Abstract
Invasive candidiasis is a common problem in premature infants that leads to high morbidity and mortality. Although Candida albicans has historically been the most prominent species involved in these infections and has therefore been the subject of the most study, Candida parapsilosis is increasing in frequency, and neonates are disproportionately affected. This article reviews unique aspects of the epidemiology of this organism as well as strategies for prophylaxis against invasive candidiasis in general. Additionally, important differences between C. parapsilosis and C. albicans are coming to light related to virulence determinants and interactions with components of host immunity. These developments are reviewed while highlighting the significant gaps in our understanding that remain to be elucidated.
Keywords: antifungal prophylaxis, antifungal resistance, Candida albicans, Candida parapsilosis, echinocandins, fluconazole, host defense, lactoferrin, virulence factors
Candida spp. are the leading cause of invasive fungal infections in premature infants, leading to death or severe neurodevelopmental impairment in a large majority of those affected [1]. Among non-albicans species, Candida parapsilosis is increasingly being recognized as an important cause of invasive candidiasis in this population, and in some centers has overtaken Candida albicans as the leading species in invasive disease [2,3]. This diploid yeast is closely related to C. albicans, but has a number of unique attributes that are the focus of this review. C. parapsilosis was formerly divided into Groups I, II and III based on a variety of observed genetic differences. Further study published in 2005 demonstrated that these groups are sufficiently different such that they warrant designation as three distinct but closely related and phenotypically indistinguishable species [4]. Thus, C. parapsilosis Groups II and III are now designated as Candida orthopsilosis and Candida metapsilosis, respectively. Although the majority of clinical isolates appear to be C. parapsilosis sensus stricto, C. orthopsilosis and C. metapsilosis have been identified in collections of clinical isolates at frequencies that vary based on geographic region [5,6]. Because these definitions are relatively recent, and careful attention to defining these species is not universal in the literature, the term ‘C. parapsilosis’ will be used to discuss these organisms for the remainder of this review.
Epidemiology
Colonization & infection
Rates of infection with C. parapsilosis vary from region to region throughout the world. The incidence of candidemia in different regions and the distribution of Candida spp. by age group was reviewed recently [7]. C. parapsilosis accounts for a wide range of Candida isolates, ranging from as little as 4% (Switzerland and one center in Brazil) to as high as 44% of all isolates (Saudi Arabia). Colonization with yeast is an important predisposing factor for developing invasive disease [8]. C. parapsilosis in particular was noted to colonize neonates later than C. albicans by several weeks [9], which is consistent with observations that C. parapsilosis is a rare cause of neonatal early-onset sepsis. In a study of 82 premature infants in the USA, four infants were found to have early stool colonization with C. parapsilosis between 1 and 2 weeks of age. All four infants developed C. parapsilosis candidemia [10]. A study in India showed similar trends, and added that central venous catheters were a risk factor for colonization. In this study, Candida tropicalis was the most common organism [8]. A French study suggested that a delayed time to total enteral nutrition is associated with C. parapsilosis colonization. Other suspected risk factors, including number of central venous catheters, duration of central venous catheters, days of antibiotics and mechanical ventilation were not significantly associated with invasive infection in this study, perhaps owing to a relatively low number of patients [11].
Because of its association with invasive infection, the source of colonization has also been an area of interest. Multiple studies have demonstrated horizontal transmission from environmental sources in outbreak settings, and hands of healthcare workers are commonly implicated [12,13]. Molecular techniques have confirmed identity between strains recovered from patients and strains recovered from colonizing sources [14]. Vertical transmission has been implicated as a source of neonatal colonization with C. albicans, with vaginal delivery as an independent risk factor. However, one study that used molecular methods to study route of colonization in premature neonates found C. parapsilosis colonization to be much more prevalent in neonates than in their mothers and no cases of vertical transmission were documented [15].
C. parapsilosis infections have been reported in virtually every sterile body site in neonates, including blood, lung, urine, retina and ascites. Unlike C. albicans, however, C. parapsilosis meningitis is relatively uncommon and is mainly associated with indwelling medical devices [16]. Clinical manifestations of disease in neonates are difficult to distinguish from other organisms causing neonatal sepsis. One study compared neonates with C. albicans fungemia and C. parapsilosis fungemia, and found that infants with C. parapsilosis were less likely to have bradycardia, hypoxemia or respiratory distress, and more likely to have hypothermia. However, the C. albicans group was more likely to have positive sterile site cultures other than blood, suggesting that involvement of other organs may have contributed to their symptoms [17].
Risk factors
Several studies have examined risk factors for development of candidemia in neonates, but few have examined C. parapsilosis in particular. Risk factors for candidemia are numerous and are listed in Table 1. While some or all of these risk factors are likely to apply to infants with C. parapsilosis, few studies focus on neonates. Those that have examined this issue compared infants with invasive C. albicans to those with invasive C. parapsilosis. Factors that have been reported to increase risk for invasive C. parapsilosis infection include use of cephalosporins [18], presence of an indwelling central venous catheter [19,20] and receipt of parenteral nutrition [20]. Factors that favor C. albicans infection over C. parapsilosis include development of oral thrush or diaper dermatitis [19]. These studies should be interpreted with caution, as each series may have been underpowered to detect significant associations. These same studies reported that C. parapsilosis was more likely to be found in the peripheral blood, and less likely to be found in the urine, on central venous catheter tips or in deep-seated infections [18]. Further investigation is warranted to clarify potentially unique risk factors for C. parapsilosis infections.
Table 1.
Risk factors for candidemia in neonates.
| Risk factor | Ref. |
|---|---|
| Extreme prematurity† | [104–106] |
| Vaginal delivery | [105] |
| Colonization with Candida | [107] |
| Apgar score <5 at 5 min | [104] |
| Shock | [104] |
| Antibiotic number >2 | [104] |
| Longer duration of antibiotics | [108] |
| Use of a third-generation cephalosporin | [106,109] |
| Parenteral nutrition >5 days | [104] |
| Lipid use >7 days | [104] |
| Central venous catheter | [104] |
| Use of H2 blockers | [104] |
| Length of stay >7 days | [104] |
| Intubation | [104] |
| Early dexamethasone | [108] |
| Abdominal surgery | [105] |
| Thrombocytopenia | [106] |
Increase in risk is inversely proportional to gestational age in weeks.
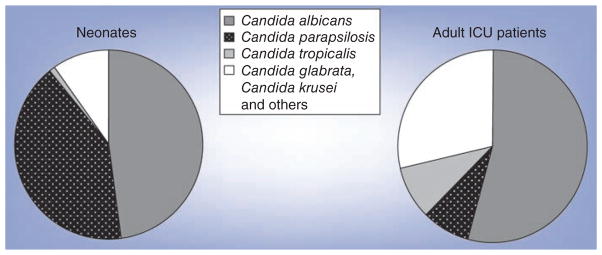
Species prevalence
Several surveillance programs exist to monitor fungal infections regionally, nationally and internationally. Overall, C. albicans is typically the leading fungal pathogen, followed by Candida glabrata [21,22]. In some settings, C. tropicalis may be the dominant fungal pathogen [23]. By contrast, studies that examined neonates or subsets of neonates, such as extremely low birth-weight infants, found C. parapsilosis as the second most common fungal pathogen (Figure 1) [1,24,25]. In fact, C. parapsilosis has surpassed C. albicans to become the dominant fungal pathogen in children and neonates in some centers [26,27]. These authors suggested that the shift may be due to a high number of patients undergoing gastrointestinal surgery and/or receiving parenteral nutrition, particularly at home [26]. As medical technology becomes more widespread and more common in out-of-hospital settings, clinicians should be aware of potential shifts in epidemiology of fungal infections.
Figure 1. Distribution of Candida species by age.
ICU: Intensive care unit.
Antifungal prophylaxis
The use of prophylactic medications, both systemic and mucosal, for prevention of invasive fungal infections has received a great deal of attention in recent years. Studies have focused on infants with low birthweight (<2500 g) or very low birthweight (<1500 g). The best-studied agent in systemic prophylaxis studies is fluconazole. In a Cochrane review last updated in 2009, eight randomized, placebo-controlled trials were included [28]. Since this update, Aydemir et al. have published another randomized trial comparing fluconazole, nystatin and placebo [29]. While these studies did not examine C. parapsilosis specifically, it was included in the aggregate end point. Limiting discussion to randomized trials included in the Cochrane review, 758 low birth weight or very low birth weight infants were included in these trials. Most (656) were included in trials of intravenous fluconazole versus placebo. The pooled effect sizes estimated that the number of infants needed to treat with fluconazole is 11 to prevent one episode of invasive candidiasis [28]. However, the reviewers noted that this estimate was driven by locations with high prevalence of invasive disease, and cautioned that the number needed to treat is considerably higher in low-prevalence locations. Indeed, the incidence of invasive candidiasis varied markedly among centers, making a unified strategy for prophylaxis difficult to develop [24]. In addition, no overall benefit to all-cause mortality was noted in the pooled studies [28].
The efficacy of mucosal antifungal prophylaxis to prevent invasive candidiasis is promising, but suffers from a lack of rigorous trials. Another Cochrane review examined the potential use of nonsystemic antifungal agents such as nystatin and miconazole for the prevention of invasive fungal infections [30]. The reviewers included four randomized studies. The pooled analysis of 1625 infants showed a protective effect for invasive fungal infection, needing to treat five infants to prevent one infection. Similar to fluconazole trials, such numbers may have been driven by large trials (948 infants) in high-prevalence areas. The results should be interpreted cautiously owing to methodological flaws. Some studies were unblinded, and one study was performed in a setting where resources did not allow for interventions such as ventilator support for infants with birthweights less than 1250 g. Similar to systemic prophylaxis, no difference was found in all-cause mortality prior to discharge [30]. A recent randomized trial comparing systemic fluconazole and oral nystatin to placebo in preterm neonates provided additional insight into this issue [29]. This study of 278 infants revealed similar decreases in invasive fungal infections, including C. parapsilosis, in both intervention groups compared with placebo. The rate of invasive infection was 16.5% in the placebo group, compared with 3.2% in the fluconazole group and 4.3% in the nystatin group. Again, there was no difference among the groups in mortality before discharge. This study suggests that nonsystemic prophylaxis may be comparable to systemic prophylaxis in reducing invasive fungal infections in very low birthweight infants.
Although the benefit of antifungal prophylaxis in reducing the incidence of invasive candidiasis in infants is well documented in settings where the baseline infection rate is high, concern regarding the possibility of inducing antifungal resistance remains. Studies in the USA and Italy have shown that there was no selection for intrinsically azole-resistant Candida spp. during periods of routine use of fluconazole [24,31]. By contrast, a national study in Finland showed an association of increased use of fluconazole with an increased proportion of the intrinsically azole-resistant C. glabrata [32]. However, this study was not limited to neonatal populations. Another Finnish study did not find a similar shift in a neonatal intensive care unit setting, but did uncover a single strain of C. parapsilosis with increasing resistance to fluconazole over a 10-year period [33]. Data on colonization from one of the early randomized controlled trials of fluconazole prophylaxis were also not entirely reassuring in this regard. The prevalence of colonization with the more azole-sensitive C. albicans (MIC90 = 0.5 μg/ml) was reduced from 43 to 7%, but colonization was replaced with the more resistant non-albicans strains, primarily C. parapsilosis (MIC90 = 8 μg/ml), which increased from 45 to 83% [34]. In another set of vulnerable patients, recipients of hematopoietic stem cell transplants and patients with acute myelogenous leukemia, Mann et al. demonstrated a shift in colonizing yeast to intrinsically resistant Candida spp. in patients receiving azole prophylaxis [35]. There was also a fourfold increase in MIC to azole drugs in colonizing strains that became invasive infections. Finally, development of resistant C. parapsilosis in the setting of fluconazole prophylaxis in an animal neonatal intensive care unit has also been reported [36].
Lactoferrin, a component of mammalian milk, is well known to have anti-infective properties. The utility of bovine lactoferrin administration for prevention of sepsis in premature neonates has received recent attention with promising results. A multicenter study conducted in Italy randomized 472 infants to lactoferrin alone or in combination with the probiotic Lactobacillus rhamnosus GG compared with placebo. Significant reductions in both late-onset sepsis and invasive Candida infections were seen in both lactoferrin groups relative to placebo [37]. Interestingly, there was no effect on fungal colonization.
Systemic prophylaxis appears to be safe and effective in reducing the incidence of invasive fungal infection. Studies involving mucosal therapy are encouraging; however, more carefully controlled studies are needed. The cost–benefit ratio of administering prophylactic antifungals depends largely upon the local prevalence of invasive fungal infection. If a center opts to use routine antifungal prophylaxis, surveillance of resistance patterns should occur to identify emergence of resistant organisms. Lactoferrin is a promising new strategy for prophylaxis that avoids the use of conventional antifungal agents and could avoid the potential complication of emerging resistance.
Treatment of neonatal candidiasis
The treatment of neonatal candidiasis is a continuously evolving field. A summary of available antifungal agents and dosing recommendations is listed in Table 2. Guidelines have been published that cover treatment of candidiasis across all ages and body sites [38] and pharmacotherapy in neonates has recently been reviewed [39]. We will focus our discussion on the newest class of antifungal agents, the echinocandins. These medications inhibit the synthesis of 1,3-β-D-glucan, a component of fungal cell walls. Member drugs include caspofungin, micafungin and anidulafungin. Although no controlled trials have compared efficacy against other antifungal classes, successful therapy with caspofungin and micafungin in neonates for invasive fungal infections, including C. parapsilosis, has been reported [40,41]. There are no data available on the use of anidulafungin in neonates. Because of lack of data, reserving use of echinocandins for situations of treatment failure, intolerable or toxic side effects or adverse events with other antifungal medications is recommended [38]. Adverse events are largely unknown in neonates, but may include hepatitis and electrolyte abnormalities.
Table 2.
Medications for use in invasive Candida infections in neonates.
| Drug class | Drug | Recommended dosing | Comments |
|---|---|---|---|
| Polyenes | Amphotericin B deoxycholate | 1–1.5 mg/kg iv. every 24 h | |
| Amphotericin B liposomal formulation | 5–7 mg/kg iv. every 24 h | ||
| Amphotericin B lipid complex | 5–7 mg/kg iv. every 24 h | ||
|
| |||
| Triazole | Fluconazole | Loading dose: 12–25mg/kg; maintenance dose: 6–12 mg/kg/dose | Interval of dosing varies by gestational age |
| Voriconazole | No data in neonates | Use with caution in renal insufficiency | |
| Itraconazole | No data in neonates | ||
| Posaconazole | No data in neonates | ||
|
| |||
| Echinocandins | Caspofungin | 25mg/m 2 iv. every 24 h or 2 mg/kg iv. every 24 h | |
| Micafungin | 7–10 mg/kg iv. every 24 h | Higher dosing warranted in infants less than 27 weeks’ gestational age and less than 14 days of life | |
| Anidulafungin | No data in neonates | ||
|
| |||
| Flucytosine | 12.5–37.5 mg/kg enterally every 6 h | Not for use in monotherapy No parenteral formulation Carries a US FDA black box warning to use with caution in renal insufficiency |
|
iv.: Intravenous.
Patterns of resistance
Resistance patterns vary from location to location, and while multinational studies have shown that widespread antifungal resistance is not yet emerging, local data may show the opposite. For example, while some studies have shown that C. parapsilosis has retained the susceptibility to amphotericin B [42], other reports have shown resistance of C. parapsilosis to this agent [40,43]. Although surveillance has not yet identified emerging azole resistance in the setting of fluconazole prophylaxis [31], ongoing monitoring is important as these trends can take many years to detect. In locations where fluconazole is used routinely for prophylaxis, systematic monitoring should be in place to determine if strains of C. parapsilosis and other Candida spp. are developing resistance to fluconazole.
Concerns have also been raised over echinocandin resistance in C. parapsilosis isolates [44], which may be associated with acquired mutations of the fks gene encoding β-D-glucan synthase [45]. It is recognized that C. parapsilosis strains tend to have decreased susceptibility to echinocandins without displaying fks gene mutations; however, these differences do not appear to be clinically significant in most cases [46]. Indeed, worldwide surveillance of invasive Candida species, including C. parapsilosis, has shown very low rates of resistance with updated Clinical Laboratory and Standards Institute guidelines [46]. Other studies have shown very low incidence of non-wild-type C. parapsilosis, suggesting overall low incidences of mutation or acquisition of mutant fks genes [47].
Virulence factors
The focus thus far has been on clinical aspects of these important infections in a neonatal population. Clinical disease is the measurable end point of a complex interplay involving properties of the microbe that contribute to its ability to cause disease in a human host and a failure of host defense mechanisms that have evolved to prevent these infections and their consequences. Because of its historical importance and its unique virulence attributes, C. albicans has been the subject of the vast majority of work related to Candida biology and interactions with the host in health and disease. Abundant knowledge has been gained as a result of these studies. However, as increasing attention has been paid to the non-albicans species in recent years, unique aspects of the virulence and host–pathogen interactions of these fungi have come to light, and significant differences from C. albicans are emerging. These observations underscore the need to study host–pathogen interactions from a narrow focus, as even closely related pathogens can yield very different results and may therefore be amenable to unique therapeutic strategies.
Virulence factors increase a microbe’s pathogenic potential by augmenting its ability to colonize and invade its host, avoid immune detection and ultimately increase morbidity and mortality. Virulence factors associated with C. parapsilosis include its ability to adhere to a wide array of biological and prosthetic surfaces, the ability to form biofilms on implanted medical devices and secretion of hydrolytic enzymes capable of causing significant tissue damage. Recent studies have also highlighted the importance of fatty acid synthesis and storage in the role of C. parapsilosis pathogenesis. This section reviews the current information regarding these attributes of C. parapsilosis.
Adhesion & biofilm formation
Candida biofilms are fungal communities of yeast associated with biological and prosthetic surfaces and are often associated with implanted medical devices [48]. Their clinical significance is underscored by the observation that C. parapsilosis biofilms are resistant to antifungal treatment and host immune responses and are associated with increased mortality [49–51]. In comparison to C. albicans biofilms, the architecture of C. parapsilosis biofilms is much simpler [52]. C. parapsilosis biofilms are not as thick as C. albicans biofilms and are often observed as monolayers or aggregates of yeast. Additionally, unlike C. albicans biofilms in which production of filamentous forms including true hyphae and pseudohyphae plays a prominent role, C. parapsilosis does not produce true hyphae, and instead exists in either yeast or pseudohyphae cell morphologies. Interestingly, biofilm formation has been linked to cell and colony morphology, where in general, C. parapsilosis pseudohyphae are more biofilm proficient than yeast [53]. The mechanisms behind C. parapsilosis pseudohyphae production are unclear although the presence of specific amino acids has been identified to play a role [54]. Finally, although C. albicans biofilms produce an extracellular matrix that is protein rich, the extracellular matrix produced by C. parapsilosis is predominately carbohydrate [52]. Despite these differences in biofilm structure, C. parapsilosis has a much higher affinity for prosthetic material than C. albicans, especially in the presence of high glucose and lipid media [55,56]. These properties may relate to the association of C. parapsilosis infection with parenteral nutrition.
Hydrolytic enzyme production
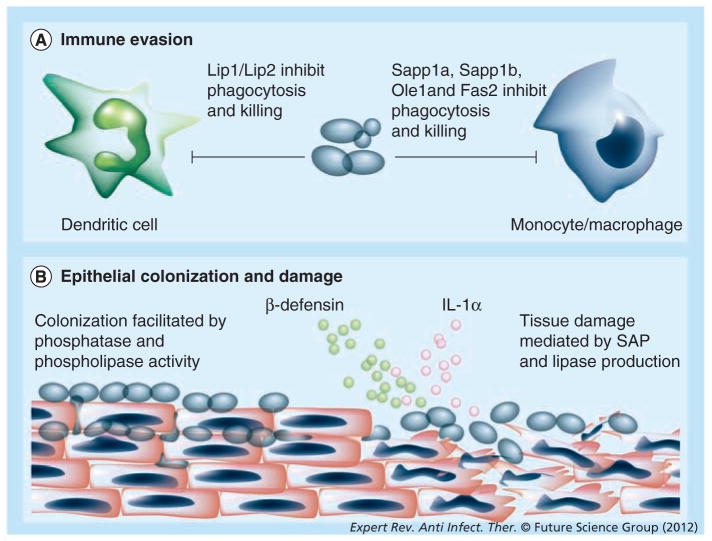
Candida species secrete hydrolytic enzymes that are associated with virulence, including secreted aspartyl proteases (SAPs), phospholipases, lipases and hemolysins. In reconstituted human tissue models of C. parapsilosis infection, inhibition of these enzymes decreased tissue damage [57,58]. In general, SAP production seems to be higher in C. albicans than C. parapsilosis, although there are some discrepancies among studies [59–61]. C. parapsilosis has three SAP genes: SAPP1, SAPP2 and SAPP3. SAPP1 has undergone gene duplication and has been referred to as SAPP1a and SAPP1b [62]. The proteolytic activity of SAPP1 and SAPP2 has been examined, with SAPP1 having an increased expression and a broader substrate specificity than SAPP2 [62,63]. The role of SAP production in C. parapsilosis systemic infections remains unclear, although SAP activity does play an important role in establishing mucocutaneous infection. Interestingly, non-blood isolates had higher protease activity than blood isolates but activity can increase during infection [58,64,65]. SAP production also plays a role in establishing vaginal infections, as non-SAP producers were incapable of causing long-term vaginitis in a rat model [66,67]. C. parapsilosis SAP production may also have a role in immune evasion, as deletion of SAPP1a and SAPP1b resulted in increased susceptibility to human serum and increased phagocytosis and killing by macrophages (Figure 2A) [62].
Figure 2. Immune response to Candida parapsilosis.
Factors involved in (A) immune evasion and (B) epithelial colonization and damage by Candida parapsilosis. See text for further details.
Lip: Lipase; SAP: Secreted aspartyl protease.
Phospholipases are important virulence factors for many fungal pathogens and contribute significantly to host damage [68]. Phospholipase activity of C. parapsilosis appears to be strain dependent as a large range of phospholipase activity has been reported [60,61,64,69–71]. The role of phospholipase in virulence remains unclear, although increased phospholipase activity is associated with increased adherence to epithelial cells (Figure 2b) [64]. Lipase production, however, is recognized to play an important role in C. parapsilosis infection. In addition to being biofilm deficient, C. parapsilosis mutants lacking lipases Cplip1 and Cplip2 also exhibited decreased virulence in a murine model of infection [72]. Interestingly, mutants exhibited decreased virulence when animals were infected by the intraperitoneal route, but not when introduced intravenously. In comparison, neonatal rats infected with C. parapsilosis lipase mutants intravenously, intragastrically and intraperitoneally had lower fungal burdens than rats infected with wild-type C. parapsilosis [73]. Cplip1 and Cplip2 double mutants also had a reduced ability to cause epithelial damage, and were phagocytosed and killed more efficiently by macrophage-like cells [72] and by dendritic cells (DCs) (Figure 2A) [74].
Hemolytic activity for C. parapsilosis, in general, has been reported to be absent or very low, although this may be a result of small sample sizes [61,75]. One study, however, looked at the hemolytic activity of 34 C. parapsilosis isolates, the majority of which exhibited weak hemolytic activity [65]. This study noted that tracheal isolates had increased hemolytic activity compared with blood isolates, indicating that perhaps hemolytic ability plays a role in colonization of specific sites. However, the role of hemolytic activity in C. parapsilosis pathogenesis remains poorly understood and more research into this area is needed.
Fatty acid synthesis & storage
Recent studies have highlighted the importance of fatty acid synthesis and storage in C. parapsilosis virulence. Surprisingly, these genes play a role in the immune evasion, biofilm formation and enhanced virulence in murine models of infection. When FAS2 (fatty acid synthase type 2) was deleted, mutant cells failed to form biofilms, were hypersensitive to human serum, and were phagocytosed and killed more efficiently by effector cells (Figure 2A) [76]. When OLE1 (stearoyl-coenzyme A desaturase 1) was deleted, mutant cells failed to produce pseudohyphae and were hypersensitive to osmotic and oxidative stress, as well as human serum [77]. In addition, ole1 mutants were phagocytosed and killed more efficiently by macrophages and exhibited decreased virulence in a murine model of infection (Figure 2A). Disruption of FIT2 (fat storage-inducing protein type 2) also increased C. parapsilosis sensitivity to osmotic stress and resulted in decreased virulence in a murine model of infection [78].
Host defense
In recent years, the number of studies focusing on host–pathogen interactions with C. parapsilosis has increased but this relationship is still poorly understood. The majority of recent studies have focused on this interaction from the pathogen’s perspective; that is, how C. parapsilosis genes or morphological traits influence interactions with the host. Very few studies have focused on the host receptors involved in the recognition and response to C. parapsilosis. Improved knowledge of these interactions will identify unique aspects of the host response to this species and could inform novel therapeutic strategies.
Receptors
To defend against pathogenic fungi, the host’s immune system must first recognize and respond to the invading pathogens by recognizing specific fungal pathogen-associated molecular patterns (PAMPs). These PAMPs are recognized by pattern recognition receptors (PRRs), which are found on a wide array of effector cells. Fungal PAMPs are generally components of the carbohydrate-rich fungal cell wall and include chitin, β-glucan and various mannan structures. PRRs include several different members of the C-type lectin receptor family and Toll-like receptors. Although recent work has defined many interactions between C. albicans PAMPs and PRRs of the innate immune system (reviewed in [79]), similarities and differences specific to C. parapsilosis remain poorly defined.
Neutrophils
Neutrophils are key mediators against fungal infection, as both neutropenic mice and humans have increased susceptibility to systemic Candida infections [80,81]. Human neutrophils underwent phagocytosis and killing of C. parapsilosis much more efficiently than C. albicans yeast, suggesting that these interactions have properties that are unique among different Candida spp. [82]. Despite the increased susceptibility of preterm neonates to infection with C. parapsilosis, neutrophils from these patients responded to Candida in a similar fashion to adult cells [83]. Neutrophil phagocytosis of C. albicans is mediated in part via β-glucan recognition by dectin-1 and complement receptor 3 [84–87], although there is an ongoing controversy about the contribution of each receptor. Neutrophil phagocytosis of C. parapsilosis, however, does not appear to be mediated by dectin-1 or complement receptor 3, as neutrophil treatment with blocking antibodies against both receptors did not inhibit phagocytosis [82]. Current work from our group has demonstrated that the lectin galectin-3 plays an important role in the increased efficiency of neutrophil phagocytosis of C. parapsilosis versus C. albicans [Linden et al., The role of galectin-3 in the human neutrophil response to candida and protection against disseminated candidiasis (2012), Submitted]. Our data suggest that C. parapsilosis induces galectin-3 secretion and that secreted galectin-3 acts as an autocrine or paracrine signal on neutrophils, augmenting phagocytosis. Interestingly, our group and others have recently found a relative deficiency of galectin-3 in neonates and decreased expression associated with lower gestational age [88]. Based on these observations, it is tempting to speculate that impaired galectin-3 expression in neonates may contribute to their enhanced susceptibility to C. parapsilosis and potentially other infections as well, but this hypothesis requires further study.
Monocytes & macrophages
Macrophages play an important role against disseminated Candida infections [89]. Interestingly, C. parapsilosis possesses several enzymes that are protective against macrophage effector functions. C. parapsilosis mutant strains deficient in Sapp1a and Sapp1b were phagocytosed and killed more efficiently by human monocytes and macrophages than their wild-type counterparts [62]. Deletion of genes involved in fatty acid synthesis, including OLE1 and FAS2 also increased phagocytosis and macrophage killing of the mutant C. parapsilosis strain compared with the wild-type (Figure 2A) [76,77,90].
Dendritic cells
Located in the skin and mucosae, DCs are an important link between innate and adaptive immunity during infection. DCs protect the host as antigen-presenting cells capable of mounting Th1, Th2 or Th17 responses against C. albicans infection [91–94]. DCs also act as ‘nonprofessional phagocytes’ capable of ingesting and killing Candida yeast. In response to C. parapsilosis, DCs produced pseudopodial protrusions called fungipods [95]. Interestingly, DCs produced more fungipods in response to C. parapsilosis than in response to C. tropicalis or C. albicans. Fungipod formation was mediated by recognition through the mannose receptor, not dectin-1 or DC-SIGN, suggesting that C. parapsilosis mannan may have unique inflammatory properties. The fungipod was suggested to aid in DC effector functions by improving particle binding and retention, possibly augmenting phagocytosis by size discrimination. Work by another group demonstrated that C. parapsilosis lipases provided protection from the immune system by inhibiting DC effector function [74]. Lipase-deficient C. parapsilosis mutants were phagocytosed and killed more efficiently by both immature and mature DCs, possibly via increased lysosome maturation (Figure 2A). Lipase activity also reduced cytokine production by DCs, as the lipase mutants induced more cytokine production than wild-type C. parapsilosis.
Epithelial cells
Adherence to host cells is a critical aspect in Candida pathogenesis, and adhesion to epithelial cells is probably important for colonization and transmission of C. parapsilosis. The majority of work focusing on C. parapsilosis interactions with epithelial cells has used oral epithelial cells, including carcinoma cell lines, primary cell lines and reconstituted oral human epithelial tissue. Adhesion of C. parapsilosis to buccal epithelial cells has been positively correlated with many factors including specific cell surface carbohydrates [96]. Although C. parapsilosis is capable of adhering to oral epithelial cells, it does not have the same invasive qualities associated with production of hyphae by C. albicans. Despite its poor ability to invade, C. parapsilosis was capable of causing extensive tissue damage in a model of reconstructed human oral epithelial tissue [58]. Colonization and invasion was strain dependent and did not depend on cell morphology (yeast vs pseudohyphae). Another group using models of both reconstituted oral epithelium and reconstituted epidermal tissue saw similar results including strain-to-strain variability, poor invasion and extensive tissue disruption and damage [57]. Interestingly, a strain producing pseudohyphae was noted to be more invasive and destructive in the oral epithelium model than yeast isolates. Because of the low number of strains used in each of these studies, the relationship of cell morphology and adherence to epithelial cells remains uncertain. Tissue destruction is mediated by C. parapsilosis production of SAP and lipases, as inhibition of these enzymes reduced epithelial damage but not invasion (Figure 2B) [57,58]. These results were confirmed using mutant strains of C. parapsilosis deficient in the lipase genes CpLIP1 and CpLIP2 [72].
Epithelial cells can discriminate between C. albicans yeast and hyphae by activation of NF-κB and different stages of the biphasic MAPK response. Epithelial cells responded to both yeast and hyphae by activation of the first MAPK phase via c-Jun activation. In response to hyphae, however, epithelial cells activated the second MAPK phase via MKP1 and c-Fos activation, which correlates with proinflammatory responses [97]. Although C. parapsilosis was capable of activating NF-κB, it failed to activate the second MAPK phase responsible for hypha recognition [98]. Notably, the C. parapsilosis strain used failed to form pseudohyphae. C. parapsilosis also induced less secretion of the proinflammatory cytokines G-CSF, GMSF and IL-6 when compared with C. albicans. C. parapsilosis was capable of causing epithelial damage and inducing secretion of the damage-associated cytokine IL-1α (Figure 2B). By contrast, another study using a filament-producing environmental strain of C. parapsilosis demonstrated that infection caused an increase in epithelial gene expression of TLR2, TLR4 and TLR6. Interestingly, these epithelial cells inhibited fungal growth, presumably by increased expression of IL-1β, TNF-α and IFN-γ and secretion of human β-defensins [99]. Taken together, these studies suggest that epithelial cells may respond differently to differing C. parapsilosis morphologies.
Endothelial cells
Interactions between Candida and endothelial cells play a critical role in the pathogenesis of disseminated candidiasis. In hematogenously disseminated disease, endothelial cells provide a barrier between pathogens circulating in blood vessels and uninfected host tissue. Though much work has focused on understanding how C. albicans escapes the vascular lumen, little work has examined the interaction of C. parapsilosis with endothelial cells. C. albicans was capable of inducing its own endocytosis by human umbilical vein endothelial cells (HUVECs) by binding of Als3 and Ssa1 to N-cadherin [100–102]. C. albicans invaded human brain micro-vascular endothelial cells (HBMECs) by again inducing its own endocytosis by endothelial recognition of Als3 and Ssa1. In comparison to HUVEC, however, HBMEC recognized Als3 using heat-shock protein GP96, a receptor expressed by HBMEC but not HUVEC [103]. Ssa1-mediated endocytosis by HBMEC occurred through an undefined receptor. The extent to which C. parapsilosis exhibits similar properties is essentially unexplored.
Expert commentary & five-year view
Systemic candidiasis remains a common problem among the immunocompromised. Disseminated disease occurs in the setting of bone marrow transplant recipients, patients undergoing chemotherapy for solid organ and hematologic malignancies, and in cases of mucosal compromise such as gastrointestinal surgery or severe burns. Neutropenia or defects in neutrophil function are known to be important risk factors in the development of invasive candidiasis, as are common components of ICU care including endotracheal intubation, central venous and urinary catheters, parenteral nutrition, broad-spectrum antibiotics and corticosteroid use. Despite the availability of current antifungal agents, the morbidity and mortality related to these infections remains unsuitably high.
Premature infants constitute another group of patients at high risk for this disease and its complications. Although they share some of the risk factors common to ICU care in other patient populations, their susceptibility to these infections is also likely to be related to unique mechanisms of compromised immunity. Despite sharing many risk factors with other patient populations that develop candidiasis, the high frequency with which C. parapsilosis is the causative organism in this population is quite unique, suggesting that features of this particular species and the neonatal host make infection with this organism more likely to occur. We have reviewed a number of features related to the pathogenesis of C. parapsilosis that differ from C. albicans, supporting the notion that this species brings a specific set of properties to the host–pathogen interface that may affect the likelihood of disease in a specific host setting. However, our understanding of how these virulence properties manifest in vivo, particularly in a neonatal host, is in its earliest stages with much work still to be done.
The development and function of the innate and adaptive arms of the neonatal immune system have been the subject of much study, and many specific mechanisms that contribute to the enhanced susceptibility to infection in neonates have been described. Considerably less attention has been focused on the specific developmental deficiencies in immune function that increase the risk for fungal disease. Because quantitative or qualitative defects in neutrophil function, for example, are known to contribute to invasive fungal infection, yet neonates who develop these infections are rarely neutropenic, our group tested specific adult and infant neutrophil functions when confronted with C. parapsilosis and C. albicans [82,83]. No significant differences among preterm infant, term infant or adult neutrophils were detected in these studies, but unexpected, dramatic differences in phagocytosis efficiency of these two species were observed. Our more recent finding of a role for galectin-3 in neutrophils confronting C. parapsilosis coupled with a relative deficiency of galectin-3 in neonatal serum suggests that other mechanisms that affect neutrophil function may be at play [Linden et al. The role of galectin-3 in the human neutrophil response to candida and protection against disseminated candidiasis (2012), Submitted]. This example is meant to illustrate that gaining an understanding of how susceptibility of a specific patient population relates to a specific pathogen is likely to require study tailored to that interaction. Although important and instructive, more general observations of immune function, or responses of a host to a closely related species of pathogen need to be confirmed in the specific host–pathogen interaction of interest.
Treatment of fungal infections has relied on conventional chemotherapeutic agents for decades. The echinocandins represent the newest class of antifungal drugs, and the first to target fungal cell wall synthesis. These agents are proving to be a useful addition to the antifungal armamentarium, but they have yet to be studied rigorously in neonates. However, even with available antifungal therapies, once these infections are detected in premature infants, the result is either death or neurodevelopmental sequelae in the vast majority of infected infants [1]. The most effective method to improve these outcomes is prevention. Indeed, antifungal prophylaxis as described above has been well studied and shown to be effective, particularly in high-incidence settings. Although not observed so far, concern remains regarding the emergence of resistance or replacement of current species with fungi that carry intrinsic resistance, potentially resulting in resistance patterns that preclude the use of an entire class of antifungals. With limited options, new drug development should be encouraged well before this eventuality. Newer strategies that avoid antifungal drugs and their selective pressures are being investigated, such as lactoferrin [37]. The promising results suggest that continued investigation aimed at augmenting or replacing components of innate immunity in patients at risk may offer new strategies that are safe and effective in prevention. When prophylactic strategies fail, outcomes may also be improved by more accurate and timely diagnosis of fungal infection. Clinical signs of fungal infection are nonspecific and require a high index of suspicion. Additionally, culture-based methods are insensitive, which can lead to costly delays in initiation of appropriate therapy. Rapid diagnostics are an area of intense interest, and if developed will probably facilitate timely and accurate detection and treatment and improve outcomes.
As infections with non-albicans Candida species have increased in prevalence, so has interest in the unique properties of these organisms and how they manifest in the human host. Likewise, mechanisms of antifungal host defense that are poorly developed in neonates are beginning to be elucidated. Future work in these areas is likely to not only improve our understanding of these basic mechanisms and interactions, but inform the development of newer, more effective preventive, diagnostic and therapeutic strategies. Future innovations will not only improve the outcomes of premature infants, but will hopefully extend to broader groups of patients at risk.
Key issues.
Although Candida albicans is the most common species associated with invasive candidiasis, incidence of infection with Candida parapsilosis has increased and varies by geographic region and age group. Premature neonates have the highest reported incidence of infection with this species relative to other patients at risk.
Antifungal prophylaxis with systemic or mucosal agents in premature infants is effective at reducing rates of invasive candidiasis, particularly in high-prevalence settings. Although not evident to date, concerns about emerging resistance with these strategies remain.
Echinocandins are a useful addition to available antifungal agents, but there are few data available on their use in neonates.
Virulence properties of C. parapsilosis include its tendency to grow as a biofilm, which increases its resistance to immune cells and antifungal agents, and production of hydrolytic enzymes. Gene products involved in fatty acid synthesis and storage appear to have particular importance in C. parapsilosis virulence.
Interactions of C. parapsilosis with host cells are beginning to be defined and contrasted to prior studies with C. albicans. Neutrophils undergo phagocytosis of C. parapsilosis yeast with much higher efficiency than C. albicans yeast and galectin-3 is involved in this process. Dendritic cells undergo unique morphological changes (‘fungipods’) in response to C. parapsilosis relative to other Candida species. Epithelial cells respond to C. parapsilosis differently than C. albicans. These observations underscore the need to study these interactions in a species-specific manner.
Footnotes
For reprint orders, please contact reprints@expert-reviews.com
Financial and competing interests disclosure
Work in JM Bliss’ laboratory was supported by grants from the National Center for Research Resources (5P20RR018728-10) and the National Institute of General Medical Sciences (8P20GM103537-10) from the NIH. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1•.Benjamin DK, Jr, Stoll BJ, Fanaroff AA, et al. National Institute of Child Health and Human Development Neonatal Research Network. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics. 2006;117(1):84–92. doi: 10.1542/peds.2004-2292. Provided neurodevelopmetal follow-up of a large cohort of infants of <1000 g birthweight with invasive candidiasis and demonstrated very high rates of death or neurodevelopmental impairment. Also demonstrated higher mortality risk with delayed removal or replacement of central venous catheters. [DOI] [PubMed] [Google Scholar]
- 2.Trofa D, Gácser A, Nosanchuk JD. Candida parapsilosis, an emerging fungal pathogen. Clin Microbiol Rev. 2008;21(4):606–625. doi: 10.1128/CMR.00013-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Asbeck EC, Clemons KV, Stevens DA. Candida parapsilosis: a review of its epidemiology, pathogenesis, clinical aspects, typing and antimicrobial susceptibility. Crit Rev Microbiol. 2009;35(4):283–309. doi: 10.3109/10408410903213393. [DOI] [PubMed] [Google Scholar]
- 4.Tavanti A, Davidson AD, Gow NA, Maiden MC, Odds FC. Candida orthopsilosis and Candida metapsilosis spp. nov to replace Candida parapsilosis groups II and III. J Clin Microbiol. 2005;43(1):284–292. doi: 10.1128/JCM.43.1.284-292.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomez-Lopez A, Alastruey-Izquierdo A, Rodriguez D, et al. Barcelona Candidemia Project Study Group. Prevalence and susceptibility profile of Candida metapsilosis and Candida orthopsilosis: results from population-based surveillance of candidemia in Spain. Antimicrob Agents Chemother. 2008;52(4):1506–1509. doi: 10.1128/AAC.01595-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lockhart SR, Messer SA, Pfaller MA, Diekema DJ. Geographic distribution and antifungal susceptibility of the newly described species Candida orthopsilosis and Candida metapsilosis in comparison to the closely related species Candida parapsilosis. J Clin Microbiol. 2008;46(8):2659–2664. doi: 10.1128/JCM.00803-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7•.Falagas ME, Roussos N, Vardakas KZ. Relative frequency of albicans and the various non-albicans Candida spp among candidemia isolates from inpatients in various parts of the world: a systematic review. Int J Infect Dis. 2010;14(11):e954–e966. doi: 10.1016/j.ijid.2010.04.006. A systematic review of candidemia cases throughout the world, demonstrating marked geographic variation in the distribution of albicans and non-albicans species. [DOI] [PubMed] [Google Scholar]
- 8.Singhi S, Rao DS, Chakrabarti A. Candida colonization and candidemia in a pediatric intensive care unit. Pediatr Crit Care Med. 2008;9(1):91–95. doi: 10.1097/01.PCC.0000298643.48547.83. [DOI] [PubMed] [Google Scholar]
- 9.Parm U, Metsvaht T, Sepp E, et al. Risk factors associated with gut and nasopharyngeal colonization by common Gram-negative species and yeasts in neonatal intensive care units patients. Early Hum Dev. 2011;87(6):391–399. doi: 10.1016/j.earlhumdev.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 10.el-Mohandes AE, Johnson-Robbins L, Keiser JF, Simmens SJ, Aure MV. Incidence of Candida parapsilosis colonization in an intensive care nursery population and its association with invasive fungal disease. Pediatr Infect Dis J. 1994;13(6):520–524. doi: 10.1097/00006454-199406000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Gagneur A, Sizun J, Vernotte E, et al. Low rate of Candida parapsilosis-related colonization and infection in hospitalized preterm infants: a one-year prospective study. J Hosp Infect. 2001;48(3):193–197. doi: 10.1053/jhin.2001.1007. [DOI] [PubMed] [Google Scholar]
- 12.Hernández-Castro R, Arroyo-Escalante S, Carrillo-Casas EM, et al. Outbreak of Candida parapsilosis in a neonatal intensive care unit: a health care workers source. Eur J Pediatr. 2010;169(7):783–787. doi: 10.1007/s00431-009-1109-7. [DOI] [PubMed] [Google Scholar]
- 13.van Asbeck EC, Huang YC, Markham AN, Clemons KV, Stevens DA. Candida parapsilosis fungemia in neonates: genotyping results suggest healthcare workers hands as source, and review of published studies. Mycopathologia. 2007;164(6):287–293. doi: 10.1007/s11046-007-9054-3. [DOI] [PubMed] [Google Scholar]
- 14.Huang YC, Su LH, Wu TL, Lin TY. Genotyping analysis of colonizing candidal isolates from very-low-birthweight infants in a neonatal intensive care unit. J Hosp Infect. 2004;58(3):200–203. doi: 10.1016/j.jhin.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 15.Bliss JM, Basavegowda KP, Watson WJ, Sheikh AU, Ryan RM. Vertical and horizontal transmission of Candida albicans in very low birth weight infants using DNA fingerprinting techniques. Pediatr Infect Dis J. 2008;27(3):231–235. doi: 10.1097/INF.0b013e31815bb69d. [DOI] [PubMed] [Google Scholar]
- 16.Carter JE, Laurini JA, Evans TN, Estrada B. Neonatal Candida parapsilosis meningitis and empyema related to epidural migration of a central venous catheter. Clin Neurol Neurosurg. 2008;110(6):614–618. doi: 10.1016/j.clineuro.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Huang YC, Lin TY, Lien RI, et al. Candidaemia in special care nurseries: comparison of albicans and parapsilosis infection. J Infect. 2000;40(2):171–175. doi: 10.1053/jinf.2000.0638. [DOI] [PubMed] [Google Scholar]
- 18.Clerihew L, Lamagni TL, Brocklehurst P, McGuire W. Candida parapsilosis infection in very low birthweight infants. Arch Dis Child Fetal Neonatal Ed. 2007;92(2):F127–F129. doi: 10.1136/fnn.2006.097758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faix RG. Invasive neonatal candidiasis: comparison of albicans and parapsilosis infection. Pediatr Infect Dis J. 1992;11(2):88–93. [PubMed] [Google Scholar]
- 20.Levy I, Rubin LG, Vasishtha S, Tucci V, Sood SK. Emergence of Candida parapsilosis as the predominant species causing candidemia in children. Clin Infect Dis. 1998;26(5):1086–1088. doi: 10.1086/520277. [DOI] [PubMed] [Google Scholar]
- 21.Chow JK, Golan Y, Ruthazer R, et al. Factors associated with candidemia caused by non-albicans Candida species versus Candida albicans in the intensive care unit. Clin Infect Dis. 2008;46(8):1206–1213. doi: 10.1086/529435. [DOI] [PubMed] [Google Scholar]
- 22.Pfaller MA, Castanheira M, Messer SA, Moet GJ, Jones RN. Variation in Candida spp. distribution and antifungal resistance rates among bloodstream infection isolates by patient age: report from the SENTRY Antimicrobial Surveillance Program (2008–2009) Diagn Microbiol Infect Dis. 2010;68(3):278–283. doi: 10.1016/j.diagmicrobio.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 23.Xess I, Jain N, Hasan F, Mandal P, Banerjee U. Epidemiology of candidemia in a tertiary care centre of north India: 5-year study. Infection. 2007;35(4):256–259. doi: 10.1007/s15010-007-6144-6. [DOI] [PubMed] [Google Scholar]
- 24.Fridkin SK, Kaufman D, Edwards JR, Shetty S, Horan T. Changing incidence of Candida bloodstream infections among NICU patients in the United States: 1995–2004. Pediatrics. 2006;117(5):1680–1687. doi: 10.1542/peds.2005-1996. [DOI] [PubMed] [Google Scholar]
- 25.Clerihew L, Lamagni TL, Brocklehurst P, McGuire W. Invasive fungal infection in very low birthweight infants: national prospective surveillance study. Arch Dis Child Fetal Neonatal Ed. 2006;91(3):F188–F192. doi: 10.1136/adc.2005.082024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neu N, Malik M, Lunding A, et al. Epidemiology of candidemia at a children’s hospital, 2002 to 2006. Pediatr Infect Dis J. 2009;28(9):806–809. doi: 10.1097/INF.0b013e3181a0d78d. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez D, Almirante B, Park BJ, et al. Barcelona Candidemia Project Study Group. Candidemia in neonatal intensive care units: Barcelona, Spain. Pediatr Infect Dis J. 2006;25(3):224–229. doi: 10.1097/01.inf.0000202127.43695.06. [DOI] [PubMed] [Google Scholar]
- 28••.Clerihew L, Austin N, McGuire W. Prophylactic systemic antifungal agents to prevent mortality and morbidity in very low birth weight infants. Cochrane Database Syst Rev. 2007;(4):CD003850. doi: 10.1002/14651858.CD003850.pub3. A systematic review demonstrating efficacy of systemic antifungal prophylaxis for prevention of candidiasis in premature neonates. [DOI] [PubMed] [Google Scholar]
- 29.Aydemir C, Oguz SS, Dizdar EA, et al. Randomised controlled trial of prophylactic fluconazole versus nystatin for the prevention of fungal colonisation and invasive fungal infection in very low birth weight infants. Arch Dis Child Fetal Neonatal Ed. 2011;96(3):F164–F168. doi: 10.1136/adc.2009.178996. [DOI] [PubMed] [Google Scholar]
- 30.Austin N, Darlow BA, McGuire W. Prophylactic oral/topical non-absorbed antifungal agents to prevent invasive fungal infection in very low birth weight infants. Cochrane Database Syst Rev. 2009;(4):CD003478. doi: 10.1002/14651858.CD003478.pub3. [DOI] [PubMed] [Google Scholar]
- 31.Manzoni P, Leonessa M, Galletto P, et al. Routine use of fluconazole prophylaxis in a neonatal intensive care unit does not select natively fluconazole-resistant Candida subspecies. Pediatr Infect Dis J. 2008;27(8):731–737. doi: 10.1097/INF.0b013e318170bb0c. [DOI] [PubMed] [Google Scholar]
- 32.Poikonen E, Lyytikäinen O, Anttila VJ, et al. Secular trend in candidemia and the use of fluconazole in Finland, 2004–2007. BMC Infect Dis. 2010;10:312. doi: 10.1186/1471-2334-10-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarvikivi E, Lyytikäinen O, Soll DR, et al. Emergence of fluconazole resistance in a Candida parapsilosis strain that caused infections in a neonatal intensive care unit. J Clin Microbiol. 2005;43(6):2729–2735. doi: 10.1128/JCM.43.6.2729-2735.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaufman D, Boyle R, Hazen KC, Patrie JT, Robinson M, Donowitz LG. Fluconazole prophylaxis against fungal colonization and infection in preterm infants. N Engl J Med. 2001;345(23):1660–1666. doi: 10.1056/NEJMoa010494. [DOI] [PubMed] [Google Scholar]
- 35.Mann PA, McNicholas PM, Chau AS, et al. Impact of antifungal prophylaxis on colonization and azole susceptibility of Candida species. Antimicrob Agents Chemother. 2009;53(12):5026–5034. doi: 10.1128/AAC.01031-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoder BA, Sutton DA, Winter V, Coalson JJ. Resistant Candida parapsilosis associated with long term fluconazole prophylaxis in an animal model. Pediatr Infect Dis J. 2004;23(7):687–688. doi: 10.1097/01.inf.0000128777.22022.47. [DOI] [PubMed] [Google Scholar]
- 37••.Manzoni P, Stolfi I, Messner H, et al. Italian Task Force for the Study and Prevention of Neonatal Fungal Infections–the Italian Society of Neonatology. Bovine lactoferrin prevents invasive fungal infections in very low birth weight infants: a randomized controlled trial. Pediatrics. 2012;129(1):116–123. doi: 10.1542/peds.2011-0279. A multicentered randomized controlled trial demonstrating that oral administration of bovine lactoferrin from birth in premature infants significantly lowered the incidence of invasive candidiasis, with no effect on colonization. [DOI] [PubMed] [Google Scholar]
- 38.Pappas PG, Kauffman CA, Andes D, et al. Infectious Diseases Society of America. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(5):503–535. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Testoni D, Smith PB, Benjamin DK., Jr The use of antifungal therapy in neonatal intensive care. Clin Perinatol. 2012;39(1):83–98. doi: 10.1016/j.clp.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yalaz M, Akisu M, Hilmioglu S, Calkavur S, Cakmak B, Kultursay N. Successful caspofungin treatment of multidrug resistant Candida parapsilosis septicaemia in an extremely low birth weight neonate. Mycoses. 2006;49(3):242–245. doi: 10.1111/j.1439-0507.2006.01220.x. [DOI] [PubMed] [Google Scholar]
- 41.Odio CM, Araya R, Pinto LE, et al. Caspofungin therapy of neonates with invasive candidiasis. Pediatr Infect Dis J. 2004;23(12):1093–1097. [PubMed] [Google Scholar]
- 42.González GM, Elizondo M, Ayala J. Trends in species distribution and susceptibility of bloodstream isolates of Candida collected in Monterrey, Mexico, to seven antifungal agents: results of a 3-year (2004 to 2007) surveillance study. J Clin Microbiol. 2008;46(9):2902–2905. doi: 10.1128/JCM.00937-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zaoutis TE, Foraker E, McGowan KL, et al. Antifungal susceptibility of Candida spp. isolated from pediatric patients: a survey of 4 children’s hospitals. Diagn Microbiol Infect Dis. 2005;52(4):295–298. doi: 10.1016/j.diagmicrobio.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 44.Moudgal V, Little T, Boikov D, Vazquez JA. Multiechinocandin- and multiazole-resistant Candida parapsilosis isolates serially obtained during therapy for prosthetic valve endocarditis. Antimicrob Agents Chemother. 2005;49(2):767–769. doi: 10.1128/AAC.49.2.767-769.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghannoum MA, Chen A, Buhari M, et al. Differential in vitro activity of anidulafungin, caspofungin and micafungin against Candida parapsilosis isolates recovered from a burn unit. Clin Microbiol Infect. 2009;15(3):274–279. doi: 10.1111/j.1469-0691.2008.02660.x. [DOI] [PubMed] [Google Scholar]
- 46.Pfaller MA, Boyken L, Hollis RJ, et al. In vitro susceptibility of invasive isolates of Candida spp. to anidulafungin, caspofungin, and micafungin: six years of global surveillance. J Clin Microbiol. 2008;46(1):150–156. doi: 10.1128/JCM.01901-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pfaller M, Boyken L, Hollis R, et al. Use of epidemiological cutoff values to examine 9-year trends in susceptibility of Candida species to anidulafungin, caspofungin, and micafungin. J Clin Microbiol. 2011;49(2):624–629. doi: 10.1128/JCM.02120-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blankenship JR, Mitchell AP. How to build a biofilm: a fungal perspective. Curr Opin Microbiol. 2006;9(6):588–594. doi: 10.1016/j.mib.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 49.Giovanna P, Dimitrios P, Giovanni DD, Fabio R, Rosaria CM. Ambroxol influences voriconazole resistance of Candida parapsilosis biofilm. FEMS Yeast Res. 2012;12(4):430–438. doi: 10.1111/j.1567-1364.2012.00792.x. [DOI] [PubMed] [Google Scholar]
- 50.Tumbarello M, Posteraro B, Trecarichi EM, et al. Biofilm production by Candida species and inadequate antifungal therapy as predictors of mortality for patients with candidemia. J Clin Microbiol. 2007;45(6):1843–1850. doi: 10.1128/JCM.00131-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Katragkou A, Chatzimoschou A, Simitsopoulou M, Georgiadou E, Roilides E. Additive antifungal activity of anidulafungin and human neutrophils against Candida parapsilosis biofilms. J Antimicrob Chemother. 2011;66(3):588–591. doi: 10.1093/jac/dkq466. [DOI] [PubMed] [Google Scholar]
- 52.Silva S, Henriques M, Martins A, Oliveira R, Williams D, Azeredo J. Biofilms of non-Candida albicans Candida species: quantification, structure and matrix composition. Med Mycol. 2009;47(7):681–689. doi: 10.3109/13693780802549594. [DOI] [PubMed] [Google Scholar]
- 53.Laffey SF, Butler G. Phenotype switching affects biofilm formation by Candida parapsilosis. Microbiology (Reading, Engl) 2005;151(Pt 4):1073–1081. doi: 10.1099/mic.0.27739-0. [DOI] [PubMed] [Google Scholar]
- 54.Kim SK, El Bissati K, Ben Mamoun C. Amino acids mediate colony and cell differentiation in the fungal pathogen Candida parapsilosis. Microbiology (Reading, Engl) 2006;152(Pt 10):2885–2894. doi: 10.1099/mic.0.29180-0. [DOI] [PubMed] [Google Scholar]
- 55.Nosek J, Holesova Z, Kosa P, Gacser A, Tomaska L. Biology and genetics of the pathogenic yeast Candida parapsilosis. Curr Genet. 2009;55(5):497–509. doi: 10.1007/s00294-009-0268-4. [DOI] [PubMed] [Google Scholar]
- 56.Silva S, Negri M, Henriques M, Oliveira R, Williams DW, Azeredo J. Adherence and biofilm formation of non-Candida albicans Candida species. Trends Microbiol. 2011;19(5):241–247. doi: 10.1016/j.tim.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 57.Gácser A, Schäfer W, Nosanchuk JS, Salomon S, Nosanchuk JD. Virulence of Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis in reconstituted human tissue models. Fungal Genet Biol. 2007;44(12):1336–1341. doi: 10.1016/j.fgb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 58.Silva S, Henriques M, Oliveira R, et al. Characterization of Candida parapsilosis infection of an in vitro reconstituted human oral epithelium. Eur J Oral Sci. 2009;117(6):669–675. doi: 10.1111/j.1600-0722.2009.00677.x. [DOI] [PubMed] [Google Scholar]
- 59.Bramono K, Yamazaki M, Tsuboi R, Ogawa H. Comparison of proteinase, lipase and alpha-glucosidase activities from the clinical isolates of Candida species. Jpn J Infect Dis. 2006;59(2):73–76. [PubMed] [Google Scholar]
- 60.D’Eça A, Júnior, Silva AF, Rosa FC, Monteiro SG, de Maria Silva Figueiredo P, de Andrade Monteiro C. In vitro differential activity of phospholipases and acid proteinases of clinical isolates of Candida. Rev Soc Bras Med Trop. 2011;44(3):334–338. doi: 10.1590/s0037-86822011005000036. [DOI] [PubMed] [Google Scholar]
- 61.Issa SY, Badran EF, Aqel KF, Shehabi AA. Epidemiological characteristics of Candida species colonizing oral and rectal sites of Jordanian infants. BMC Pediatr. 2011;11:79. doi: 10.1186/1471-2431-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Horváth P, Nosanchuk JD, Hamari Z, Vágvölgyi C, Gácser A. The identification of gene duplication and the role of secreted aspartyl proteinase 1 in Candida parapsilosis virulence. J Infect Dis. 2012;205(6):923–933. doi: 10.1093/infdis/jir873. [DOI] [PubMed] [Google Scholar]
- 63.Hrusková-Heidingsfeldová O, Dostál J, Majer F, Havlíkova J, Hradilek M, Pichová I. Two aspartic proteinases secreted by the pathogenic yeast Candida parapsilosis differ in expression pattern and catalytic properties. Biol Chem. 2009;390(3):259–268. doi: 10.1515/BC.2009.034. [DOI] [PubMed] [Google Scholar]
- 64.Dagdeviren M, Cerikcioglu N, Karavus M. Acid proteinase, phospholipase and adherence properties of Candida parapsilosis strains isolated from clinical specimens of hospitalised patients. Mycoses. 2005;48(5):321–326. doi: 10.1111/j.1439-0507.2005.01145.x. [DOI] [PubMed] [Google Scholar]
- 65.França EJ, Furlaneto-Maia L, Quesada RM, Favero D, Oliveira MT, Furlaneto MC. Haemolytic and proteinase activities in clinical isolates of Candida parapsilosis and Candida tropicalis with reference to the isolation anatomic site. Mycoses. 2011;54(4):e44–e51. doi: 10.1111/j.1439-0507.2009.01825.x. [DOI] [PubMed] [Google Scholar]
- 66.Agatensi L, Franchi F, Mondello F, et al. Vaginopathic and proteolytic Candida species in outpatients attending a gynaecology clinic. J Clin Pathol. 1991;44(10):826–830. doi: 10.1136/jcp.44.10.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.De Bernardis F, Mondello F, San Millàn R, Pontòn J, Cassone A. Biotyping and virulence properties of skin isolates of Candida parapsilosis. J Clin Microbiol. 1999;37(11):3481–3486. doi: 10.1128/jcm.37.11.3481-3486.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kuhn DM, Chandra J, Mukherjee PK, Ghannoum MA. Comparison of biofilms formed by Candida albicans and Candida parapsilosis on bioprosthetic surfaces. Infect Immun. 2002;70(2):878–888. doi: 10.1128/iai.70.2.878-888.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gokce G, Cerikcioglu N, Yagci A. Acid proteinase, phospholipase, and biofilm production of Candida species isolated from blood cultures. Mycopathologia. 2007;164(6):265–269. doi: 10.1007/s11046-007-9053-4. [DOI] [PubMed] [Google Scholar]
- 70.Marcos-Arias C, Eraso E, Madariaga L, Quindós G. In vitro activities of natural products against oral Candida isolates from denture wearers. BMC Complement Altern Med. 2011;11:119. doi: 10.1186/1472-6882-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Matsumoto FE, Gandra RF, Ruiz LS, et al. Yeasts isolated from blood and catheter in children from a public hospital of São Paulo, Brazil. Mycopathologia. 2002;154(2):63–69. doi: 10.1023/a:1015540224658. [DOI] [PubMed] [Google Scholar]
- 72.Gácser A, Trofa D, Schäfer W, Nosanchuk JD. Targeted gene deletion in Candida parapsilosis demonstrates the role of secreted lipase in virulence. J Clin Invest. 2007;117(10):3049–3058. doi: 10.1172/JCI32294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73••.Trofa D, Soghier L, Long C, Nosanchuk JD, Gacser A, Goldman DL. A rat model of neonatal candidiasis demonstrates the importance of lipases as virulence factors for Candida albicans and Candida parapsilosis. Mycopathologia. 2011;172(3):169–178. doi: 10.1007/s11046-011-9429-3. Described a useful animal model for neonatal candidiasis in which neonates are more susceptible than adults and further demonstrated the role of Candida parapsilosis lipase in virulence. [DOI] [PubMed] [Google Scholar]
- 74.Nagy I, Filkor K, Németh T, Hamari Z, Vágvölgyi C, Gácser A. In vitro interactions of Candida parapsilosis wild type and lipase deficient mutants with human monocyte derived dendritic cells. BMC Microbiol. 2011;11:122. doi: 10.1186/1471-2180-11-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seneviratne CJ, Wong SS, Yuen KY, et al. Antifungal susceptibility and virulence attributes of bloodstream isolates of Candida from Hong Kong and Finland. Mycopathologia. 2011;172(5):389–395. doi: 10.1007/s11046-011-9444-4. [DOI] [PubMed] [Google Scholar]
- 76.Nguyen LN, Trofa D, Nosanchuk JD. Fatty acid synthase impacts the pathobiology of Candida parapsilosis in vitro and during mammalian infection. PLoS ONE. 2009;4(12):e8421. doi: 10.1371/journal.pone.0008421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nguyen LN, Nosanchuk JD. Lipid droplet formation protects against gluco/ lipotoxicity in Candida parapsilosis: an essential role of fatty acid desaturase Ole1. Cell Cycle. 2011;10(18):3159–3167. doi: 10.4161/cc.10.18.16932. [DOI] [PubMed] [Google Scholar]
- 78.Nguyen LN, Hamari Z, Kadereit B, et al. Candida parapsilosis fat storage-inducing transmembrane (FIT) protein 2 regulates lipid droplet formation and impacts virulence. Microbes Infect. 2011;13(7):663–672. doi: 10.1016/j.micinf.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 79.Netea MG, Maródi L. Innate immune mechanisms for recognition and uptake of Candida species. Trends Immunol. 2010;31(9):346–353. doi: 10.1016/j.it.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 80.Dutta A, Palazzi DL. Candida non-albicans versus Candida albicans fungemia in the non-neonatal pediatric population. Pediatr Infect Dis J. 2011;30(8):664–668. doi: 10.1097/INF.0b013e318213da0f. [DOI] [PubMed] [Google Scholar]
- 81.Mahieu LM, Van Gasse N, Wildemeersch D, Jansens H, Ieven M. Number of sites of perinatal Candida colonization and neutropenia are associated with nosocomial candidemia in the neonatal intensive care unit patient. Pediatr Crit Care Med. 2010;11(2):240–245. doi: 10.1097/PCC.0b013e3181b808fb. [DOI] [PubMed] [Google Scholar]
- 82•.Linden JR, Maccani MA, Laforce-Nesbitt SS, Bliss JM. High efficiency opsoninindependent phagocytosis of Candida parapsilosis by human neutrophils. Med Mycol. 2010;48(2):355–364. doi: 10.1080/13693780903164566. Demonstrated phagocytosis and killing of C. parapsilosis yeast by human neutrophils that was far more efficient than of Candida albicans yeast. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Destin KG, Linden JR, Laforce-Nesbitt SS, Bliss JM. Oxidative burst and phagocytosis of neonatal neutrophils confronting Candida albicans and Candida parapsilosis. Early Hum Dev. 2009;85(8):531–535. doi: 10.1016/j.earlhumdev.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kennedy AD, Willment JA, Dorward DW, Williams DL, Brown GD, DeLeo FR. Dectin-1 promotes fungicidal activity of human neutrophils. Eur J Immunol. 2007;37(2):467–478. doi: 10.1002/eji.200636653. [DOI] [PubMed] [Google Scholar]
- 85.Li X, Utomo A, Cullere X, et al. The β-glucan receptor Dectin-1 activates the integrin Mac-1 in neutrophils via Vav protein signaling to promote Candida albicans clearance. Cell Host Microbe. 2011;10(6):603–615. doi: 10.1016/j.chom.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van Bruggen R, Drewniak A, Jansen M, et al. Complement receptor 3, not Dectin-1, is the major receptor on human neutrophils for beta-glucan-bearing particles. Mol Immunol. 2009;47(2–3):575–581. doi: 10.1016/j.molimm.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 87.van Bruggen R, Zweers D, van Diepen A, et al. Complement receptor 3 and Toll-like receptor 4 act sequentially in uptake and intracellular killing of unopsonized Salmonella enterica serovar Typhimurium by human neutrophils. Infect Immun. 2007;75(6):2655–2660. doi: 10.1128/IAI.01111-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Demmert M, Faust K, Bohlmann MK, et al. Galectin-3 in cord blood of term and preterm infants. Clin Exp Immunol. 2012;167(2):246–251. doi: 10.1111/j.1365-2249.2011.04509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gow NA, Netea MG, Munro CA, et al. Immune recognition of Candida albicans beta-glucan by dectin-1. J Infect Dis. 2007;196(10):1565–1571. doi: 10.1086/523110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nguyen LN, Gacser A, Nosanchuk JD. The stearoyl-coenzyme A desaturase 1 is essential for virulence and membrane stress in Candida parapsilosis through unsaturated fatty acid production. Infect Immun. 2011;79(1):136–145. doi: 10.1128/IAI.00753-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.d’Ostiani CF, Del Sero G, Bacci A, et al. Dendritic cells discriminate between yeasts and hyphae of the fungus Candida albicansImplications for initiation of T helper cell immunity in vitro and in vivo. J Exp Med. 2000;191(10):1661–1674. doi: 10.1084/jem.191.10.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rizzetto L, Kuka M, De Filippo C, et al. Differential IL-17 production and mannan recognition contribute to fungal pathogenicity and commensalism. J Immunol. 2010;184(8):4258–4268. doi: 10.4049/jimmunol.0902972. [DOI] [PubMed] [Google Scholar]
- 93.Robinson MJ, Osorio F, Rosas M, et al. Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J Exp Med. 2009;206(9):2037–2051. doi: 10.1084/jem.20082818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Saijo S, Ikeda S, Yamabe K, et al. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity. 2010;32(5):681–691. doi: 10.1016/j.immuni.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 95•.Neumann AK, Jacobson K. A novel pseudopodial component of the dendritic cell anti-fungal response: the fungipod. PLoS Pathog. 2010;6(2):e1000760. doi: 10.1371/journal.ppat.1000760. Described a novel pseudopodial protrusion from dendritic cells in response to fungal stimuli that was species specific; the response was strongest for C. parapsilosis while absent for C. albicans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lima-Neto RG, Beltrão EI, Oliveira PC, Neves RP. Adherence of Candida albicans and Candida parapsilosis to epithelial cells correlates with fungal cell surface carbohydrates. Mycoses. 2011;54(1):23–29. doi: 10.1111/j.1439-0507.2009.01757.x. [DOI] [PubMed] [Google Scholar]
- 97.Moyes DL, Runglall M, Murciano C, et al. A biphasic innate immune MAPK response discriminates between the yeast and hyphal forms of Candida albicans in epithelial cells. Cell Host Microbe. 2010;8(3):225–235. doi: 10.1016/j.chom.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98••.Moyes DL, Murciano C, Runglall M, Kohli A, Islam A, Naglik JR. Activation of MAPK/c-Fos induced responses in oral epithelial cells is specific to Candida albicans and Candida dubliniensis hyphae. Med Microbiol Immunol. 2012;201(1):93–101. doi: 10.1007/s00430-011-0209-y. Expanded on previous work showing that epithelial cells distinguish C. albicans hyphae from yeast through activation of the second phase of the biphasic MAPK response (c-Fos activation). This study demonstrated that the MAPK/c-Fos response is specific to hyphae and does not occur with nonhyphal Candida spp. including C. parapsilosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bahri R, Curt S, Saidane-Mosbahi D, Rouabhia M. Normal human gingival epithelial cells sense C. parapsilosis by toll-like receptors and module its pathogenesis through antimicrobial peptides and proinflammatory cytokines. Mediators Inflamm. 2010;2010:940383. doi: 10.1155/2010/940383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Phan QT, Fratti RA, Prasadarao NV, Edwards JE, Jr, Filler SG. N-cadherin mediates endocytosis of Candida albicans by endothelial cells. J Biol Chem. 2005;280(11):10455–10461. doi: 10.1074/jbc.M412592200. [DOI] [PubMed] [Google Scholar]
- 101.Phan QT, Myers CL, Fu Y, et al. Als3 is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. PLoS Biol. 2007;5(3):e64. doi: 10.1371/journal.pbio.0050064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sun JN, Solis NV, Phan QT, et al. Host cell invasion and virulence mediated by Candida albicans Ssa1. PLoS Pathog. 2010;6(11):e1001181. doi: 10.1371/journal.ppat.1001181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu Y, Mittal R, Solis NV, Prasadarao NV, Filler SG. Mechanisms of Candida albicans trafficking to the brain. PLoS Pathog. 2011;7(10):e1002305. doi: 10.1371/journal.ppat.1002305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Saiman L, Ludington E, Pfaller M, et al. Risk factors for candidemia in neonatal intensive care unit patients. The National Epidemiology of Mycosis Survey study group. Pediatr Infect Dis J. 2000;19(4):319–324. doi: 10.1097/00006454-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 105.Shetty SS, Harrison LH, Hajjeh RA, et al. Determining risk factors for candidemia among newborn infants from population-based surveillance: Baltimore, Maryland, 1998–2000. Pediatr Infect Dis J. 2005;24(7):601–604. doi: 10.1097/01.inf.0000168751.11375.d6. [DOI] [PubMed] [Google Scholar]
- 106.Benjamin DK, Jr, DeLong ER, Steinbach WJ, Cotton CM, Walsh TJ, Clark RH. Empirical therapy for neonatal candidemia in very low birth weight infants. Pediatrics. 2003;112(3 Pt 1):543–547. doi: 10.1542/peds.112.3.543. [DOI] [PubMed] [Google Scholar]
- 107.Huang YC, Li CC, Lin TY, et al. Association of fungal colonization and invasive disease in very low birth weight infants. Pediatr Infect Dis J. 1998;17(9):819–822. doi: 10.1097/00006454-199809000-00014. [DOI] [PubMed] [Google Scholar]
- 108.Pera A, Byun A, Gribar S, Schwartz R, Kumar D, Parimi P. Dexamethasone therapy and Candida sepsis in neonates less than 1250 grams. J Perinatol. 2002;22(3):204–208. doi: 10.1038/sj.jp.7210699. [DOI] [PubMed] [Google Scholar]
- 109.Benjamin DK, Jr, Ross K, McKinney RE, Jr, Benjamin DK, Auten R, Fisher RG. When to suspect fungal infection in neonates: A clinical comparison of Candida albicans and Candida parapsilosis fungemia with coagulase-negative staphylococcal bacteremia. Pediatrics. 2000;106(4):712–718. doi: 10.1542/peds.106.4.712. [DOI] [PubMed] [Google Scholar]