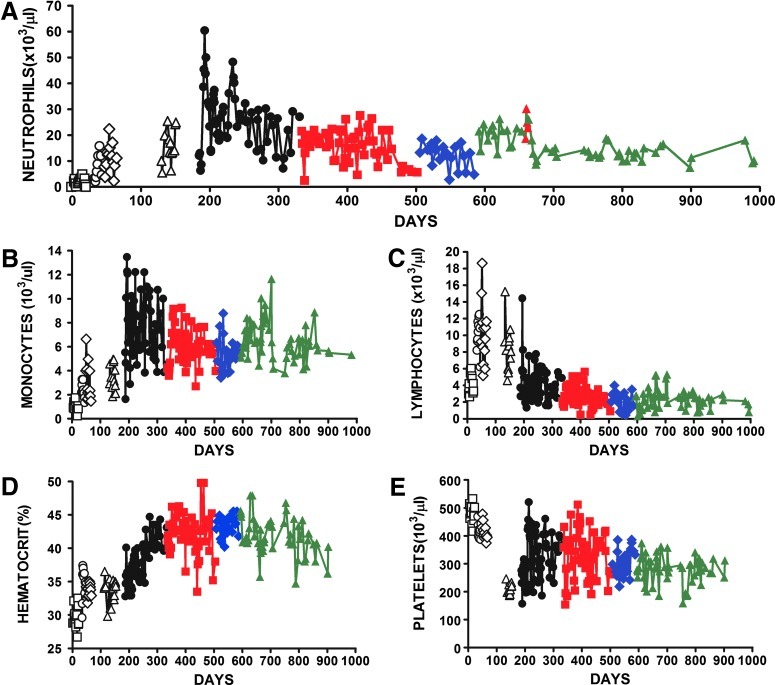
FIG. 2.
Gray collie blood cell counts. Serial blood cell counts of a gray collie dog before and after treatment with recombinant canine G-CSF and G-CSF-lentivirus. Pretreatment values (open squares). Increasing doses of recombinant canine G-CSF administered subcutaneously were 0.5 μg/kg (open circles), 1.0 μg/kg (open diamonds), and 2.0 μg/kg (open triangles). Four sequential doses of G-CSF-lentivirus at 3×107 infectious units per kilogram body weight were administered intramuscularly: first virus (solid black circles), second virus (solid red squares), third virus (solid blue diamonds), and fourth virus (solid green triangles). Shown are absolute neutrophil counts (A), monocytes (B), lymphocytes (C), hematocrit (D), and platelets (E). Red solid triangles in (A) are neutrophil values after subcutaneous administration of five sequential doses of recombinant canine G-CSF at 2.5 μg/kg. Color images available online at www.liebertpub.com/hum
Recombinant canine G-CSF
To determine the effects of recombinant canine G-CSF we administered three escalating doses of rcG-CSF (0.5, 1.0, and 2.0 μg/kg, given subcutaneously) over periods of 13, 17, and 22 days, respectively, and monitored blood cell counts (Table 2 and Fig. 2). Serial blood cell counts showed significant increases in neutrophils after 0.5-μg/kg rG-CSF administration with a mean of 7.13±4.79×103 cells/μl (p<0.001; Table 2 and Fig. 2A). Neutrophil levels after rG-CSF administration at 1.0 and 2.0 μg/kg showed increases, with mean counts of 11.21±5.16×103 and 15.85±6.14×103 cells/μl, respectively, which were significantly elevated (p<0.05) over cell numbers after the preceding dose (Table 2 and Fig. 2A). Serial blood cell counts also showed significant increases in WBCs after 0.5-μg/kg rG-CSF administration, with a mean of 19.76±6.20×103 cells/μl (p<0.001; Table 2). WBC levels after rG-CSF administration at 1.0 and 2.0 μg/kg showed increases, with mean counts of 23.77±4.53 and 26.01±5.55×103 cells/μl, respectively, and these were significantly elevated over the counts recorded during no treatment (p<0.001; Table 2).
After administration of 0.5-μg/kg rcG-CSF monocyte numbers were significantly increased to 2.38±0.61×103 cells/μl, more than the level of 1.00±0.40×103 cells/μl observed in the nontreatment period (p>0.001; Table 2 and Fig. 2B). Monocyte numbers were not significantly different at 2.96±1.54×103 and 3.50±1.06×103 cells/μl, respectively, subsequent to injections of rcG-CSF at 1.0 and 2.0 μg/kg (p>0.1; Table 2 and Fig. 2B). The pretreatment mean lymphocyte level was 4.21±1.17×103 cells/μl, which was significantly increased by rcG-CSF administration at 0.5 μg/kg to 10.22±1.95×103 cells/μl (p<0.001; Table 2 and Fig. 2C). Although lymphocyte counts decreased after G-CSF administrations at 1.0 and 2.0 μg/kg they were not significantly different (Table 2 and Fig. 2C). After administration of rcG-CSF at 0.5 μg/kg the percent hematocrit (Hct) was significantly increased to 34.8±2.51% over the nontreatment level of 29.64±1.57% (p<0.001; Table 2 and Fig. 2D). However, the next two levels of rcG-CSF gave Hct values of 33.72±0.90 and 33.73±1.73%, respectively, which were not significantly changed (Table 2 and Fig. 2D). During the period when recombinant G-CSF was administered the gray collie dog was healthy and free of infection.
G-CSF-lentivirus administration
Recombinant canine G-CSF administration was then stopped, and we administered four doses of G-CSF-lentivirus (intramuscularly) at 107 IU/kg body weight. As in the study of the normal dog, lentivirus was administered in equal aliquots to each hind leg muscle. After the first lentivirus treatment the mean neutrophil count was elevated to 26.00±11.0×103 cells/μl (n=64) recorded over a 246-day period; this was significantly increased over the mean value during recombinant G-CSF treatment (p<0.001; Table 2 and Fig. 2A). Lentivirus administration was repeated a further three times and blood cell counts were recorded (Table 2 and Fig. 2). The mean neutrophil counts significantly decreased after the second and third injections to 15.80±6.14×103 cells/μl (p<0.001) and 11.52±4.90×103 cells/μl (p<0.05), but increased to 15.21±4.50×103 cells/μl (p<0.05) after the fourth virus injection (Table 2 and Fig. 2A). After each injection, tests for virus-neutralizing antibodies were negative, as we observed with the normal dog.
To determine whether it was possible to increase neutrophil production and blood cell counts above the virus-mediated response, Sugar was administered rcG-CSF subcutaneously on days 662 to 666 (2.5 μg/kg) after the fourth virus administration (Fig. 2A). The neutrophil count during rcG-CSF administration was 24.56±4.26×103 cells/μl (n=5), which was significantly elevated compared with 18.87±3.81×103 cells/μl (n=4) immediately preceding cytokine administration (p<0.05) and 12.51±4.12×103 cells/μl (n=4) immediately after (p<0.05). These data indicate that the gray collie was able to increase neutrophil production in response to rcG-CSF while overexpressing neutrophils after G-CSF lentivirus administration, suggesting the ability to form neutrophils had not been affected by virus treatment. The mean WBC count after the first virus administration was 39.95±13.10×103 cells/μl, which was significantly elevated over the pretreatment count of 7.32±2.25×103 cells/μl (p<0.001; Table 2). The WBC count after the virus administration was nearly 4-fold higher than the normal dog level of 9.80±2.40×103 cells/μl (Lee et al., 1993). The WBC counts decreased successively after the second and third virus administrations to 25.83±6.80×103 and 19.66±5.74×103 cells/μl, respectively, and increased to 24.63±5.24×103 cells/μl after the fourth virus, mirroring the changes we observed in neutrophil counts (Table 2 and Fig. 2A).
After the first virus administration monocyte counts increased to a mean value of 7.50±2.55×103 cells/μl, which was significantly elevated over the pretreatment value of 1.00±0.40 cells/μl (p<0.01; Table 2 and Fig. 2B). After the next three virus injections monocyte counts remained elevated with mean values of 5.97±1.43×103, 5.39±1.31×103, and 6.39±1.53×103 cells/μl, respectively (Table 2 and Fig. 2B). All treatments resulted in increased monocyte levels over that reported for normal dogs, 0.58±0.21×103 cells/μl (Lee et al., 1993). Previous reports have described increases in monocyte counts after recombinant G-CSF administration to both patients and normal subjects, although less than the cell counts we observed (Frampton et al., 1994; Anderlini et al., 1996). After the four lentivirus administrations lymphocyte numbers were significantly reduced in comparison with rcG-CSF treatment (p<0.001; Table 2 and Fig. 2C) but were not different from pretreatment levels (p>0.1; Table 2 and Fig. 2C). Lymphocyte numbers after the second, third, and fourth virus injections were within the range of 2.60±0.80×103 cells/μl recorded for normal dogs (Lee et al., 1993).
The mean hematocrit after the first lentivirus administration was 37.71±3.18%, which was significantly increased over the value of 33.73±1.73% recorded after the highest level of rcG-CSF administered (p<0.001; Table 2 and Fig. 2D). Hematocrits were increased after the next two virus administrations to 42.21±3.04 and 43.33±1.42%, respectively, but only the former was significantly different (p<0.001; Table 2 and Fig. 2D). The fourth and final virus administration resulted in a significant decrease in hematocrit to 41.73±2.87% (p<0.05; Table 2 and Fig. 2D). However, all the hematocrits were within the normal range for a dog, and these increased levels may be attributable to the increasing maturity and overall better health status of the dog. G-CSF-lentivirus treatment resulted in significantly decreased platelet counts compared with the pretreatment level (p<0.001; Table 2 and Fig. 2E). However, these platelet values after virus treatments were within the range of 367.00±100.00×103 cells/μl described for normal dogs (Lee et al., 1993).
The lentivirus injections into the gray collie dog were not associated with clinical signs of inflammation, infection, or fever. After treatment with lentivirus the dog had few problems with recurrent fevers or infections. After lentivirus administration the dog continued to grow and gain weight and was no longer housed in a pathogen-free environment. Sugar was adopted from our institute and survived for 19 months without any ill health. However, she died suddenly and unexpectedly of a liver tumor at 57 months of age. We showed that both liver and kidney were free of provirus, using a sensitive real-time PCR assay, indicating that her death from liver tumor was probably unrelated to lentivirus administration.
Discussion
We have shown in a healthy normal dog that four consecutive lentivirus administrations induced sequential increases in neutrophil production that were sustained for more than 6 years. After the first virus administration of 107 IU/kg, the mean neutrophil count was 27.75±3.00×103 cells/μl, and that was similar to the neutrophil level of 25.70±1.38×103 cells/μl recorded after administration of rcG-CSF at 2 μg/kg, suggesting the equivalency of these doses. We established this virus dose as one-tenth of that administered previously to two affected gray collie dogs that induced significant increases in neutrophil production of 29.23±12.93×103 and 12.13±4.26×103 cells/μl (Yanay et al., 2003, 2006). After the first virus administration the collie dog showed significant increases in blood neutrophils to 26.00±11.00×103 cells/μl and maintained elevated neutrophil counts for 27 months when housed at our institution. Dose escalation of rcG-CSF administration to a gray collie gave sustained increases in neutrophil counts. This finding suggests that there is no inherent limit to neutrophil production in canine cyclic neutropenia, an interpretation supported by the observation that administration of rcG-CSF at 2.5 μg/kg to Sugar after the fourth virus treatment also induced significant increases in neutrophil production.
The neutrophil levels of the normal dog increased after each virus administration whereas the gray collie dog showed mean increases only after the first and fourth injections. This may be related to the abnormal hematopoietic system of gray collies and, although responsive to G-CSF, the collies are probably always less responsive than normal dogs.
Sugar survived for 57 months, an old age for a dog with cyclic neutropenia, and died unexpectedly of a liver tumor. At autopsy, her liver tumor was free of provirus, strongly suggesting that the liver tumor was not related to lentivirus administration. In two other gray collie dogs treated with lentivirus we also showed that the cause of death was not related to virus administration (Yanay et al., 2003, 2006). We have previously observed that renal failure, attributable to amyloidosis, is often the cause of death in these dogs, if they do not die from bacterial infections (Hammond et al., 1990).
Although we observed some changes in other blood cell counts in both the normal dog and the gray collie dog, the changes were minor compared with the changes in blood neutrophils, a finding consistent with the natural role of G-CSF in regulating neutrophil production (Morstyn et al., 2001). Some of the observed changes in hematocrit and lymphocyte counts may have been age related, because both dogs entered the study at a very young age. Importantly, in a 6-year period of observation lentivirus treatment was not associated with anemia, lymphocytopenia, monocytopenia, or other changes suggesting adverse effects on hematopoiesis. Although some reports suggest there may be complications from long-term administration of G-CSF in patients with severe congenital neutropenia or cyclic neutropenia (Jeha et al., 2000; Sotomatsu et al., 2000), we believe that the marrow defect determines the risk of acute myelogenous leukemia, and not the G-CSF therapy (Dale et al., 2006). An important observation from these data, together with our previous studies of two affected dogs (Yanay et al., 2003, 2006), is that a total of 9.5 years of sustained virus-mediated G-CSF delivery showed persistence of the neutrophil effect and absence of evidence for malignant transformation. This is also a unique but important model for examining the risk of myelodysplasia or leukemia with high long-term expression of G-CSF.
Previously in dogs, lentivirus-mediated transgene expression has been sustained for 5 years after transduction of multilineage long-term repopulating cells, providing support for the use of such vectors for gene therapy studies in patients (Enssle et al., 2010; Matrai et al., 2010). In our study the target tissue was relatively nonproliferating muscle, which probably has a lower potential for tumor formation than rapidly dividing hematopoietic cells. We have previously shown in rats that G-CSF- and erythropoietin (EPO)-lentivirus can be consecutively administered intramuscularly to provide sustained transgene expression (Brzezinski et al., 2007), and our current data in a large animal model support the notion that readministration of lentivirus provides sustained gene expression without adverse effects. Neutrophil counts of ≥500 cells/μl provide treatment for neutropenic patients (Morstyn et al., 2001), and our studies suggest this cell number could be attained with a lentivirus dose considerably less than the 107 IU/kg used in this study. The study of large-animal models has been important for the successful translation of gene therapy protocols to the clinic (Bauer et al., 2009). Overall, these data from a normal dog and a gray collie dog suggest the absence of immune response to lentivirus administered intramuscularly and indicate that dose escalation may be applied to achieve a desired level of neutrophil production toward the treatment of patients with severe chronic neutropenia.
Acknowledgments
This work was supported by U.S. National Institutes of Health grant DK43727-12 (Osborne). The authors thank Paul and Michelle Tennis for introducing them to Sugar, and Mike Powers for taking Buster to the countryside. The authors thank the Department of Comparative Medicine for expert care of the dogs and Amgen (Thousand Oaks, CA) for recombinant canine G-CSF.
Author Disclosure Statement
Ofer Yanay and William R.A. Osborne report no competing financial interests. David C. Dale, M.D., has served as consultant and speaker for and has received research funds from Amgen.
References
- Anderlini P. Przepiorka D. Champlin R. Korbling M. Biological and clinical effects of granulocyte colony-stimulating factor in normal individuals. Blood. 1996;88:2819–2825. [PubMed] [Google Scholar]
- Barry S. Brzezinski M. Yanay O., et al. Sustained elevation of neutrophils in rats induced by lentivirus-mediated G-CSF delivery. J. Gene Med. 2005;7:1510–1516. doi: 10.1002/jgm.807. [DOI] [PubMed] [Google Scholar]
- Barry S.C. Seppen J. Ramesh N., et al. Lentiviral and murine retroviral transduction of T-cells for expression of human CD40 ligand. Hum. Gene Ther. 2000;11:323–332. doi: 10.1089/10430340050016058. [DOI] [PubMed] [Google Scholar]
- Barry S.C. Harder B. Brzezinski M., et al. Lentivirus vectors encoding both central polypurine tract and posttranscriptional regulatory element provide enhanced transduction and transgene expression. Hum. Gene Ther. 2001;12:1103–1108. doi: 10.1089/104303401750214311. [DOI] [PubMed] [Google Scholar]
- Bauer T.R. Adler R.L. Hickstein D.D. Potential large animal models for gene therapy of human genetic diseases of immune and blood cell systems. ILAR J. 2009;50:168–186. doi: 10.1093/ilar.50.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzezinski M. Yanay O. Waldron L., et al. G-CSF-lentivirus administration in rats provided sustained elevated neutrophil counts and subsequent EPO-lentivirus administration increased hematocrits. J. Gene Med. 2007;9:571–578. doi: 10.1002/jgm.1050. [DOI] [PubMed] [Google Scholar]
- Cavazzana-Calvo M. Payen E. Negre O., et al. Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia. Nature. 2010;467:318–322. doi: 10.1038/nature09328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale D.C. Ward S.B. Kimball H.R. Wolff S.M. Studies on neutrophil production and turnover in grey collie dogs with cyclic neutropenia. J. Clin. Invest. 1972;51:2190–2196. doi: 10.1172/JCI107026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale D.C. Rosenberg P.S. Alter B.P. Lineage-specific hematopoietic growth factors. N. Engl. J. Med. 2006;355:526–527. [PubMed] [Google Scholar]
- Enssle J. Trobridge G. Keyser K.A., et al. Stable marking and transgene expression without progression to monoclonality in canine long-term hematopoietic repopulating cells transduced with lentiviral vectors. Hum. Gene Ther. 2010;21:397–403. doi: 10.1089/hum.2009.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faldyna M. Levá L. Knötigová P. Toman M. Lymphocyte subsets in peripheral blood of dogs—a flow cytometric study. Vet. Immunol. Immunopathol. 2001;82:23–37. doi: 10.1016/s0165-2427(01)00337-3. [DOI] [PubMed] [Google Scholar]
- Frampton J.E. Lee C.R. Faulds D. Filgrastim: A review of its pharmacological properties and therapeutic efficacy in neutropenia. Drugs. 1994;48:731–760. doi: 10.2165/00003495-199448050-00007. [DOI] [PubMed] [Google Scholar]
- Hammond W.P. Boone T.C. Donahue R.E., et al. A comparison of treatment of canine cyclic hematopoiesis with recombinant human G-CSF, GM-CSF, and IL-3 and canine G-CSF. Blood. 1990;76:523–532. [PubMed] [Google Scholar]
- Jeha S. Chan K.W. Aprikyan A.G., et al. Spontaneous remission of granulocyte colony-stimulating factor–associated leukemia in a child with severe congenital neutropenia. Blood. 2000;96:3647–3649. [PubMed] [Google Scholar]
- Jones J.B. Lange R.D. Cyclic hematopoiesis: Animal models. Exp. Hematol. 1983;11:571–580. [PubMed] [Google Scholar]
- Lee G.R. Bithell T.C. Foerster J., et al. Wintrobe's Clinical Haematology. Lea & Febiger; London: 1993. [Google Scholar]
- Lejnieks D.V. Han S.W. Ramesh N., et al. Granulocyte colony-stimulating factor expression from transduced vascular smooth muscle cells provides sustained neutrophil increases in rats. Hum. Gene Ther. 1996;7:1431–1436. doi: 10.1089/hum.1996.7.12-1431. [DOI] [PubMed] [Google Scholar]
- Levine B.L. Humeau L.M. Boyer J., et al. Gene transfer in humans using a conditionally replicating lentiviral vector. Proc. Natl. Acad. Sci. U.S.A. 2006;103:17372–17377. doi: 10.1073/pnas.0608138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lothrop C.D. Warren D.J. Souza L.M., et al. Correction of canine cyclic hematopoiesis with recombinant human granulocyte colony-stimulating factor. Blood. 1988;72:1324–1328. [PubMed] [Google Scholar]
- Lyman G.H. Pegfilgrastim: A granulocyte colony-stimulating factor with sustained duration of action. Expert Opin. Biol. Ther. 2005;5:1635–1646. doi: 10.1517/14712598.5.12.1635. [DOI] [PubMed] [Google Scholar]
- Matrai J. Chuah M.K.L. Vandendriessche T. Recent advances in lentiviral vector development and applications. Mol. Ther. 2010;18:477–490. doi: 10.1038/mt.2009.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morstyn G. Foote M.A. Walker T. Molineux G. Filgrastim (r-metHuG-CSF) in the 21st century: SD/01. Acta Haematol. 2001;105:151–155. doi: 10.1159/000046557. [DOI] [PubMed] [Google Scholar]
- Osborne W.R.A. Geary R. Lau S., et al. Transduced vascular smooth muscle cells in a canine model of gene therapy. Clin. Res. 1993;41:194A. [Google Scholar]
- Seppen J. Barry S.C. Klingspoor J.H., et al. Apical gene transfer into quiescent human and canine polarized intestinal epithelial cells by lentiviral vectors. J. Virol. 2000;74:7642–7645. doi: 10.1128/jvi.74.16.7642-7645.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppen J. Barry S.C. Harder B. Osborne W.R.A. Lentivirus administration to rat muscle provides efficient sustained expression of erythropoietin. Blood. 2001;98:594–596. doi: 10.1182/blood.v98.3.594. [DOI] [PubMed] [Google Scholar]
- Soneoka Y. Cannon P.M. Ramsdale E.E., et al. A transient three-plasmid expression system for the production of high-titer retroviral vectors. Nucleic Acids Res. 1995;23:628. doi: 10.1093/nar/23.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotomatsu M. Kanazawa T. Ogawa C., et al. Complication of rapidly progressive glomerulonephritis in severe congenital neutropenia treated with long-term granulocyte colony-stimulating factor (filgrastim) Br. J. Haematol. 2000;110:234–235. doi: 10.1046/j.1365-2141.2000.02072-1.x. [DOI] [PubMed] [Google Scholar]
- Wang G.P. Levine B.L. Binder G.K., et al. Analysis of lentiviral vector integration in HIV+ study subjects receiving autologous infusions of gene modified CD4+ T cells. Mol. Ther. 2009;17:844–850. doi: 10.1038/mt.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welles E.G. Hall A.S. Carpenter D.M. Canine complete blood counts: A comparison of four in-office instruments with the ADVIA 120 and manual differential counts. Vet. Clin. Pathol. 2009;38:20–29. doi: 10.1111/j.1939-165X.2008.00084.x. [DOI] [PubMed] [Google Scholar]
- Yanay O. Barry S.C. Katen L.J., et al. Treatment of canine cyclic neutropenia by lentivirus-mediated G-CSF delivery. Blood. 2003;102:2046–2052. doi: 10.1182/blood-2002-12-3722. [DOI] [PubMed] [Google Scholar]
- Yanay O. Brzezinski M. Christensen J., et al. An adult dog with cyclic neutropenia treated by lentivirus- mediated delivery of granulocyte colony-stimulating factor. Hum. Gene Ther. 2006;17:464–469. doi: 10.1089/hum.2006.17.464. [DOI] [PubMed] [Google Scholar]