Abstract
The neural correlates of creativity are poorly understood. Freestyle rap provides a unique opportunity to study spontaneous lyrical improvisation, a multidimensional form of creativity at the interface of music and language. Here we use functional magnetic resonance imaging to characterize this process. Task contrast analyses indicate that improvised performance is characterized by dissociated activity in medial and dorsolateral prefrontal cortices, providing a context in which stimulus-independent behaviors may unfold in the absence of conscious monitoring and volitional control. Connectivity analyses reveal widespread improvisation-related correlations between medial prefrontal, cingulate motor, perisylvian cortices and amygdala, suggesting the emergence of a network linking motivation, language, affect and movement. Lyrical improvisation appears to be characterized by altered relationships between regions coupling intention and action, in which conventional executive control may be bypassed and motor control directed by cingulate motor mechanisms. These functional reorganizations may facilitate the initial improvisatory phase of creative behavior.
Hip-Hop music, in particular rap, has had a huge cultural impact in western society, especially among the young, since its appearance four decades ago. Freestyle rap, a popular form, requires an artist to freely improvise rhyming lyrics and novel rhythmic patterns, guided by the instrumental beat – a particularly challenging form of spontaneous artistic creativity.
It has been proposed that artistic creativity is itself a twofold process, in which an initial improvisatory phase, characterized by spontaneous generation of novel material, is followed by a period of focused re-evaluation and revision1. The neural correlates of the improvisatory phase are poorly understood1,2,3,4,5,6,7,8 Freestyle rap thus provides a unique opportunity to study this initial, improvisatory phase at the interface of music and language.
In an attempt to identify the neural correlates of spontaneous lyrical improvisation in this context we compared freestyle (improvised) to conventional (rehearsed) performance, using functional magnetic resonance imaging (fMRI). Utilizing spatial independent component analysis (sICA) methods9 recently developed in this laboratory to effectively remove imaging artifacts associated with connected speech or song, has made it possible to study this unique genre using fMRI for the first time. Importantly, in order to study spontaneous lyrical improvisation in its most natural form, our design evaluated the natural and ecologically valid process: freestyle artists producing freestyle rap, unencumbered by unrelated cognitive demands.
Spontaneous improvisation is a complex cognitive process that shares features with what has been characterized as a ‘flow’ state10. It has been suggested that the frontal lobe, may play a central role in the improvisatory process, although the nature of its contributions is unclear2. On this basis, in addition to its other characteristics, we expected the neural correlates of lyrical improvisation to include changes in prefrontal activity that might enable spontaneous creative activity through effects on systems that regulate attention, affect, language and motor control.
Our results support these predictions and provide a novel model for improvisation characterized by functional changes within a large-scale network that is anchored in the frontal lobe. This pattern – activation of medial and deactivation of dorsolateral cortices – may provide a context in which self-generated action is freed from the conventional constraints of supervisory attention and executive control, facilitating the generation of novel ideas11. Importantly, altered relationships within the prefrontal cortex appear to have widespread functional consequences, affecting motivation, emotion, language as well as motor control, and may generalize to other forms of spontaneous creative behavior.
Results
Subjects were scanned while they performed two tasks, each of which used an identical 8-bar musical background track: 1) spontaneous, improvised freestyle rap (improvised) and 2) conventional performance of an overlearned, well-rehearsed set of lyrics (conventional). All figures and tables presented here are in Montreal neurological institute (MNI) space and are thresholded at a family-wise error rate less than 0.05 based on Monte Carlo simulations.
Language measures
Subjects' scores on verbal fluency tests administered prior to the scanning sessions [29.3 ± 6.8 (mean ± s.d.) words generated in one minute on semantic; and 58.0±11.1 in three minutes on phonological tests] were above the 80th percentile12 in each instance. This highlights the importance of superior linguistic skills in this genre, which requires rapid online formulation of meaningful, rhyming words and phrases within a prescribed tempo and rhythm.
GLM contrast of improvised vs. conventional conditions
To determine the neural correlates of spontaneous lyrical improvisation, we first compared improvised and conventional conditions directly using the general linear model (GLM) (Figure 1, a, b and Table 1). Improvised performance was characterized by significant increases in activity of the medial prefrontal cortex (MPFC), extending from the frontopolar cortex to the pre-supplementary motor area (pre-SMA) and decreases in the dorsolateral prefrontal cortex (DLPFC), extending from the orbital to superior regions. Medial prefrontal activations were lateralized to the left hemisphere; lateral prefrontal deactivations were lateralized to the right) (Figure 1c).
Figure 1. Activity related to improvisation: Results of the group level, random effects analysis of variance (ANOVA) comparing improvised and conventional conditions are illustrated.
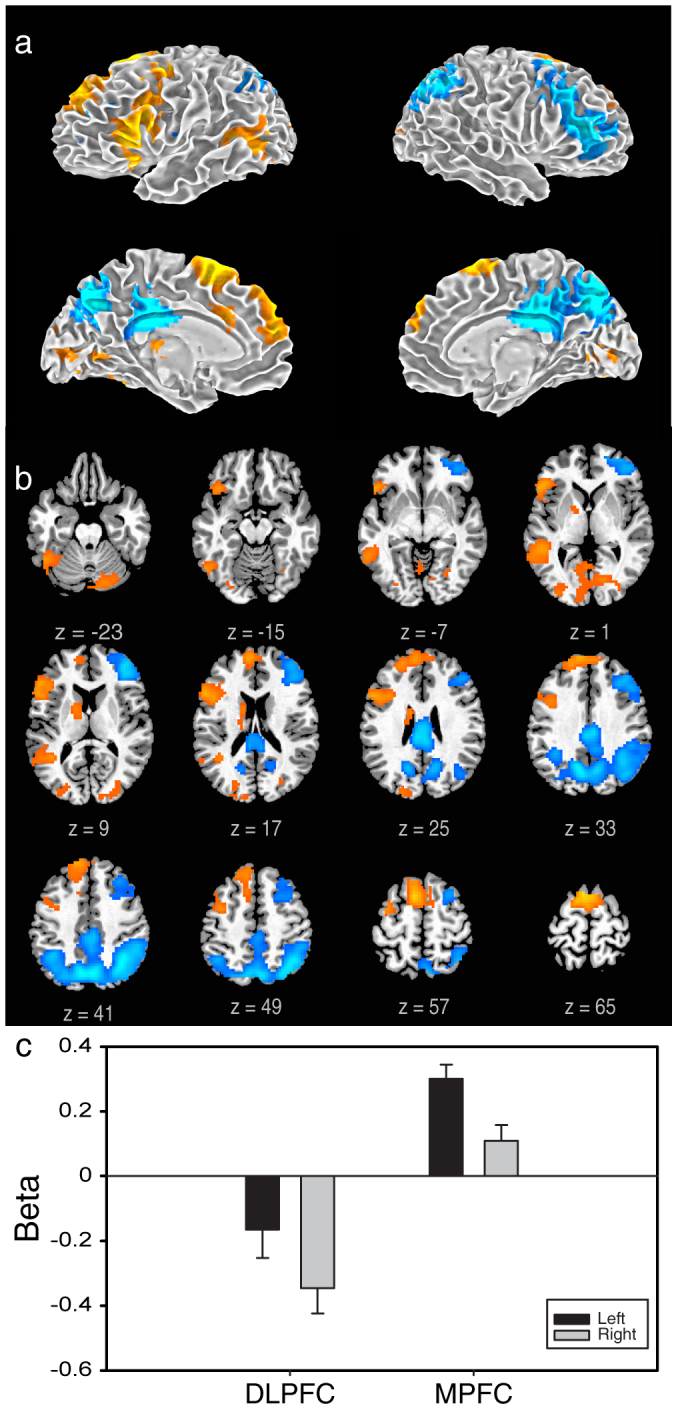
Warm colors represent significant increases in BOLD signal during improvised, cool colors represent significant decreases. Results, revealing dissociated frontal and left lateralized patterns, are displayed (a) on a 3D brain surface rendered using SUMA (SUrface MApping) and (b), as axial slices with planes of section relative to the bi-commissural line indicated. The bar plot (c) illustrates the mean values ± standard errors of signal changes (improvised-conventional) in DLPFC and MPFC ROIs defined in the GLM contrast and homotopic ROIs in the contralateral hemisphere. MPFC activations were strongest on the left, while activations in the right hemisphere were sub-threshold, and DLPFC deactivations were strongest on the right, while left hemisphere regions were non-significantly decreased. It should be noted that the activity in the DLPFC was significantly attenuated when the improvised condition was compared directly to an implicit baseline, indicating that this finding was not simply produced by contrast with the conventional condition (in which activity in the DLPFC did not itself differ from baseline).
Table 1. Results of GLM contrast of improvised and conventional conditions.
| Region | Side | BA | x | y | z | t value |
|---|---|---|---|---|---|---|
| Improvised>Conventional | ||||||
| MPFC_ventral | L | 10 | −6 | 57 | 18 | 5.10 |
| MPFC_middle | L | 9 | −9 | 57 | 33 | 5.60 |
| MPFC_dorsal | L | 8 | −15 | 48 | 42 | 4.63 |
| Pre-SMA | L | 6 | −6 | 15 | 66 | 7.39 |
| CMA/ACC | L | 32 | −8 | 18 | 47 | 3.74 |
| PMd | L | 6 | −39 | 0 | 54 | 5.37 |
| IFG_Orbitalis | L | 47 | −51 | 24 | −6 | 5.03 |
| IFG_Triangularis | L | 45 | −48 | 30 | 3 | 5.60 |
| IFG_Opercularis | L | 44 | −54 | 15 | 18 | 5.10 |
| Posterior MTG | L | 21 | −48 | −45 | 3 | 5.06 |
| Posterior STG/STS | L | 22 | −45 | −39 | 6 | 3.92 |
| Fusiform | L | 37 | −45 | −57 | −24 | 4.05 |
| Caudate | L | −12 | −3 | 21 | 4.00 | |
| Pallidum | L | −18 | 0 | 3 | 3.29 | |
| Lingual | L | 17 | −6 | −66 | −3 | 3.22 |
| IOG | L | 18 | −33 | −90 | 0 | 3.63 |
| Lingual | R | 17 | 15 | −75 | 0 | 3.78 |
| IOG | R | 18 | 33 | −84 | 3 | 3.19 |
| Cerebellum_Crus1 | R | 27 | −75 | −33 | 5.60 | |
| Improvised<Conventional | ||||||
| Inferior DLPFC/MFG | R | 10 | 39 | 48 | 9 | −7.25 |
| Superior DLPFC/MFG | R | 9 | 34 | 29 | 37 | −5.25 |
| IPS | R | 7 | 35 | −65 | 42 | −7.74 |
| SPL | R | 7 | 33 | −66 | 48 | −7.53 |
| Precuneus | R | 7 | 3 | −69 | 42 | −8.47 |
| PCC | R | 23 | 6 | −39 | 24 | −5.74 |
| Angular gyrus | R | 39 | 36 | −57 | 42 | −8.51 |
| Supramarginal gyrus | R | 40 | 51 | −42 | 42 | −6.41 |
| IPS | L | 7 | −32 | −65 | 40 | −4.89 |
| SPL | L | 7 | −15 | −75 | 45 | −6.86 |
| Precuneus | L | 7 | −6 | −69 | 39 | −8.61 |
| PCC | L/R | 23 | 0 | −33 | 27 | −6.81 |
| Supramarginal gyrus | L | 40 | −36 | −48 | 39 | −6.86 |
Regions of interest, hemisphere, Brodmann numbers, and MNI coordinates indicating local maxima of significant activations are tabulated with associated t-scores. MPFC = medial prefrontal cortex, DLPFC = dorsolateral prefrontal cortex, pre-SMA = pre-supplementary motor area, CMA = cingulate motor area, PMd = dorsal premotor cortex, IFG = inferior frontal gyrus, MTG = middle temporal gyrus, STG/STS = superior temporal gyrus/sulcus, PCC = posterior cingulate cortex, IPS = intraparietal sulcus, SPL = superior parietal lobule, IOG = inferior occipital gyrus
The improvised condition was also associated with increased activity in perisylvian areas in the left hemisphere, including inferior frontal gyrus (LIFG), middle temporal (MTG) and superior temporal (STG) gyri, and intervening superior temporal sulcus (STS) and fusiform gyrus. Improvised performance was in addition associated with left lateralized activation of motor areas; these included the left cingulate motor area (CMA), pre-SMA, dorsal premotor cortex (PMd), head and body of the caudate nucleus, and globus pallidus, and the right posterior cerebellum and vermis. Indices of articulatory movements did not differ between conditions: there were no significant differences in the number of syllables produced during improvised and conventional performance [994±103 (mean ± s.d.) and 1035±98 total syllables respectively].
Parametric modulation
We applied parametric modulation methods to determine how the innovative quality of performance might modulate these activity patterns. Using blinded ratings of performance quality (Table 2), we found significant associations between these measures and activity in the posterior and middle MTG and STS, the left MPFC, specifically lateral Brodmann area (BA) 9, a region near superior frontal sulcus, and the posterior cingulate cortex (PCC) (Figure 2).
Table 2. Guidelines for evaluating innovation in performance.
| Factors considered | |
|---|---|
| Creative use of Language | Did the participant generate a narrative? |
| Did the participant vary the types of content? | |
| Did the participant avoid crutch phrases? (“you know”) | |
| Did the participant incorporate humor? | |
| Did the participant incorporate “uncommon” words? | |
| Did the participant expand upon topics that were introduced? | |
| Did the participant employ multi-syllabic rhymes schemes? | |
| Was the participant able to maintain a coherent rhyme scheme over longer periods of time (greater than two bars)? | |
| Creative use of Rhythm | Did the participant vary rhythmic patterns? |
| Were the varied patterns connected to rhyme schemes? | |
| Was the participant able to rap at a rapid rate? | |
| Was the participant able to maintain a complex rhythm over longer period of time? | |
| Was the participant able to use rhythm that resulted in innovative “phrasing” (e.g. extending rhyme schemes past their natural beginning and end points)? |
Figure 2. Activations associated with innovative performance: Results of a parametric analysis relating BOLD activations to measures of innovative performance are illustrated.
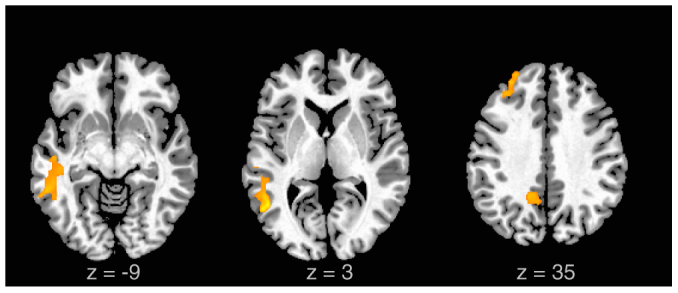
Performance scores, indexing creative use of language and rhythm were positively associated with BOLD signal increases in left middle and posterior MTG, left superior MPFC, and precuneus/PCC during Improvised.
Functional connectivity
Using a seed selected from the left lateral MPFC (guided by the parametric modulation results outlined above), we detected stronger negative correlations between activity in the MPFC and the ventral DLPFC (Figure 3a, indicating that the dissociated, reciprocal changes noted in these prefrontal areas in the above GLM contrasts are not independent; changes in one are tightly coupled to changes in the other). Similarly, activity in the MPFC was anticorrelated with activity in the intraparietal sulcus (IPS).
Figure 3. Functional connections associated with improvisation: Results of connectivity analyses (improvised vs.conventional) are illustrated.
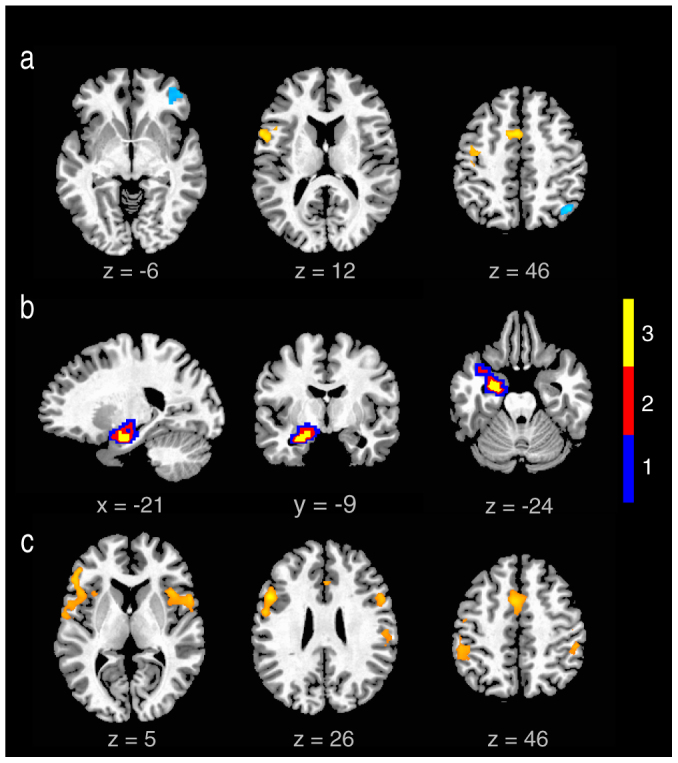
In (a) the MPFC (−19,49,33, see Methods for more details) is used as a seed region, revealing stronger negative correlations (cool colors) with the DLPFC and IPS, and stronger positive correlations (warm colors) with the LIFG, PMd and CMA (cluster extending into the pre-SMA, not pictured) during the improvised condition. Three separate connectivity maps were then generated using peak coordinates (of regions positively correlated with MPFC derived from the above analysis) from LIFG (−54, 12, 21, t = 4.60), CMA (−3, 9, 48, t = 4.49) and pre-SMA (0, 9, 66, t = 4.05) as seeds. The conjunction of these maps (b) reveals that each of these regions is more positively connected to the left amygdala during improvised. [The PMd was not]. In (b), blue highlights voxels in the amygdala with that were significantly more correlated with any one of the three seed regions; red with any two of these regions; yellow with all three. In (c) the peak AMG voxel from the above conjunction map (−21 −9 −24) was used as a seed. The results indicate that during the improvised condition, the left amygdala is itself connected to a wide array of regions in both hemispheres, including the insula, IFG, IPL and ACC.
On the other hand, we detected stronger positive correlations between activity in the MPFC and the anterior perisylvian (LIFG) and cortical motor areas including cingulate motor area and adjacent anterior cingulate cortex (ACC), the pre-SMA and the dorsal lateral premotor area (PMd) in the contrast of improvised vs. conventional conditions.
To trace the extensions of this network, we selected secondary seeds – i.e. from the inferior frontal and cortical premotor areas that were positively correlated with MPFC activity – and found that the left IFG and medial premotor areas were in turn positively correlated with activity in the left (but not the right) amygdala (AMG) (Figure 3b). By selecting an AMG seed from the peak in the conjunction of connectivity maps of LIFG, CMA and pre-SMA (Figure 3b), we found that the left AMG was positively connected to an extended network (Figure 3c), that included the right IFG and inferior parietal lobules (IPL) and anterior insula in both left and right hemispheres.
Changes in activation patterns over time
To explore the possible evolution of the creative process over time, we compared the contrast between improvised and conventional conditions at the beginning and end (first and last measures) of each eight bar segment (Figure 4). Activity in the left prefrontal, premotor, anterior perisylvian language areas and amygdala identified in the initial GLM contrasts, was significantly higher at onset. In contrast, activations were greater in the right hemisphere by the final measure. The latter were found in regions that had been deactivated in the initial GLM contrasts, including the frontal eye fields and contiguous portions of the DLPFC, the dorsal premotor area, IPS, IPL and precuneus.
Figure 4. Activations associated with evolution of improvisation in time: Activity selectively related to improvisation at the beginning vs. the end of each 8 bar segment is illustrated.
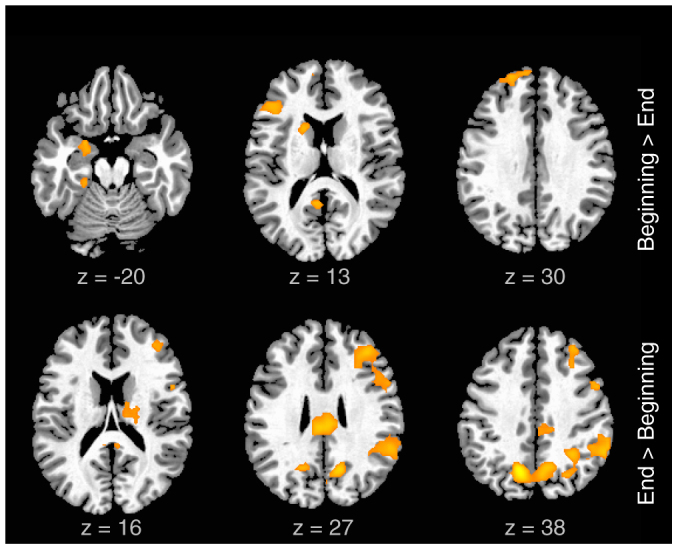
The first row (positive contrast of improvised vs. conventional, 1st bar vs. 8th bar) shows that left hemisphere regions (including AMG, IFG, MPFC and caudate) identified in previous analyses, were more strongly activated during the first than the final measure of each segment. The second row (positive contrast of improvised vs. conventional, 8th bar vs. 1st bar) indicates that during the final measure, activations were relatively stronger in right hemisphere regions (including the inferior and middle frontal gyri, the posterior parietal cortex and other posterior paramedian areas).
Discussion
In this study, we used fMRI to investigate the neural correlates of spontaneous lyrical improvisation by comparing spontaneous freestyle rap to conventional rehearsed performance. Our results reveal characteristic patterns of activity associated with this novel form of lyrical improvisation, and may also provide more general insights into the creative process itself. It has been suggested that the creative behaviors could occur in two stages: an improvisatory phase characterized by generation of novel material and a phase in which this material is re-evaluated and revised1. The present study may provide clues to the mechanisms that underlie the initial, improvisatory phase. Our results suggest a model in which an elementary reorganization of brain activity facilitates improvisation and may generalize to other forms of spontaneous creative behavior.
The most striking feature of lyrical improvisation detected by direct comparison of freestyle and conventional performance, in large part consistent with the previous study of melodic improvisation5, was a dissociated pattern of activity within the prefrontal cortex: increases in activity throughout the MPFC, extending from the frontal pole to the border of the pre-SMA, and simultaneous decreases in the DLPFC, from its orbital to superior regions. The implications of this dissociation are discussed below, in the context of subsequent analyses.
A second salient feature of improvisation revealed by the GLM contrasts was a marked lateralization of task-related changes in the BOLD signal. For example, the medial prefrontal activations just noted were stronger in the left hemisphere, while dorsolateral prefrontal deactivations were stronger on the right. Similarly, additional task-related activations in language and motor areas were strongly lateralized to the left hemisphere, while additional deactivations in superior frontal and parietal areas were lateralized to the right.
Activation of left hemisphere language areas (in inferior frontal and posterior middle and superior temporal gyri) was predicted and is perhaps unsurprising given the nature of the genre. However it should be noted that activation here indicates enhanced activity, over and above levels observed during conventional performance, and so is not related to language processing per se. Instead activation of language-related cortices likely reflects the unique demands of freestyle improvisation, which requires rapid online selection of novel words13 and phrases that rhyme14.
Increased activity in other left hemisphere regions associated with motor control (including medial and lateral premotor cortices, cingulate motor area and basal ganglia) does not appear to be related to increases in movement per se: there were no significant differences in quantitative indices of motor activity, including the number of syllables produced, during improvised and conventional conditions, and no condition-dependent differences in activity of the primary motor cortex were observed. Activity in these regions may instead reflect spontaneous phonetic encoding and articulation of rapidly selected words during improvisation. Enhanced activity in the caudate may also support rapid online sequencing of ongoing behaviors in this condition15. Freestyle improvisation also requires that the articulation of words and phrases be spontaneously incorporated into established rhythmic patterns; this process might place additional demand on these regions. Additionally, both the cerebellar hemisphere and vermis, selectively activated during improvised performance, have been associated with maintenance of rhythmic patterns in working memory16. The brain regions activated in association with rhyming and rhythmic variations may provide clues to mechanisms underlying the effects of musical intervention in clinical populations17.
The widespread changes identified by the foregoing analysis are suggestive but incomplete. The questions that follow – how are these concurrent activations and deactivations related to one another; are they integrated in a meaningful way? – were addressed using connectivity analyses.
The connectivity results revealed strong positive correlations between activity in a primary seed region in the MPFC (located within in the large cluster identified in the GLM contrast, selection was guided by the results of parametric modulation analyses) and inferior frontal and cortical premotor areas. To explore the potential extensions of this network, we tracked the extended connections of the inferior frontal and premotor regions themselves. Using each as a seed in subsequent analyses, we found that these regions were themselves positively correlated with activity in the left amygdala and the amygdala itself was strongly coupled to an extended network that included the right IFG, and IPL and anterior insula in both left and right hemispheres. The connectivity analyses therefore suggested the emergence of a more widespread, large-scale network that might play a role in lyrical improvisation.
Taken together, functional connectivity and GLM results provide a broader context in which to understand the dissociation of activity in medial and lateral prefrontal cortices and the ways in which this pattern might facilitate improvisation: The frontal midline cortices, selectively activated during improvisation, regulate motivational incentive, intentionality and drive18,19, and in this context the MPFC operates at the interface of intention and action – synthesizing information, encoding goals and guiding self-generated, stimulus-independent behaviors20,21,22,23,24. Normally, the expression of these behaviors is modulated by interactions between medial and lateral prefrontal regions19,25 – the MPFC provides a signal to the DLPFC, where information is processed prior to its gaining access to the motor system. In this way, the lateral prefrontal regions maintain executive control, consciously monitoring and implementing adjustments in an ongoing performance in order to ensure that actions conform to explicit goals26,27.
Here however, conventional interactions between medial and lateral prefrontal cortices appear to be markedly altered: given that BOLD signal in these regions is anticorrelated, the increases in MPFC activity appear to be tightly coupled to decreases in the DLPFC. We propose that this dissociated pattern reflects a state in which internally motivated, stimulus-independent behaviors are allowed to unfold in the absence of conscious volitional control.
There are a number of potential routes from the medial prefrontal cortex to motor effector areas in which the DLPFC could be bypassed. One parallel pathway that provides direct access to the motor system is via the dense projections from the MPFC to the CMA28,29, a premotor region that combines cognitive and affective information to orchestrate behavior.
Accordingly, we found that both the ACC and CMA (including the speech-related posterior rostral cingulate zone (RCZp)30) were significantly activated along with the MPFC during improvised performance, while activity in the DLPFC was significantly attenuated. At the same time, activity in MPFC and the cingulate areas was strongly correlated during improvised but not conventional conditions. An alternative, direct route through cingulate pathways into the motor system may allow the medial frontal regions to generate novel, exploratory behaviors31, bypassing conventional executive controls and thereby providing the cognitive flexibility necessary for successful improvisation.
It is interesting in this context that self-generated, stimulus independent behaviors appear to be initiated by midline frontal regions well before subjects consciously experience the intention to act20,32. In the absence of processing by lateral prefrontal regions – where a sense of agency could be constructed post-hoc – ongoing actions, moment to moment decisions and adjustments in performance may be experienced as having occurred outside of conscious awareness. This is not inconsistent with the experience of many artists who describe the creative process as seemingly guided by an outside agency.
In addition, the patterns we observe may reflect alterations in the activity of attentional systems: deactivations in superior portions of the DLPFC during the improvised condition (in the vicinity of the frontal eye fields) were accompanied by significant decreases in activity in the IPS. Together, these regions constitute elements of a supervisory attentional system, the so-called dorsal attention network33. This suggests that the conscious, deliberate, top-down attentional processes mediated by this network may be attenuated during improvisation, consistent with the notion that a state of defocused attention enables the generation of novel, unexpected associations that underlie spontaneous creative activity11. What monitoring and attentional processes do occur during improvisation may be mediated by the cingulate system, which remains active while DLPFC and parietal activity is reduced.
Beyond the interaction of the MPFC and DLPFC, the functional interconnections between medial prefrontal cortex, IFG, medial premotor areas and the amygdala, suggest that spontaneous lyrical improvisation is associated with emergence of a network that integrates motivation, language, emotion and motor function. The simultaneous coupling of the amygdala to inferior parietal lobules and insulae indicates that this network also incorporates regions that play a role in multimodal sensory processing and the representation of subjective experience34, and that, as a whole, this entire network is more effectively coupled during spontaneous creative behavior – perhaps facilitating what has been described as a psychological ‘flow’ state10 (which describes a subject's complete immersion in creative activity, typified by focused self-motivation, positive emotional valence, and loss of self-consciousness).
Supplementary analyses revealed additional, noteworthy patterns: The results of parametric modulation analyses indicated that innovative performance – incorporation of features such as inventive wordplay or novel rhythms into the improvisation – is associated with increased activity in a subset of left hemisphere regions (Figure 2) including the posterior and middle MTG and STS, and the MPFC. This suggests that regions that may correspond to the location of the mental lexicon (in which words and their semantic features are stored35, likely consistent with subjects' superior performance on verbal fluency tests), and regions that play a role in motivation, drive and self–organized behavior, may play a prominent role in the innovative use of language and rhythm. Interestingly, parametric modulation also highlighted an area not implicated in the GLM contrasts, the left posterior cingulate cortex (PCC), which has been shown to play a role, along with the MPFC, in self-motivated or self-referential behaviors36.
We also observed interesting, systematic differences in the patterns of activity in the first and last measures of the eight bar segments that constitute the basic unit of this musical form. Surprisingly we found that activity in the set of left prefrontal, premotor, anterior perisylvian language areas and amygdala reported above, was relatively higher at onset, but that activations in general appeared to shift to the right hemisphere by the final measure. This indicates first of all that the network related to motivation, emotion and language identified above may be more strongly engaged in initiating the improvisation.
What the relative increases in activity in the right hemisphere at the end of each segment indicate is however not clear. It is interesting that many of these increases were found in regions that were deactivated in the principal improvised-conventional contrast reported above. The time dependent increases in activity of frontal eye fields and IPS might reflect a re-emergence of top-down attentional processing at the end of each improvisational sequence, and increasing activity in the dorsolateral prefrontal cortices might reflect an increase in executive functions mediated by these regions. It is possible that rule based behaviors (e.g. attention to metric structure, selection of final lyrical elements) may be more important, and may re-engage these regulatory mechanisms, at the end of each 8 bar segment. It is clear nevertheless that the notion that simple attenuation of attention and executive control supports improvisation may be an oversimplification and that these processes seem to vary in a more complex way over time. The mechanisms underlying these interactions between musical improvisation and temporal structure clearly warrant further investigation.
As noted above, creativity may actually be a biphasic process involving initial free generation and subsequent revision of novel material1. Here we have examined only the first, spontaneous or improvisational phase. As we report, improvisation, contrasted with conventional performance, was in general associated with relative decreases in activity in supervisory attentional and executive systems. Were our subjects to actively reevaluate and revise the lyrics they had improvised, we might predict activation of these systems in support of evaluative processes that more likely require attention to and conscious, goal-directed revision of the original material. Indeed, a recent imaging study of graphic design did show activation of executive systems including the DLPFC specifically during subjects' evaluation of their prior creative outputs1.
Compared to previous studies of musical improvisation by Ullen and his colleagues4, Berkowitz & Ansari3 and Brown et al.7, our results differ in one fundamental way. While elegantly designed in order to enforce tight experimental control, these studies used conditions that were less spontaneous and may have imposed additional attentional and mnemonic demands (e.g improvised material had to be memorized as it was generated and reproduced during a subsequent scanning run4); this might in part account for the activation of the DLPFC reported in these studies. In contrast, we observed significant deactivation of the DLPFC (along with activation of the MPFC) and it is possible that this pattern may emerge when spontaneous improvisation takes place without the superimposition of secondary cognitive tasks.
In summary, the functional reorganization we observe – in which the medial prefrontal cortices may guide behavior in the absence of conscious attention and effect motor control through alternate cingulate pathways – is one feature of a larger network, linking intention, affect, language and action, that may underlie and facilitate the initial, improvisatory phase of creative behaviors. We speculate that the neural mechanisms illustrated here could be generalized to explain the cognitive processes of other spontaneous artistic forms, which can be tested in future studies across disciplines.
Methods
Subjects
Twelve male freestyle artists (mean age, 30.3 yr; range, 23–36 yr) were studied. Participants had at least 5 years of professional experience, defined as performing in front of an audience, or recording projects for public consumption, and receiving payment for this work. The range of professional experience across participants was 5 to 18 years, 9.8 ± 4.3 (mean ± s.d.). All participants were right-handed native speakers of American English. Written informed consent was obtained for all participants under a protocol approved by the Institutional Review Board (NIH 92-DC-0178).
Experimental design
The set of lyrics used in the conventional condition was selected by two co-authors. These lyrics were easy to memorize, and participants had not been exposed to them before the experiments. A recording of material, which was performed on the background instrumental track used in the experiments, was sent to the participants to memorize one week before the experiments. Prior to the imaging experiments, participants were asked to perform phonemic (generating words beginning with a specific letter) and categorical (animal naming) verbal fluency tests12. Participants then went through a training session, in order to make sure they performed all experimental conditions correctly before scanning. The participants were asked not to move their heads or other parts of their body during the scan. In order to constrain head motion, foam pads were used for support in the head coil. In both pilot and actual experiments, debriefing indicated that participants' performance was not affected by the motion restraints.
In the conventional condition, the participants were asked to rap the memorized lyrics on the 8-bar instrumental track. In the improvised condition, lyrics were improvised spontaneously, on the same instrumental track. An 8-bar instrumental track at 85 beats per minute was created by a co-author and repeatedly used as the background music for the whole experiment. A two-beat auditory cue and a visual prompt were placed at the beginning of the eighth bar to indicate the end of the 8 bars. Participants performed two sessions during the scan, of which each included 6 blocks (22.53s per block) of improvised and conventional conditions per session in an alternating box-car design.
MRI scanning
T2*-weighted BOLD images were acquired on a General Electric (GE) Signa HDxt 3.0 Tesla scanner (GE Healthcare, Waukesha, WI, USA) with an 8-channel High Resolution Brain Coil. Anatomical images were acquired using a magnetization-prepared rapid gradient-echo (MPRAGE) sequence. A single-shot gradient-echo EPI sequence was used for functional imaging: the acceleration factor of ASSET (Array Spatial Sensitivity Encoding Technique) = 2, TR (repetition time) = 2000 ms, TE (echo time) = 30 ms, flip-angle = 90°, 64×64 matrix, FOV (field of view) = 227 mm, 4 dummy scans. 40 interleaved sagittal slices with a thickness of 4 mm were used to cover whole brain. Because the majority of head motion during overt speech production is in the sagittal plane (especially “nodding”), off-plane motion was minimized by this setup and the advantage of in-plane image registration37 was maximized. The audio of participants' performances were recorded by a FOMRI™ II noise canceling optical microphone (Optoacoustics, Or Yehuda, Israel).
Data analysis
Time-locked, denoised auditory recordings were collected during each block from all participants. Syllables produced in each block were measured for both conditions by detecting syllable nuclei based on salient voiced peaks. After the experiment, auditory recordings acquired during improvised blocks were evaluated blindly by two experienced musicians who assessed the creative use of language and rhythm, assigning a consensus score using 10-point scale (Table 2), for use in the parametric modulation analyses.
The structural image of each subject was first segmented and normalized into MNI space using the tissue probability maps (TPMs) in SPM8 (Wellcome Department of Imaging Neuroscience, London, UK, http://www.fil.ion.ucl.ac.uk/spm/)38. In-plane registration, slice-time correction and volumetric rigid-body registration were sequentially applied to the functional datasets. Such traditional motion correction algorithms are effective in correcting misalignments caused by bulk head movements, but not motion-related susceptibility artifacts associated with overt speech production. To minimize the latter, spatial independent component analysis (sICA) was applied to the motion and slice-time corrected functional data on each subject level9. In sICA, each BOLD image was treated as a mixture of multiple spatially independent signal and noise sources. The number of components in each dataset was estimated by minimum description length (MDL) criterion39. The systematic classification of artifactual and neuronal ICA components was based on their degree of spatial clustering, location of major positively weighted clusters and neighborhood connectedness between positively and negatively weighted clusters. The noise components identified by a human-expert using these criterions and their variances were subtracted from the original dataset. Inter-rater reliability was assessed among five raters (including the current rater) by Fleiss' kappa test in an independent dataset consisting of 18 subjects. The Fleiss' kappa value of 0.9696 indicated almost perfect agreement among the raters. Afterward, the denoised data were normalized into MNI space at a voxel size of 3 x 3 x 3 mm by applying the transforms derived from the structural image normalization, and smoothed to a target full-width-half-max (FWHM) of 10 mm.
At the subject level, the GLM was implemented using SPM8. Separate regressors were constructed by convolving the box-car function of each condition with the canonical hemodynamic response function. In addition to task regressors, a nuisance covariate of the whole-brain mean signal was used to account for the global BOLD signal fluctuations induced by changes in PCO2 during continuous overt speech production40,41,42. To identify the effect of innovative performance, a separate GLM model was built with the addition of a regressor of performance scores for all improvised blocks. To estimate the evolution of improvised performance over time, in addition to the main regressors, additional regressors were added indicating 1st and 8th bar for both conventional and improvised conditions. In each case, a group-level voxel-wise random-effects ANOVA model was used to draw statistical inferences at the population level.
A seed based functional connectivity analysis was performed on the residual time-series of each voxel output from each participant's GLM. A band-pass filter of 0.045–0.1 Hz was applied on the residual in MATLAB (version R2010A, The MathWorks Inc., Natick, Massachusetts) to ensure that estimated connectivity between regions was not affected by high-frequency physiological noise or low-frequency fluctuations caused by scanner signal drifts and stimulus on-off manipulations. The data for each condition were shifted by three volumes to account for the delay (approximately 6 seconds) of the hemodynamic response, and then concatenated. For each condition, a correlation map was generated in AFNI43 by calculating the Pearson's correlation coefficient between the eigenvector of time series of all voxels within a 5-mm sphere centered at the seed's coordinate, and each voxel's time series in the brain. The correlation coefficients were then Fisher's z-transformed and input in a random-effect ANOVA model to compare the connectivity changes between the two task conditions at a group level in SPM8. For both GLM and connectivity analyses, Monte Carlo simulations were used to determine cluster size threshold for family-wise error correction.
To select the seed of MPFC for the functional connectivity analysis, we took into account both the GLM results (improvised vs. conventional) and parameter modulation indices of innovative performance. Since the cluster of MFPC activation derived from the improvised vs. conventional contrast was large, extending from the frontal pole to the pre-SMA, we divided this cluster into 6 sub-regions, using the division between the medial and superior frontal (lateral) gyri defined in the PickAtlas44 and between areas along inferior-superior axis corresponding to BA 10, 9 or 8 defined in the Talariach Daemon45 and in the work of Petrides and Pandya46,47. The parameter modulation results indicated that lateral BA 9 sub-region was most strongly associated with the creative use of language and rhythm, and we therefore selected the center of mass (−19 49 33) in this sub-region as the MPFC seed. To further explore the extensions of this network, we investigated secondary connectivity patterns of all regions that were more positively connected to the MPFC in the improvised vs. conventional contrast. More details can be found in the legend to Figure 3.
Author Contributions
S.L., A.R.B., H.M.C., Y.X., D.A.R. and M.W.E. designed the study. S.L. and A.R.B. interpreted the data and wrote the paper. S.L., H.M.C. and M.G.E. collected fMRI and behavioural data. K.E.S., D.A.R. and M.W.E. conducted behavioural data analysis. S.L. conducted neuroimaging analyses with the help of Y.X. and H.M.C. All authors commented on the manuscript.
Acknowledgments
This study was funded by NIH intramural program. The authors thank Steven Wise for critical discussions and comments on the manuscript. We also thank the rap musicians who participated in the study.
References
- Ellamil M., Dobson C., Beeman M. & Christoff K. Evaluative and generative modes of thought during the creative process. Neuroimage (2011). [DOI] [PubMed] [Google Scholar]
- Dietrich A. & Kanso R. A review of EEG, ERP, and neuroimaging studies of creativity and insight. Psychol Bull 136, 822–848 (2010). [DOI] [PubMed] [Google Scholar]
- Berkowitz A. L. & Ansari D. Generation of novel motor sequences: the neural correlates of musical improvisation. Neuroimage 41, 535–543 (2008). [DOI] [PubMed] [Google Scholar]
- Bengtsson S. L., Csikszentmihalyi M. & Ullen F. Cortical regions involved in the generation of musical structures during improvisation in pianists. J Cogn Neurosci 19, 830–842 (2007). [DOI] [PubMed] [Google Scholar]
- Limb C. J. & Braun A. R. Neural substrates of spontaneous musical performance: an FMRI study of jazz improvisation. PLoS One 3, e1679 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Manzano O. & Ullen F. Goal-independent mechanisms for free response generation: Creative and pseudo-random performance share neural substrates. Neuroimage 59, 772–780 (2012). [DOI] [PubMed] [Google Scholar]
- Brown S., Martinez M. J. & Parsons L. M. Music and language side by side in the brain: a PET study of the generation of melodies and sentences. Eur J Neurosci 23, 2791–2803 (2006). [DOI] [PubMed] [Google Scholar]
- Berkowitz A. L. & Ansari D. Expertise-related deactivation of the right temporoparietal junction during musical improvisation. Neuroimage 49, 712–719 (2010). [DOI] [PubMed] [Google Scholar]
- Xu Y., AbdulSabur N., Liu S., Chow H. M. & Braun A. R. Denoising the speaking brain: Characterizing and removing imaging artifacts in BOLD fMRI of continuous overt speech production. in Poster presented at the 3rd Annual Neurobiology of Language Conference, Annapolis, MD. [Google Scholar]
- Csikszentmihalyi M. Creativity: flow and the psychology of discovery and invention. 1st edn, (HarperCollinsPublishers, 1996). [Google Scholar]
- Martindale C. in Handbook of creativity (ed R. J. Sternberg) Ch. 7, 137–152 (Cambridge University Press., 1999).
- Tombaugh T. N., Kozak J. & Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol 14, 167–177 (1999). [PubMed] [Google Scholar]
- Thompson-Schill S. L., D'Esposito M., Aguirre G. K. & Farah M. J. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc Natl Acad Sci U S A 94, 14792–14797 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindell A. K. & Lum J. A. Priming vs. rhyming: orthographic and phonological representations in the left and right hemispheres. Brain Cogn 68, 193–203 (2008). [DOI] [PubMed] [Google Scholar]
- Van den Bercken J. H. & Cools A. R. Evidence for a role of the caudate nucleus in the sequential organization of behavior. Behav Brain Res 4, 319–327 (1982). [DOI] [PubMed] [Google Scholar]
- Jerde T. A., Childs S. K., Handy S. T., Nagode J. C. & Pardo J. V. Dissociable systems of working memory for rhythm and melody. Neuroimage 57, 1572–1579 (2011). [DOI] [PubMed] [Google Scholar]
- Schlaug G., Norton A., Marchina S., Zipse L. & Wan C. Y. From singing to speaking: facilitating recovery from nonfluent aphasia. Future Neurol 5, 657–665 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss D. T. in The human frontal lobes : functions and disorders (eds Bruce L. Miller & Jeffrey L. Cummings) Ch. 19, 292–305 (Guilford Press., 2007).
- Kouneiher F., Charron S. & Koechlin E. Motivation and cognitive control in the human prefrontal cortex. Nat Neurosci 12, 939–945 (2009). [DOI] [PubMed] [Google Scholar]
- Soon C. S., Brass M., Heinze H. J. & Haynes J. D. Unconscious determinants of free decisions in the human brain. Nat Neurosci 11, 543–545 (2008). [DOI] [PubMed] [Google Scholar]
- Ramnani N. & Owen A. M. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat Rev Neurosci 5, 184–194 (2004). [DOI] [PubMed] [Google Scholar]
- Tsujimoto S., Genovesio A. & Wise S. P. Evaluating self-generated decisions in frontal pole cortex of monkeys. Nat Neurosci 13, 120–126 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passingham R. E., Bengtsson S. L. & Lau H. C. Medial frontal cortex: from self-generated action to reflection on one's own performance. Trends Cogn Sci 14, 16–21 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggard P. Human volition: towards a neuroscience of will. Nat Rev Neurosci 9, 934–946 (2008). [DOI] [PubMed] [Google Scholar]
- Ridderinkhof K. R., Ullsperger M., Crone E. A. & Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science 306, 443–447 (2004). [DOI] [PubMed] [Google Scholar]
- Miller E. K. & Cohen J. D. An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24, 167–202 (2001). [DOI] [PubMed] [Google Scholar]
- Tanji J. & Hoshi E. Role of the lateral prefrontal cortex in executive behavioral control. Physiol Rev 88, 37–57 (2008). [DOI] [PubMed] [Google Scholar]
- Petrides M. & Pandya D. N. Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. J Neurosci 27, 11573–11586 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates J. F. & Goldman-Rakic P. S. Prefrontal connections of medial motor areas in the rhesus monkey. J Comp Neurol 336, 211–228 (1993). [DOI] [PubMed] [Google Scholar]
- Picard N. & Strick P. L. Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex 6, 342–353 (1996). [DOI] [PubMed] [Google Scholar]
- Daw N. D., O'Doherty J. P., Dayan P., Seymour B. & Dolan R. J. Cortical substrates for exploratory decisions in humans. Nature 441, 876–879 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried I., Mukamel R. & Kreiman G. Internally generated preactivation of single neurons in human medial frontal cortex predicts volition. Neuron 69, 548–562 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M. & Shulman G. L. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 3, 201–215 (2002). [DOI] [PubMed] [Google Scholar]
- Craig A. D. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 3, 655–666 (2002). [DOI] [PubMed] [Google Scholar]
- Levelt W. J. Spoken word production: a theory of lexical access. Proc Natl Acad Sci U S A 98, 13464–13471 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K. N. et al. The neural correlates of direct and reflected self-knowledge. Neuroimage 28, 797–814 (2005). [DOI] [PubMed] [Google Scholar]
- Huang J., Carr T. H. & Cao Y. Comparing cortical activations for silent and overt speech using event-related fMRI. Human Brain Mapping 15, 39–53 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J. & Friston K. J. Unified segmentation. Neuroimage 26, 839–851 (2005). [DOI] [PubMed] [Google Scholar]
- Li Y. O., Adali T. & Calhoun V. D. Estimating the number of independent components for functional magnetic resonance imaging data. Human Brain Mapping 28, 1251–1266 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey P. M., Macey K. E., Kumar R. & Harper R. M. A method for removal of global effects from fMRI time series. Neuroimage 22, 360–366 (2004). [DOI] [PubMed] [Google Scholar]
- Birn R. M., Diamond J. B., Smith M. A. & Bandettini P. A. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage 31, 1536–1548 (2006). [DOI] [PubMed] [Google Scholar]
- Hoit J. D. & Lohmeier H. L. Influence of continuous speaking on ventilation. J Speech Lang Hear Res 43, 1240–1251 (2000). [DOI] [PubMed] [Google Scholar]
- Cox R. W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research 29, 162–173 (1996). [DOI] [PubMed] [Google Scholar]
- Maldjian J. A., Laurienti P. J., Kraft R. A. & Burdette J. H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19, 1233–1239 (2003). [DOI] [PubMed] [Google Scholar]
- Lancaster J. L., Summerlin J. L., Rainey L., Freitas C. S. & Fox P. T. The Talairach Daemon a database server for talairach atlas labels. Neuroimage 5 (1997). [Google Scholar]
- Petrides M., Tomaiuolo F., Yeterian E. H. & Pandya D. N. The prefrontal cortex: Comparative architectonic organization in the human and the macaque monkey brains. Cortex 48, 46–57 (2012). [DOI] [PubMed] [Google Scholar]
- Petrides M. & Pandya D. N. in Handbook of neuropsychology (eds François Boller & Jordan Grafman) pp. 17–59 (Elsevier., 1994).


