Abstract
Hsp20 (heat-shock protein of 20 kDa; HspB6) is a cardioprotective agent which combats a number of pathophysiological processes in the heart, including hypertrophy, apoptosis and ischaemia/reperfusion injury. The cardioprotective actions of Hsp20 require its phosphorylation by PKA (cAMP-dependent protein kinase) on Ser16. Although the extracellular stimuli that promote cAMP-responsive phosphorylation of Hsp20 are well known, less is understood about the molecular processes that regulate this modification. AKAPs (A-kinase-anchoring proteins) physically compartmentalize PKA to specific locations within a cell to both direct PKA phosphorylation toward selected substrates and to orchestrate downstream signalling events. In the present study we used PKA anchoring disruptor peptides to verify that an AKAP underpins the cardioprotective phosphorylation of Hsp20. Biochemical and immunofluorescence techniques identify the cytosolic protein AKAP-Lbc (AKAP13) as the anchoring protein responsible for directing PKA phosphorylation of Hsp20 on Ser16. Gene silencing and rescue experiments establish that AKAP-Lbc-mediated PKA phosphorylation of Hsp20 is crucial to the anti-apoptotic effects of the Hsp. Thus AKAP-Lbc may serve an ancillary cardioprotective role by favouring the association of PKA with Hsp20.
Keywords: AKAP-Lbc, cAMP, cAMP-dependent protein kinase (PKA), cardioprotection, heat-shock protein of 20 kDa (Hsp20), phosphodiesterase (PDE)
INTRODUCTION
Hsps (heat-shock proteins) are a diverse group of chaperone proteins that are upregulated in response to various types of cellular stress. Hsp20 (Hsp of 20 kDa; also known as HspB6), a small Hsp, is widely recognized as a principle mediator of cardioprotective signalling [1, 2].Hsp20 has been shown to protect against cardiac ischaemia–reperfusion injury [3, 4], hypertrophy [5] and β-agonist-induced cardiomyocyte apoptosis [6], and also plays a role in inhibiting platelet aggregation [7] and modulation of smooth muscle relaxation [8, 9]. The cardioprotective actions of Hsp20 require its phosphorylation by PKA (cAMP-dependent protein kinase) on Ser16. Biochemical studies have shown that phospho-Ser16-Hsp20 levels are elevated in ischaemic myocardium [3] and failing human hearts [10]. In contrast, naturally occurring mutations that reduce Ser16 phosphorylation of Hsp20 are associated with attenuation of its anti-apoptotic effects [11]. Thus factors that contribute to regulation of PKA phosphorylation on Ser16 of Hsp20 are of potential therapeutic interest.
Although the extracellular stimuli that promote cAMP-responsive phosphorylation of Hsp20 are well known, less is understood about the subcellular signalling events regulating this modification. PKA phosphorylation events in cells are tightly regulated both spatially and temporally by complex networks of signalling enzymes and anchoring proteins [12]. PDEs (phosphodiesterases) are enzymes that hydrolyse cAMP and cGMP, allowing the generation of spatial cyclic nucleotide gradients. Local increases in cAMP lead to the dissociation of the regulatory and catalytic subunits of PKA, therefore cAMP-specific PDEs such as the PDE4 family can control PKA activity [13, 14]. Recently, a direct association has been demonstrated between Hsp20 and PDE4 in the heart. Moreover, disruption of this interaction was sufficient to induce PKA phosphorylation of Hsp20 in resting cells and to protect against cardiomyocyte hypertrophy, thereby emphasizing an important role for these signal-terminating enzymes in the removal of cAMP around Hsp20 [15]. AKAPs (A-kinase anchoring proteins) offer an additional level of regulation of PKA phosphorylation events, as they permit the localized activation of this kinase in the vicinity of selected substrates [16]. When G-protein-coupled receptors, adenylate cyclases, PDEs and other signalling enzymes associate with AKAPs, they create local hubs for the dynamic production and metabolism of cAMP [17–19]. Individual AKAPs have also been linked to defined roles in cardiac function, such as targeting PKA to ion channels to regulate channel phosphorylation and ion conductance [20–22], and organization of transcriptional events that are involved in the myocardial hypertrophic response [23, 24].
Although Ser16 phosphorylation of Hsp20 by PKA is known to be required for its cardioprotective effects, no AKAP has been linked to the co-ordination of this key phosphorylation event. Using biochemical and immunofluorescence techniques, we report a direct interaction between Hsp20 and the cytosolic AKAP-Lbc. We show that AKAP-Lbc scaffolding of PKA facilitates Ser16 phosphorylation of the Hsp. Functional validation of this event is provided by evidence that association of Hsp20 with AKAP-Lbc is required to confer the protective effects of Hsp20 on cardiomyocyte apoptosis.
EXPERIMENTAL
Materials
Stearated Ht31 and Ht31P peptides were from Promega. Isoproterenol was from Sigma. Phospho-Ser16 Hsp20 and digoxigenin–HRP (horseradish peroxidase) antibodies were from Abcam. Total Hsp20 antibodies were from Millipore. Anti-V5 antibodies were from Invitrogen. Anti-AKAP-Lbc (V096) antibody was described previously [25]. Anti-HspB6 antibodies (Abnova), Alexa Fluor® 488 donkey anti-rabbit and 594 donkey anti-mouse antibodies (Molecular Probes) were used for immunocytochemical staining. pEGFP-N1-FLAG-Akap-Lbc and pEGFP-N1-AKAP-Lbc ΔPKA plasmids were described previously [25]. Hsp20 phospho-site mutant plasmids are described in [15].
Cell culture, plasmid and siRNA (small interfering RNA) transfection and cell lysis
HEK (human embryonic kidney)-293 cells were cultured in DMEM (Dulbecco’s modified Eagle’s medium) supplemented with 10% (v/v) FBS (fetal bovine serum), 2 mM l-glutamine and 1% penicillin/10 mg/ml streptomycin. Cells were transiently transfected using Transit-LT1 (Mirus) and harvested after 24 h in HEK-293 lysis buffer [25 mM Hepes, 50 mM NaCl, 2.5 mM EDTA, 50 mM NaF, 30 mM sodium pyrophosphate, 10% (v/v) glycerol and 1% (v/v) Triton X-100, pH 7.5] containing 1× complete EDTA-free protease inhibitor cocktail (Roche). NRVMs (neonatal rat ventricular cardiomyocytes) were isolated from 1–5-day-old Sprague–Dawley rats by mincing ventricular tissue in ice-cold ADS buffer (120 mM NaCl, 20 mM Hepes, 1 mM NaH2PO4, 5 mM glucose, 5.4 mM KCl and 0.8 mM MgSO4, pH 7.4) and dissociating cells with 0.06% pancreatin (Sigma) and 0.03 % type II collagenase (Worthington). Digestions were stopped by the addition of FBS. Cells were suspended in DMEM/Medium 199 (4:1) supplemented with 10% (v/v) horse serum, 5% (v/v) FBS, 2 mM l-glutamine and penicillin/streptomycin and pre-plated for 2 h at 37°C and 5% CO2 to allow preferential attachment of cardiac fibroblasts. Non-adherent cardiomyocytes were then resuspended in fresh plating medium and seeded on to tissue culture dishes coated with 1% (w/v) gelatin (Sigma). After 24 h, plating medium was replaced with low serum medium comprising DMEM/M199 (4:1) supplemented with 5%(v/v) horse serum, 2 mM l-glutamine and penicillin/streptomycin. Cells were transiently transfected using the TransFectin lipid reagent (Bio-Rad Laboratories) according to the manufacturer’s instructions. All animal procedures were performed in accordance with the University of Glasgow animal ethics guidelines. HCMs (human cardiac myocytes) were cultured in HCM medium supplemented with 5% (v/v) FBS, 1% Cardiac Myocyte Growth Supplement and penicillin/streptomycin (all from ScienCell). For siRNA-mediated knockdown of AKAP-Lbc expression, cells were transfected with a silencer select validated siRNA oligonucleotide for AKAP-Lbc (5′-GCAUAUUGCUUGUAACUCA-3′) or control siRNA (Ambion) using Dharmafect I (Dharmacon) for 72 h prior to harvesting. Cardiomyocytes were harvested in lysis buffer (50 mM Tris, 150 mM NaCl, 5 mM EDTA, 50 mM NaF, 12 mM sodium deoxycholate, 1% Nonidet P40 and 0.1% SDS, pH 7.4) containing protease inhibitors.
Co-immunoprecipitation
Cellular lysates were equalized to 1 mg of protein/ml in lysis buffer. Lysates were pre-cleared by incubating with Protein A/G beads (Santa Cruz Biotechnology) with end-over-end rotation for 30 min, then beads were pelleted at 16000 g for 1 min. Proteins were immunoprecipitated with 1–4 µg of specific antibody or an equal amount of isotype-matched non-specific IgG (Cell Signaling Technology). Samples were washed with 1 ml of lysis buffer three times, then resolved by SDS/PAGE and transferred on to nitrocellulose.
Immunocytochemical staining and confocal microscopy
NRVMs were cultured on glass coverslips coated with 1 µg/cm2 mouse laminin (BD Biosciences). Cells were fixed with ice-cold 4%(w/v) paraformaldehyde in PBS for 10 min, washed twice for 5 min in PBS, then permeabilized for 20 min at room temperature (20°C) in 0.1% Triton X-100 in PBS. Non-specific binding sites were blocked by incubating with 0.2 % gelatin and 0.3 % BSA in PBS for 30 min. Cells were stained with the appropriate primary antibodies for 2 h at room temperature, then washed three times for 10 min in PBS, and incubated with fluorescently labelled secondary antibodies for 1 h at room temperature. Cells were washed once in PBS, mounted on to glass slides using ProLong Gold antifade reagent (Molecular Probes) and imaged using a ×63 Zeiss oil immersion objective on a Zeiss Pascal LSM510 laser-scanning confocal microscope (Carl Zeiss). Image files were collated using Zeiss Pascal software.
Caspase-3 activity assay
The Caspase-Glo 3/7 luminescence assay (Promega) was used to determine caspase-3 activity in NRVMs pretreated for 24 h with 10 µM isoproterenol. Relative luminescence was measured in 96-well white-walled tissue culture plates (Corning) on a Mithras LB940 plate reader (Berthold Technologies). Data was collected with MikroWin 2000 software. Purified caspase-3 enzyme (Enzo Life Sciences) was used as a positive control. Luminescence was measured for cell media and Caspase-Glo reagent alone to obtain a blank value which was subtracted from other measurements. Each experimental condition was measured in triplicate and results averaged to obtain an n of 1. A total n of 3 experiments was performed.
Statistical analysis
Bands were quantified using the NIH ImageJ software. Data represent means ± S.E.M. of three independent experiments, unless otherwise indicated. Statistical significance was calculated using an unpaired two-tailed t test or one-way ANOVA. A P value <0.05 (*) was considered significant and P < 0.01 (**) was considered highly significant.
RESULTS AND DISCUSSION
An AKAP facilitates phosphorylation of Hsp20 on Ser16
PKA phosphorylation of Hsp20 on Ser16 is cardioprotective [6, 11, 26]. Since AKAPs physically compartmentalize protein kinase A to specific locations within the cell, we reasoned that a particular anchoring protein may tether PKA to favour the phosphorylation of Hsp20.
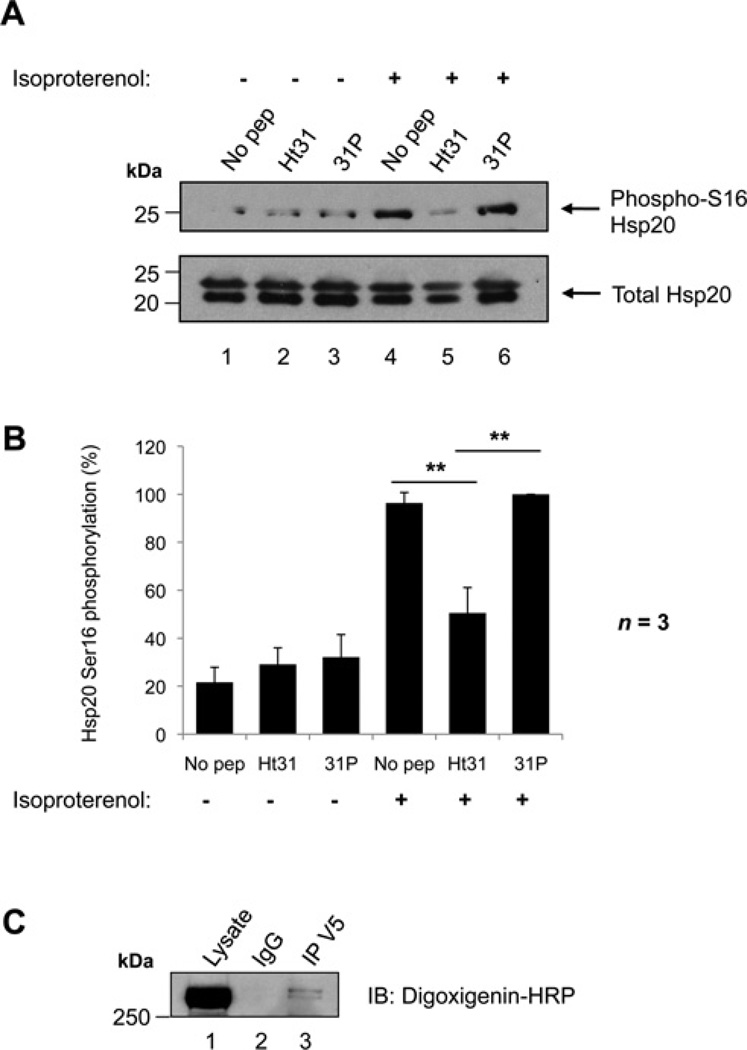
We employed two approaches to investigate whether an AKAP was required to mediate Ser16 phosphorylation of Hsp20 by PKA (Figure 1). First, the ability of a synthetic peptide (Ht31) to disrupt PKA–AKAP interactions was tested. This reagent binds PKA RII subunits with high affinity, competing with endogenous AKAPs for the interaction [27]. PKA anchoring by AKAPs was disrupted using Ht31 in NRVMs, which express Hsp20 endogenously. A proline derivative of Ht31, Ht31P, which is unable to bind RII, was used as a negative control. NRVMs were treated with cell-permeable Ht31 peptide, Ht31P peptide or left untreated (Figure 1A, lanes 1–3). Cells were also stimulated with isoproterenol to activate PKA prior to lysis (Figure 1A, lanes 4–6), and the proportion of phospho-Ser16 Hsp20 to total Hsp20 determined by Western immunoblotting. As expected, Hsp20 Ser16 phosphorylation was low under basal conditions, but increased 4.5-fold upon isoproterenol stimulation in untreated cells (Figure 1A, upper panel, lanes 1 and 4, and Figure 1B). Likewise, agonist-dependent Hsp20 Ser16 phosphorylation was elevated 3.2-fold in Ht31P-treated cells (Figure 1A, upper panel, lanes 3 and 6, and Figure 1B). In contrast, pretreatment with Ht31 only promoted a 1.7-fold increase in Hsp20 phosphorylation under the same conditions (Figure 1A, lanes 2 and 5, and Figure 1B). This effect was significantly lower than those observed upon no peptide treatment (P < 0.01) or in the presence of the Ht31P peptide control (P < 0.01). Thus it appears that Ser16 phosphorylation of Hsp20 is facilitated by an AKAP.
Figure 1. Phosphorylation of Hsp20 on Ser16 is facilitated by an AKAP.
(A) Ht31 treatment of NRVMs. Cells were treated with either 50 µM Ht31 peptide, Ht31P control peptide, or no peptide as indicated for 30 min, and then immediately harvested (lanes 1–3) or stimulated with 1 nM isoproterenol for 5 min prior to harvesting (lanes 4–6) to activate PKA and promote Hsp20-Ser16 phosphorylation. Cellular lysates were separated by SDS/PAGE and transferred on to nitrocellulose. Membranes were probed with anti-(phospho-Ser16 Hsp20) (upper panel) or anti-(total Hsp20) antibodies (lower panel). Immunoblots are representative of three independent experiments. (B) Quantification of data in (A). Bands were quantified using NIH ImageJ software. The amount of phospho-Ser16 Hsp20 was normalized to the amount of total Hsp20 (detected as a doublet in these cells), and is expressed as a percentage of the maximum phosphorylation measured. The results are the means ± S.E.M. of three independent experiments. **P < 0.01 indicates a highly significant difference between Ht31 peptide treatment compared with no peptide treatment, and Ht31 peptide treatment compared with 31P control peptide treatment. (C) RII overlay of an Hsp20 immunoprecipitation. Hsp20–V5 was transfected in to HEK-293 cells. Cellular lysates were immunoprecipitated with anti-V5 antibody or isotype-matched IgG, separated by SDS/PAGE and transferred on to nitrocellulose. Immunoprecipitates were probed with digoxigenin-labelled PKA RII subunits followed by anti-digoxigenin–HRP to detect RII-binding proteins. Molecular masses in kDa are indicated to the left-hand side of Western blots.
Secondly, an RII overlay approach was used to identify RII-binding proteins associated with Hsp20 (Figure 1C). Hsp20–V5 was transfected into HEK-293 cells and immunoprecipitated from cellular lysates with anti-V5 antibodies. Immune complexes were resolved by SDS/PAGE, and proteins were subjected to overlay with digoxigenin-labelled RII subunits to detect AKAPs. Hsp20– V5 co-precipitated with a RII-binding protein with a molecular mass of approximately 300 kDa (Figure 1C, lane 3).
Hsp20 binds to the cytosolic AKAP Lbc
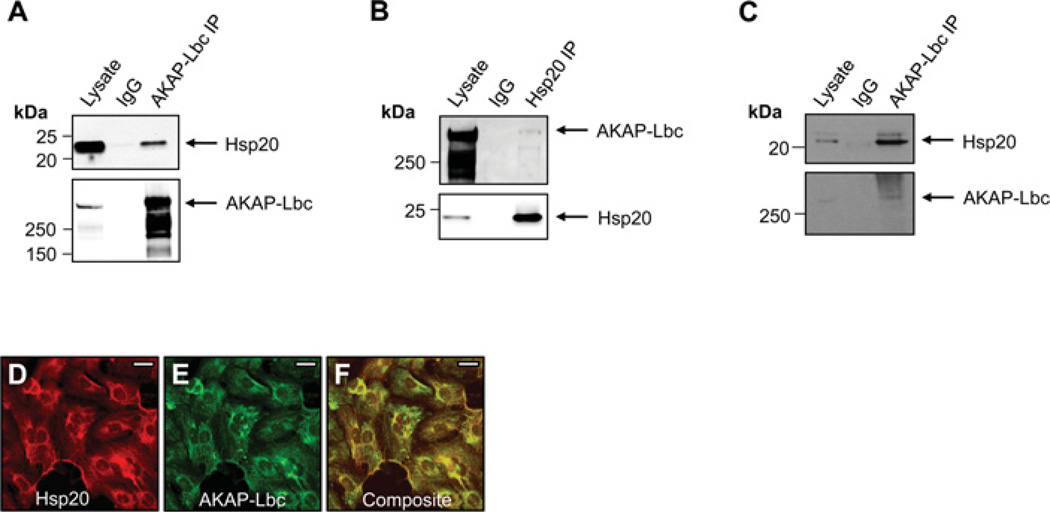
Next, we sought to identify the AKAP associated with Hsp20. A number of AKAPs are known to play important pathophysiological roles in the heart, including mAKAP (muscle-selective AKAP) [19], AKAP-Lbc [23], AKAP18 [21], AKAP79 [20] and Yotiao [28]. However, on the basis of molecular mass, AKAP-Lbc seemed to be the mostly likely candidate anchoring protein for Hsp20 (Figure 1C). Unlike other anchoring proteins, AKAP-Lbc predominantly resides in the cytoplasm where it serves as a scaffold for PKA [25], PKD (protein kinase D) and PKC (protein kinase C) isoforms [29]. Furthermore, it functions as a GEF (guanine-nucleotide-exchange factor) for Rho [25]. To determine whether AKAP-Lbc interacted with Hsp20, we undertook co-immunoprecipitation experiments. Hsp20–V5 was co-transfected into HEK-293 cells with FLAG–AKAP-Lbc (Figures 2A and 2B). Immunoprecipitation of AKAP-Lbc was performed with anti-FLAG antibodies, immune complexes were washed, separated by SDS/PAGE, and the amount of AKAP-Lbc immunoprecipitated was determined by Western immunoblotting with anti-FLAG (Figure 2A, lower panel). Co-immunoprecipitation of Hsp20–V5 was clearly detectable by immunoblotting with anti-V5 antibody (Figure 2A, upper panel). The reciprocal experiment was also performed. Hsp20–V5 was immunoprecipitated from cells using anti-V5 antibody (Figure 2B, lower panel), and immune complexes were immunoblotted for AKAP-Lbc with anti-FLAG antibody (Figure 2B, upper panel). AKAP-Lbc was identified in the Hsp20 immune complex, supporting the notion that both proteins exist in complex in cells.
Figure 2. Interaction of Hsp20 with AKAP-Lbc.
(A) Co-immunoprecipitation of Hsp20 with AKAP-Lbc in HEK-293 cells. Plasmids encoding Hsp20–V5 and FLAG–AKAP-Lbc were co-transfected into cells. Cell lysates were immunoprecipitated with anti-FLAG antibodies or normal IgG, and immunoblotted with anti-Hsp20 antibodies (upper panel) or anti-FLAG antibodies (lower panel). (B) Reciprocal co-immunoprecipitation of AKAP-Lbc with Hsp20 in HEK-293 cells. Cell lysates were immunoprecipitated with anti-V5 antibodies or normal IgG and indicated proteins were identified by immunoblotting. (C) Endogenous co-immunoprecipitation of Hsp20 with AKAP-Lbc in neonatal rat ventricular cardiac myocytes. Lysates were immunoprecipitated with anti-AKAP-Lbc antibodies or isotype-matched IgG, and immunoblotted as shown. Molecular masses in kDa are indicated to the left-hand side of Western blots. (D–F) Co-distribution of Hsp20 and AKAP-Lbc in heart cells. (D) Immunofluorescent image (red) of neonatal rat ventricular cardiac myocytes labelled with anti-Hsp20 antibodies followed by Alexa Fluor®, secondary antibodies. (E) Immunostaining of AKAP-Lbc (green). (F) Composite image (yellow). Scale bars represent 20 µM.
Evidence for this association in a more physiological context was provided by experiments performed in cardiac myocytes. Endogenous AKAP-Lbc was immunoprecipitated from NRVM lysate with anti-AKAP-Lbc antibodies (Figure 2C, lower panel). Immunoblot detection confirmed the presence of native Hsp20 in AKAP-Lbc immune complexes (Figure 2C, upper panel). Immunofluorescence microscopy was used to define the subcellular distributions of both proteins in cells. AKAP-Lbc has previously been shown to localize to the cytosol and perinuclear region of NRVMs [23]. NRVMs were fixed and immunostained for Hsp20 and AKAP-Lbc as indicated (Figures 2D–2F). Hsp20 (red, Figure 2D) and AKAP-Lbc (green, Figure 2E) displayed strikingly similar overlapping subcellular distributions on a composite image (yellow, Figure 2F). Taken together, these observations provide independent cell-based evidence for the association between cardiac Hsp20 and AKAP-Lbc.
AKAP-Lbc scaffolding of PKA is required for Ser16 phosphorylation of Hsp20
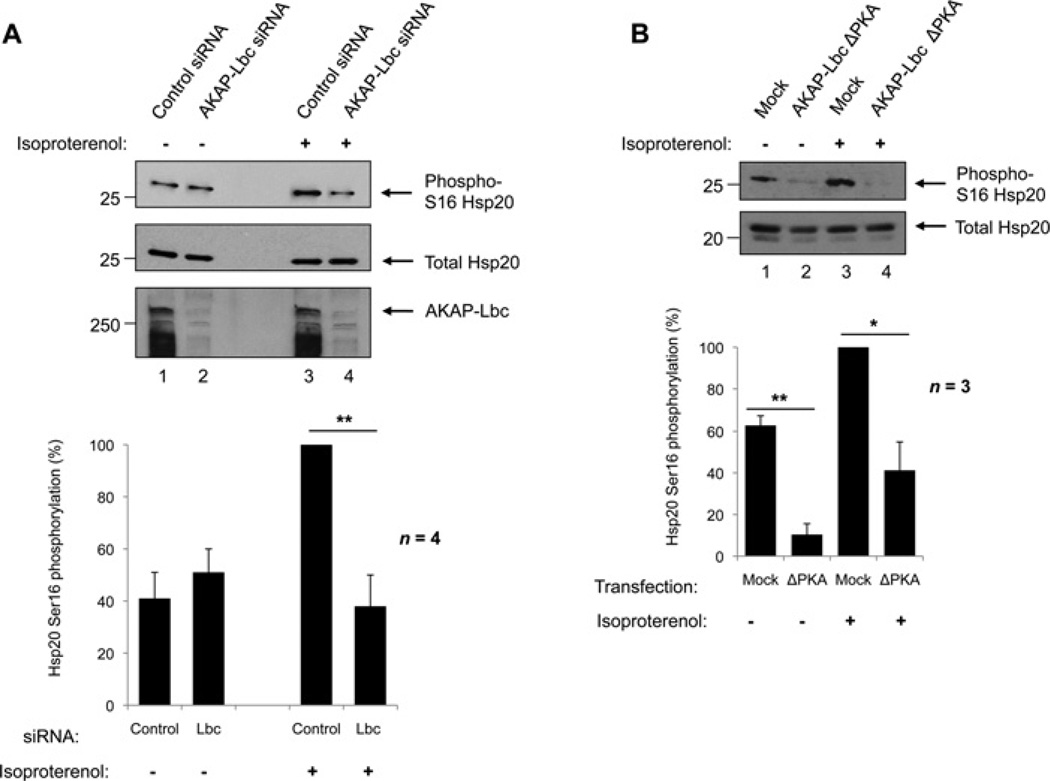
To confirm that AKAP-Lbc organizes a pool of PKA that can phosphorylate Hsp20 on Ser16, a RNA interference strategy was used (Figure 3A). Cardiac myocytes were transfected with siRNAs against AKAP-Lbc to specifically deplete cells of the AKAP, or scrambled control siRNAs. After 72 h, cells were stimulated with 1 nM isoproterenol to increase cAMP levels and activate PKA. Effective AKAP-Lbc gene silencing was determined by immunoblotting of cellular lysates (Figure 3A, third panel). Detection of total Hsp20 was used as a loading control (Figure 3A, middle panel). As expected, levels of phospho-Ser16 Hsp20 were lower in resting cells, and were not affected by AKAP-Lbc knockdown (Figure 3A, top panel, lanes 1 and 2). Upon activation of PKA, Hsp20 became rapidly phosphorylated in cells expressing AKAP-Lbc (Figure 3A, upper panel, lane 3); however, this increase in Ser16 phosphorylation was abolished in cells where expression of AKAP-Lbc was knocked down (Figure 3A, upper panel, lane 4) (P < 0.01), indicating that AKAP-Lbc facilitates PKA phosphorylation of Hsp20 on Ser16.
Figure 3. Selective knockdown of AKAP-Lbc and overexpression of a PKA-binding mutant of AKAP-Lbc (ΔPKA) attenuate Ser16 phosphorylation of Hsp20.
(A) siRNA knockdown of AKAP-Lbc in human cardiac myocytes. Cells were transfected with siRNAs against AKAP-Lbc or scrambled control siRNAs for 72 h, then immediately harvested (lanes 1 and 2) or stimulated with 1 nM isoproterenol for 5 min prior to harvesting to activate PKA (lanes 3 and 4). Top panels: Cellular lysates were immunoblotted for phospho-Ser16 Hsp20 and total Hsp20 as shown. The efficiency of the AKAP-Lbc knockdown was determined by immunoblotting with anti-AKAP-Lbc antibodies. Bottom panel: Quantification of phospho-Ser16 and total Hsp20 levels. Values were expressed as a percentage of maximum phosphorylation. The results represent the means ± S.E.M. of four independent experiments. **P < 0.01 indicates a highly significant difference between isoproterenol-stimulated cells treated with AKAP-Lbc siRNA compared with control siRNA. (B) Transfection of neonatal cardiac myocytes with an AKAP-Lbc PKA anchoring mutant (AKAP-Lbc ΔPKA) or vehicle only (mock). At 24 h after transfection, the indicated cells were left unstimulated (lanes 1 and 2) or stimulated with 1 nM isoproterenol (lanes 3 and 4). Top panels: cells were harvested and lysates immunoblotted for phospho-Ser16 and total Hsp20 as shown. Bottom panel: bands were quantified and results were expressed as in (A). *P < 0.05; **P < 0.01.
A role for AKAP-Lbc in the localized control of PKA phosphorylation of Hsp20 was confirmed in NRVMs using an AKAP-Lbc PKA-anchoring mutant, ΔPKA (Figure 3B). AKAP-Lbc ΔPKA contains two proline mutations (A1251P and I1260P) that disrupt the secondary structure of the RII-binding domain, rendering the AKAP unable to bind PKA [25, 30]. NRVMs were transfected with AKAP-Lbc ΔPKA or vehicle only (Figure 3B, lanes 1 and 2). Cells were stimulated with 1 nM isoproterenol to activate PKA (Figure 3B, lanes 3 and 4), and levels of Ser16-phosphorylated Hsp20 were assessed by immunoblotting. As expected, a robust increase in cAMP-dependent phosphorylation of Hsp20 at Ser16 was observed in isoproterenol-stimulated cells transfected with vehicle alone when compared with unstimulated controls (Figure 3B, upper panel, lanes 1 and 3, and histogram). Levels of Hsp20 Ser16 phosphorylation were significantly reduced in both isoproterenol-stimulated and unstimulated cells transfected with AKAP-Lbc ΔPKA (P < 0.05 and P < 0.01 respectively). This is consistent with the PKA-anchoring mutant of AKAP-Lbc exerting a dominant interfering effect by competing with the endogenous anchoring protein for the interaction with Hsp20.
Hsp20 Ser16 phosphorylation facilitated by AKAP-Lbc is cardioprotective
Ser16 phosphorylation has been shown to be necessary for the cardioprotective actions of Hsp20, including cardiomyocyte protection from β-agonist-induced apoptosis. Therefore in this final section we wanted to establish whether anchored PKA phosphorylation of Hsp20 contributes to the cardioprotective actions of this Hsp. Overexpression of a mutant version of Hsp20 that mimics constitutive phosphorylation at Ser16 (Hsp20-S16D) has previously been shown to protect against cardiomyocyte apoptosis induced by chronic β-adrenergic stimulation, through a mechanism involving reduced activation of caspase-3 [6]. Conversely, cells expressing phospho-null Hsp20 mutants (Hsp20-S16A) exhibit no protection from apoptosis [6], underlining the importance of this phosphorylation event.
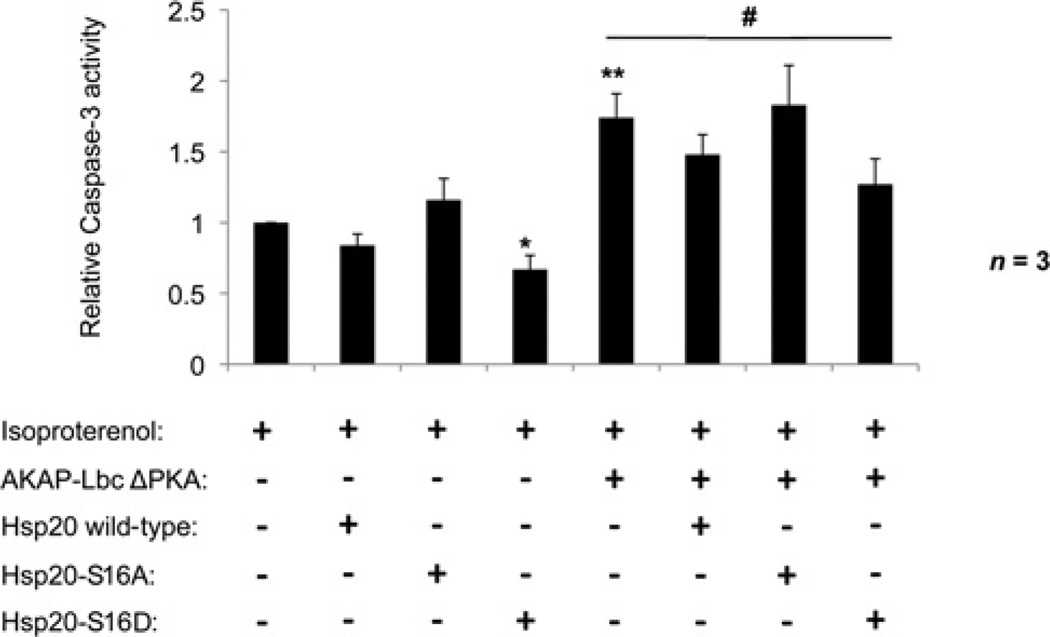
The functional relevance of the Hsp20–AKAP-Lbc interaction in cardioprotection was investigated in NRVMs chronically stimulated with 10µM isoproterenol to induce apoptosis. Cells were transfected with the AKAP-Lbc ΔPKA anchoring-defective mutant, and the ability of either wild-type Hsp20 or various Hsp20 phospho-site mutants to rescue this phenotype was determined by their co-transfection (Figure 4). Cellular apoptosis was measured using a luminescence assay of caspase-3 activity, which is directly proportional to apoptosis. Consistent with PKA phosphorylation of Hsp20 being required for its cardioprotective effects, transfection of Hsp20-S16D alone significantly reduced caspase-3 activity relative to untransfected control cells (*P < 0.05). In contrast, transfection of the non-phosphorylatable Hsp20-S16A mutant had no effect. NRVMs transfected with AKAP-Lbc ΔPKA alone displayed a 1.74 ± 0.17-fold increase in caspase-3 activity over untransfected cells (**P < 0.01), indicating a role for PKA phosphorylation co-ordinated by AKAP-Lbc in protecting against apoptosis in the heart. Co-transfection of cells with wild-type Hsp20 resulted in a 15% reduction in caspase-3 activity, and co-transfection of Hsp20-S16D significantly reduced caspase-3 activity (and therefore apoptosis) by 27% in these cells (#P < 0.05). Importantly, co-expression of the non-phosphorylatable Hsp20-S16A mutant had no effect on apoptosis and was associated with similar caspase-3 activation as NRVMs expressing AKAP-Lbc ΔPKA alone. Thus we propose that AKAP-Lbc anchoring of PKA to facilitate Hsp20 Ser16 phosphorylation is required to confer the anti-apoptotic actions of the Hsp.
Figure 4. Overexpression of an Hsp20 phospho-mutant mimicking constitutive phosphorylation protects against AKAP-Lbc ΔPKA-induced cardiomyocyte apoptosis.
NRVMs were transfected with AKAP-Lbc ΔPKA, wild-type Hsp20 and Hsp20 phospho-mutants (S16A and S16D) as indicated. Cells were stimulated with 10 µM isoproterenol for 24 h to induce apoptosis [6]. Caspase-3 activity was measured as a marker of apoptosis using the Caspase-Glo 3/7 luminescence assay. The results presented are from three independent experiments and are normalized to caspase-3 activity in untransfected isoproterenol-stimulated cells. *P < 0.05, **P < 0.01 compared with untransfected cells. #P < 0.05 compared with AKAP-Lbc ΔPKA-transfected cells.
Conclusions
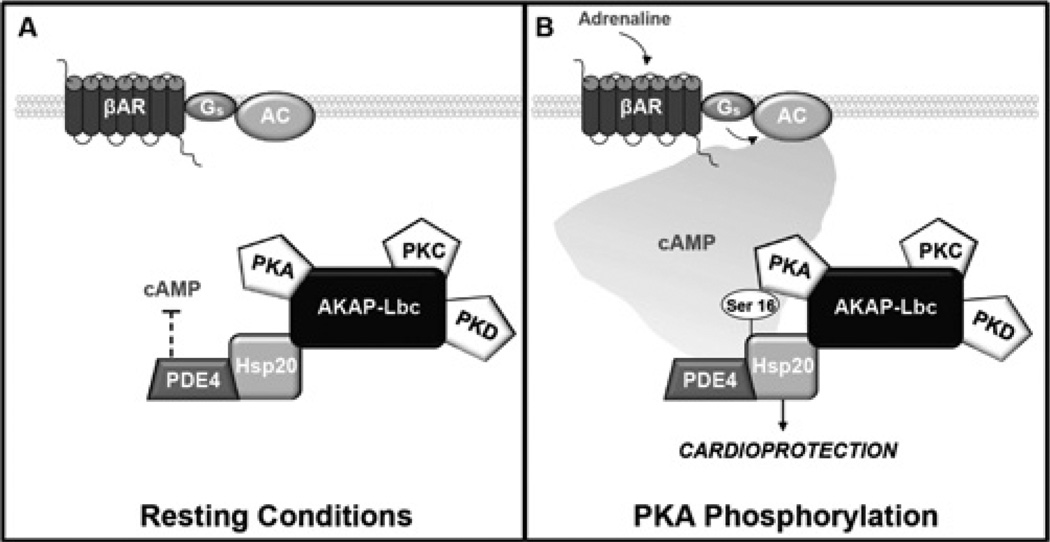
PKA phosphorylation events co-ordinated by AKAPs are integral to a range of essential cardiac processes, including modulation of cardiac excitation–contraction coupling and calcium handling [21, 24, 31, 32], cell survival and cardioprotection [6, 26]. In the present study we identify a previously unrecognized role for AKAP-Lbc in the organization of cardioprotective phosphorylation events. We show that AKAP-Lbc exists in a complex with the cardioprotective chaperone Hsp20 in the heart, and anchored PKA phosphorylation of Hsp20 on Ser16 is required to confer the anti-apoptotic effects of the Hsp. These findings extend our previous observations that Hsp20 associates with members of the cAMP-specific PDE4 family, and that PDE4 activity modulates cAMP-dependent phosphorylation of Hsp20 on Ser16 [15]. The results from the present study also suggest that an AKAP-Lbc–Hsp20–PDE4 complex resides in the cytoplasm of cardiac cells. Under resting conditions, the level of Hsp20 phosphorylation is maintained at basal levels by the actions of the associated PDE (Figure 5A). During times of stress, stimulation of β-adrenergic signalling pathways results in elevated levels of cAMP, which swamp this signal termination mechanism to trigger phosphorylation of Hsp20 on Ser16 by a pool of AKAP-Lbc-tethered PKA (Figure 5B). Hsp20 can also be phosphorylated on Ser16 by PKG (cGMP-dependent protein kinase). Activation of PKG occurs transiently in response to nitric oxide stimulation and a consequent rise in intracellular cGMP levels. PKG phosphorylation of Hsp20 has previously been linked with the modulation of smooth muscle relaxation [33, 34]. Although beyond the scope of the present study, it will be important to establish whether PKG is also part of this or another macromolecular complex, and whether related macromolecular assemblies exist in smooth muscle cells.
Figure 5. Schematic representation of the macromolecular complex coordinated by AKAP-Lbc in the heart.
Hsp20 interacts with AKAP-Lbc and PDE4 family members. (A) Under resting conditions, PDE4 bound to Hsp20 hydrolyses cAMP, preventing activation of local PKA, and Hsp20 remains largely unphosphorylated. (B) Upon β-adrenergic stimulation, local cAMP increases and activates AKAP-Lbc-bound PKA, which phosphorylates Hsp20. Phosphorylation of Hsp20 on Ser16 enhances its cardioprotective effects, including protection against apoptosis. AC, adenylyl cyclase; βAR, β-adrenergic receptor; Gs, stimulatory G-protein.
Our discovery of an association between Hsp20 and AKAP-Lbc provides a molecular context that favours local phosphorylation of the Hsp to protect against β-agonist-induced apoptosis in cardiac myocytes. These observations are in agreement with earlier work where apoptosis was attenuated following adenoviral-mediated overexpression of Hsp20. Full protection from apoptosis was gained following expression of the phospho-mimic (Hsp20-S16D); however, no advantage was conferred by expression of the phospho-null mutant (Hsp20-S16A) [6]. The mechanisms underlying this effect are thought to involve direct suppression of a pro-apoptotic ASK1 (apoptosis signal-regulating kinase 1)/p38 MAPK (mitogen-activated protein kinase) signalling cascade [5]. Interestingly, AKAP-Lbc has recently been shown to associate with several MAPKs, including p38α, and to promote its activation by RhoA in response to α1-adrenergic signals [35]. Thus the AKAP-Lbc–Hsp20 association may promote cardioprotective signalling by anchoring Hsp20 in the vicinity of p38 to oppose this activation.
The importance of the AKAP-Lbc–Hsp20 association in cardioprotection may not be confined to modulation of apoptosis. Hsp20 is also known to protect against ischaemia–reperfusion injury [3, 4], and cardiac hypertrophy [5, 15]. AKAP-Lbc has previously been implicated in the development of cardiac hypertrophy via its co-ordination of a PKD-dependent pathway [23]. The anchoring protein facilitates activation of PKD by PKC, which is then released from AKAP-Lbc by a mechanism involving direct PKA phosphorylation of the AKAP [29]. This pool of PKD then translocates to the nucleus, where it promotes phosphorylation and nuclear export of class II histone deacetylases, leading to enhanced transcription of hypertrophic genes [23]. Expression of AKAP-Lbc is also upregulated in hypertrophic NRVMs, and this has been proposed to augment activation of PKD and promote development of cardiac hypertrophy [23]. Thus AKAP-Lbc may co-ordinate a complex signalling network whereby activation of anchored PKD favours a hypertrophic phenotype, and anchored PKA activation, with subsequent phosphorylation of Hsp20, opposes these effects.
AKAP-Lbc is a multi-domain protein that contains tandem DH (Dbl homology) and PH (pleckstrin homology) domains. The DH domain is associated with guanine-nucleotide exchange activity for small GTPases such as Rho, which is an established mediator of cardiac hypertrophy and apoptosis [36–38]. The Rho-GEF activity of AKAP-Lbc is inhibited by PKA phosphorylation of the AKAP on Ser1565 [39]. Anchored PKA has been shown to phosphorylate AKAP-Lbc on Ser1565 in response to forskolin stimulus [39, 40], and it could be envisaged that factors that promote PKA phosphorylation of AKAP-Lbc and inhibition of its Rho-GEF activity might also promote cardioprotective phosphorylation of Hsp20.
Recently, an essential role for AKAP-Lbc in cardiac development has been identified [41]. Cardiomyocytes isolated from AKAP-Lbc (AKAP13)-null mice exhibited deficient sarcomere formation, and cardiac development arrested at embryonic day 9–9.5, consistent with the involvement of AKAP-Lbc-co-ordinated pathways in cardiomyocyte differentiation [41]. Hsps are among the first proteins expressed in embryogenesis [42], and it is tempting to speculate that a Hsp recruited to AKAP-Lbc could act as a molecular chaperone and aid in sarcomeric formation. Future studies will be directed towards identifying which proteins are stabilized by AKAP-Lbc-associated Hsp20 in the heart. Hsp20 was recently shown to associate with protein phosphatase 1 to modulate calcium handling at the sarcoplasmic reticulum [43], and it should also be determined whether anchored phosphatase activity is present in the AKAP-Lbc–Hsp20 complex to regulate dephosphorylation of this cardioprotective chaperone.
ACKNOWLEDGEMENTS
H.V.E. initiated these studies as a Leducq Foundation visiting scientist at the University of Washington. We thank Donelson Smith and Jon Day for helpful discussions, and Catherine Pawson for advice on immunocytochemistry.
FUNDING
H.V.E., J.D.S. and G.S.B. were supported by the Leducq Foundation [grant number cycAMP ObCDV]. Support in part was provided by the National Institutes of Health [grant number HL088366 (to J.D.S.)].
Abbreviations used
- AKAP
A-kinase-anchoring protein
- DH
Dbl homology
- DMEM
Dulbecco’s modified Eagle’s medium
- FBS
fetal bovine serum
- GEF
guanine-nucleotide-exchange factor
- HCM
human cardiac myocyte
- HEK
human embryonic kidney
- HRP
horseradish peroxidase
- Hsp
heat-shock protein
- MAPK
mitogen-activated protein kinase
- NRVM
neonatal rat ventricular cardiomyocyte
- PDE
phosphodiesterase
- PKA
cAMP-dependent protein kinase
- PKC
protein kinase C
- PKD
protein kinase D
- PKG
cGMP-dependent protein kinase
- siRNA
small interfering RNA
Footnotes
AUTHOR CONTRIBUTION
Helen Edwards performed and analysed all of the experiments. Helen Edwards, John Scott and George Baillie designed the experiments and wrote the paper.
REFERENCES
- 1.Edwards HV, Cameron RT, Baillie GS. The emerging role of HSP20 as a multifunctional protective agent. Cell. Signalling. 2011;23:1447–1454. doi: 10.1016/j.cellsig.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Fan GC, Kranias EG. Small heat shock protein 20 (HspB6) in cardiac hypertrophy and failure. J. Mol. Cell. Cardiol. 2011;51:574–577. doi: 10.1016/j.yjmcc.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan GC, Ren XP, Qian J, Yuan QY, Nicolaou P, Wang Y, Jones WK, Chu GX, Kranias EG. Novel cardioprotective role of a small heat-shock protein, Hsp20, against ischemia/reperfusion injury. Circulation. 2005;111:1792–1799. doi: 10.1161/01.CIR.0000160851.41872.C6. [DOI] [PubMed] [Google Scholar]
- 4.Fan GC, Ren XP, Qian J, Yuan QY, Wang Y, Jones WK, Chu GX, Kranias EG. Cardiac-specific overexpression of small heat-shock protein Hsp20 alleviates ischemia/reperfusion injury and myocardial dysfunction. Circulation. 2004;110:1439. [Google Scholar]
- 5.Fan GC, Yuan QY, Song GJ, Wang YG, Chen GL, Qian J, Zhou XY, Lee YJ, Ashraf M, Kranias EG. Small heat-shock protein Hsp20 attenuates β-agonist-mediated cardiac remodeling through apoptosis signal-regulating kinase 1. Circ. Res. 2006;99:1233–1242. doi: 10.1161/01.RES.0000251074.19348.af. [DOI] [PubMed] [Google Scholar]
- 6.Fan GC, Chu GX, Mitton B, Song QJ, Yuan QY, Kranias EG. Small heat-shock protein Hsp20 phosphorylation inhibits β-agonist-induced cardiac apoptosis. Circ. Res. 2004;94:1474–1482. doi: 10.1161/01.RES.0000129179.66631.00. [DOI] [PubMed] [Google Scholar]
- 7.Niwa M, Kozawa O, Matsuno H, Kato K, Uematsu T. Small molecular weight heat shock-related protein, HSP20, exhibits an anti-platelet activity by inhibiting receptor-mediated calcium influx. Life Sci. 2000;66:PL7–PL12. doi: 10.1016/s0024-3205(99)00566-4. [DOI] [PubMed] [Google Scholar]
- 8.Beall AC, Kato K, Goldenring JR, Rasmussen H, Brophy CM. Cyclic nucleotide-dependent vasorelaxation is associated with the phosphorylation of a small heat shock-related protein. J. Biol. Chem. 1997;272:11283–11287. doi: 10.1074/jbc.272.17.11283. [DOI] [PubMed] [Google Scholar]
- 9.Flynn CR, Komalavilas P, Tessier D, Thresher J, Niederkofler EE, Dreiza CM, Nelson RW, Panitch A, Joshi L, Brophy CM. Transduction of biologically active motifs of the small heat shock-related protein, HSP20, leads to relaxation of vascular smooth muscle. FASEB. J. 2003;17:1358–1360. doi: 10.1096/fj.02-1028fje. [DOI] [PubMed] [Google Scholar]
- 10.Qian J, Ren XP, Wang XH, Zhang PY, Jones WK, Molkentin JD, Fan GC, Kranias EG. Blockade of Hsp20 phosphorylation exacerbates cardiac ischemia/reperfusion injury by suppressed autophagy and increased cell death. Circ. Res. 2009;105:1223–1231. doi: 10.1161/CIRCRESAHA.109.200378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicolaou P, Knoll R, Haghighi K, Fan GC, Dorn GW, Hasenfuss G, Kranias EG. Human mutation in the anti-apoptotic heat shock protein 20 abrogates its cardioprotective effects. J. Biol. Chem. 2008;283:33465–33471. doi: 10.1074/jbc.M802307200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scott JD, Pawson T. Cell signaling in space and time: where proteins come together and when they’re apart. Science. 2009;326:1220–1224. doi: 10.1126/science.1175668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beavo JA, Brunton LL. Cyclic nucleotide research – still expanding after half a century. Nat. Rev. Mol. Cell. Biol. 2002;3:710–718. doi: 10.1038/nrm911. [DOI] [PubMed] [Google Scholar]
- 14.Baillie GS. Compartmentalized signalling: spatial regulation of cAMP by the action of compartmentalized phosphodiesterases. FEBS J. 2009;276:1790–1799. doi: 10.1111/j.1742-4658.2009.06926.x. [DOI] [PubMed] [Google Scholar]
- 15.Sin YY, Edwards HV, Li X, Day JP, Christian F, Dunlop AJ, Adams DR, Zaccolo M, Houslay MD, Baillie GS. Disruption of the cyclic AMP phosphodiesterase-4 (PDE4)-HSP20 complex attenuates the β-agonist induced hypertrophic response in cardiac myocytes. J. Mol. Cell. Cardiol. 2011;50:872–883. doi: 10.1016/j.yjmcc.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Welch EJ, Jones BW, Scott JD. Networking with AKAPs: context-dependent regulation of anchored enzymes. Mol. Interventions. 2010;10:86–97. doi: 10.1124/mi.10.2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fraser ID, Cong M, Kim J, Rollins EN, Daaka Y, Lefkowitz RJ, Scott JD. Assembly of an A kinase-anchoring protein-β2-adrenergic receptor complex facilitates receptor phosphorylation and signaling. Curr. Biol. 2000;10:409–412. doi: 10.1016/s0960-9822(00)00419-x. [DOI] [PubMed] [Google Scholar]
- 18.Bauman AL, Soughayer J, Nguyen BT, Willoughby D, Carnegie GK, Wong W, Hoshi N, Langeberg LK, Cooper DM, Dessauer CW, Scott JD. Dynamic regulation of cAMP synthesis through anchored PKA-adenylyl cyclase V/VI complexes. Mol. Cell. 2006;23:925–931. doi: 10.1016/j.molcel.2006.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dodge KL, Khouangsathiene S, Kapiloff MS, Mouton R, Hill EV, Houslay MD, Langeberg LK, Scott JD. mAKAP assembles a protein kinase A/PDE4 phosphodiesterase cAMP signaling module. EMBO J. 2001;20:1921–1930. doi: 10.1093/emboj/20.8.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao TY, Yatani A, DellAcqua ML, Sako H, Green SA, Dascal N, Scott JD, Hosey MM. cAMP-dependent regulation of cardiac L-type Ca2+ channels requires membrane targeting of PKA and phosphorylation of channel subunits. Neuron. 1997;19:185–196. doi: 10.1016/s0896-6273(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 21.Lygren B, Carlson CR, Santamaria K, Lissandron V, McSorley T, Litzenberg J, Lorenz D, Wiesner B, Rosenthal W, Zaccolo M, et al. AKAP complex regulates Ca2+ re-uptake into heart sarcoplasmic reticulum. EMBO Rep. 2007;8:1061–1067. doi: 10.1038/sj.embor.7401081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruehr ML, Russell MA, Ferguson DG, Bhat M, Ma JJ, Damron DS, Scott JD, Bond M. Targeting of protein kinase A by muscle A kinase-anchoring protein (mAKAP) regulates phosphorylation and function of the skeletal muscle ryanodine receptor. J. Biol. Chem. 2003;278:24831–24836. doi: 10.1074/jbc.M213279200. [DOI] [PubMed] [Google Scholar]
- 23.Carnegie GK, Soughayer J, Smith FD, Pedroja BS, Zhang F, Diviani D, Bristow MR, Kunkel MT, Newton AC, Langeberg LK, Scott JD. AKAP-Lbc mobilizes a cardiac hypertrophy signaling pathway. Mol. Cell. 2008;32:169–179. doi: 10.1016/j.molcel.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dodge-Kafka KL, Soughayer J, Pare GC, Michel JJC, Langeberg LK, Kapiloff MS, Scott JD. The protein kinase A anchoring protein mAKAP coordinates two integrated cAMP effector pathways. Nature. 2005;437:574–578. doi: 10.1038/nature03966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diviani D, Soderling J, Scott JD. AKAP-Lbc anchors protein kinase A and nucleates G α12-selective Rho-mediated stress fiber formation. J. Biol. Chem. 2001;276:44247–44257. doi: 10.1074/jbc.M106629200. [DOI] [PubMed] [Google Scholar]
- 26.Fan GC, Chu GX, Song QJ, Kranias EG. Phosphorylation of cardiac HSP20 increases contractility and protects myocytes from β-agonist-induced apoptosis. Circulation. 2003;108:541. [Google Scholar]
- 27.Carr DW, Hausken ZE, Fraser IDC, Stofkohahn RE, Scott JD. Association of the type-II cAMP-dependent protein-kinase with a human thyroid RII-anchoring protein – cloning and characterization of the RII binding domain. J. Biol. Chem. 1992;267:13376–13382. [PubMed] [Google Scholar]
- 28.Chen L, Kass RS. A-Kinase anchoring protein 9 and IKs channel regulation. J. Cardiovasc. Pharmacol. 2011;58:459–461. doi: 10.1097/FJC.0b013e318232c80c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carnegie GK, Smith FD, McConnachie G, Langeberg L, Scott JD. AKAP-Lbc nucleates a protein kinase D activation scaffold. Mol. Cell. 2004;15:889–899. doi: 10.1016/j.molcel.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 30.Smith FD, Langeberg LK, Cellurale C, Pawson T, Morrison DK, Davis RJ, Scott JD. AKAP-Lbc enhances cyclic AMP control of the ERK1/2 cascade. Nat. Cell Biol. 2010;12:1242–1287. doi: 10.1038/ncb2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leroy J, Richter W, Mika D, Castro LRV, Abi-Gerges A, Xie M, Scheitrum C, Lefebvre F, Schittl J, Mateo P, et al. Phosphodiesterase 4B in the cardiac L-type Ca2+ channel complex regulates Ca2+ current and protects against ventricular arrhythmias in mice. J. Clin. Invest. 2011;121:2651–2661. doi: 10.1172/JCI44747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang H-Y, Tao J, Shumay E, Malbon CC. G-protein-coupled receptor-associated A-kinase anchoring proteins: AKAP79 and AKAP250 (gravin) Eur. J. Cell Biol. 2006;85:643–650. doi: 10.1016/j.ejcb.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Rembold CM, Foster DB, Strauss JD, Wingard CJ, Van Eyk JE. cGMP-mediated phosphorylation of heat shock protein 20 may cause smooth muscle relaxation without myosin light chain dephosphorylation in swine carotid artery. J. Physiol. 2000;524:865–878. doi: 10.1111/j.1469-7793.2000.00865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brophy CM, Woodrum DA, Pollock J, Dickinson M, Komalavilas P, Cornwell TL, Lincoln TM. cGMP-dependent protein kinase expression restores contractile function in cultured vascular smooth muscle cells. J. Vasc. Res. 2002;39:95–103. doi: 10.1159/000057758. [DOI] [PubMed] [Google Scholar]
- 35.Cariolato L, Cavin S, Diviani D. A-kinase anchoring protein (AKAP)-Lbc anchors a PKN-based signaling complex involved in α1-adrenergic receptor-induced p38 activation. J. Biol. Chem. 2011;286:7925–7937. doi: 10.1074/jbc.M110.185645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Appert-Collin A, Cotecchia S, Nenninger-Tosato M, Pedrazzini T, Diviani D. The A-kinase anchoring protein (AKAP)-Lbc-signaling complex mediates α-1 adrenergic receptor-induced cardiomyocyte hypertrophy. Proc. Natl. Acad. Sci. U.S.A. 2007;104:10140–10145. doi: 10.1073/pnas.0701099104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown JH, Del Re DP, Sussman MA. The Rac and Rho hall of fame: a decade of hypertrophic signaling hits. Circ. Res. 2006;98:730–742. doi: 10.1161/01.RES.0000216039.75913.9e. [DOI] [PubMed] [Google Scholar]
- 38.Chang ZF, Lee HH. RhoA signaling in phorbol ester-induced apoptosis. J. Biomed. Sci. 2006;13:173–180. doi: 10.1007/s11373-005-9056-4. [DOI] [PubMed] [Google Scholar]
- 39.Jin J, Smith FD, Stark C, Wells CD, Fawcett JP, Kulkarni S, Metalnikov P, O’Donnell P, Taylor P, Taylor L, et al. Proteomic, functional, and domain-based analysis of in vivo 14-3-3 binding proteins involved in cytoskeletal regulation and cellular organization. Curr. Biol. 2004;14:1436–1450. doi: 10.1016/j.cub.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 40.Diviani D, Abuin L, Cotecchia S, Pansier L. Anchoring of both PKA and 14-3-3 inhibits the Rho-GEF activity of the AKAP-Lbc signaling complex. EMBO. J. 2004;23:2811–2820. doi: 10.1038/sj.emboj.7600287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mayers CM, Wadell J, McLean K, Venere M, Malik M, Shibata T, Driggers PH, Kino T, Guo XC, Koide H, et al. Rho guanine nucleotide exchange factor AKAP13 (BRX) is essential for cardiac development in mice. J. Biol. Chem. 2010;285:12344–12354. doi: 10.1074/jbc.M110.106856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neuer A, Spandorfer SD, Giraldo P, Dieterle S, Rosenwaks Z, Witkin SS. The role of heat shock proteins in reproduction. Hum. Reprod. Update. 2000;6:149–159. doi: 10.1093/humupd/6.2.149. [DOI] [PubMed] [Google Scholar]
- 43.Qian J, Vafiadaki E, Florea SM, Singh VP, Song W, Lam CK, Wang Y, Yuan Q, Pritchard TJ, Cai W, et al. Small heat shock protein 20 interacts with protein phosphatase-1 and enhances sarcoplasmic reticulum calcium cycling. Circ. Res. 2011;108:1429–1438. doi: 10.1161/CIRCRESAHA.110.237644. [DOI] [PMC free article] [PubMed] [Google Scholar]