Abstract
Background
Increased availability of genetic risk information may lead the public to give precedence to genetic causation over behavioral/environmental factors, decreasing behavior change motivation. Few population-based data inform these concerns.
Purpose
We assess the association of family history, behavioral risks, and causal attributions for diseases and the perceived value of pursuing information emphasizing health habits or genes.
Method
1959 healthy adults completed a survey that assessed behavioral risk factors, family history, causal attributions of eight diseases, and health information preferences.
Results
Participants’ causal beliefs favored health behaviors over genetics. Interest in behavioral information was higher than in genetic information. As behavioral risk factors increased, inclination toward genetic explanations increased; interest in how health habits affect disease risk decreased.
Conclusions
Those at greatest need for behavior change may hold attributions that diminish interest in behavior change information. Enhancing understanding of gene-environment influences could be explored to increase engagement with health information.
Keywords: genetic testing, attribution, family history, behavioral risk factors
The completion of the Human Genome Project and the consequent acceleration of genomic discovery is generating a tsunami of genetic data linking gene variants to a number of common health conditions [1-3]. This emerging evidence quantifying modest contributions of genetics to the occurrence of common health conditions has raised questions about how this information might influence psychosocial and behavioral outcomes [4-7].To date, awareness of direct to consumer testing offering this information to the public remains modest (32.6%) and uptake is low (2.5%), but this is likely to change [8]. One notable concern is that genetic risk information might prompt individuals to over-ascribe common health conditions to genetics, downplaying the contribution of well-known behavioral/environmental factors. Specifically, misinterpretation of genetic information may undermine public health efforts to promote the behavior change needed to prevent common health conditions [5,9]. For example, feedback of genetic test results may incline individuals to endorse pharmacological over behavioral modification options for risk reduction [10,11]. While these concerns proliferate, little theoretically-based research exists to inform the debate.
Two theoretical models, Marteau and Weinman's [12] adaptation of the Common Sense Model and Griffin et al.'s [13] Model of Risk Information Seeking and Processing to the Development of Preventive Behaviors, suggest mechanisms through which individual's beliefs about the causes of health conditions and their own personal risk factors might influence the value placed on genetic and behavioral health information. The Common Sense Model, described in detail elsewhere [14], holds that when presented with risk information, individuals construct cognitive representations (e.g., beliefs about disease causation), emotional responses (e.g., worry) and strategies to reduce the perceived threat of the information. The interplay of these processes can prompt adaptive coping strategies [14] such as taking steps to reduce the threat by seeking information about ways to change risk-conferring health habits [15]. Griffin et al.'s model suggests that personal experience with risk, such as through acknowledgement of family history or behavioral risk factors, could influence cognitive and emotional variables and in turn, preferences for health information. These personal experiences with risk can prompt actions to reduce disease risk [16].
These same processes, however, can result in defensive responses if information is deemed too threatening [15]. Those most at risk are often the most likely to downplay and distance themselves from threatening health information and to interpret health information in a defensive way [17-20]. These defensive or biased responses, in turn, may influence health information preferences and subsequent information seeking [12]. Consequently, Marteau and Weinman [12] argued that prior to genetic testing, an individual's pre-existing causal attributions about genetics and behaviors as contributors to health outcomes should be understood if we are to anticipate and potentially offset these defensive responses.
Most studies of causal attributions of health conditions have been done in small, self-selected samples of higher risk or affected individuals, often focusing on a single disease [21-24]. The advent of genetic susceptibility testing for multiple common conditions, and its potential to be applied to motivate individuals towards behavior change [1], raises important questions about how this information might be interpreted by healthy adults in the population. It has been suggested that individualized genetic information may be especially salient and threatening, and more likely to prompt defensive responses than risk assessment based on family history of disease. These defenses might be exacerbated if individuals have behavioral risk factors as well. Of particular concern is that defensive responses might prompt individuals to favor genetic explanations for common health conditions over behavioral causes, and diminish their interest in information on well-proven behavioral contributors to these conditions.
Education, Attributions and Information Preferences
Different population subgroups may hold different causal attributions and place varying value on sources of health information. It is possible, for example, that educational attainment may relate to both the beliefs about the relative contributions of genetics and behavior to health conditions and the effects on subsequent health information preferences. While the issue has not been examined directly, there are several reasons why education may impact these outcomes. Education is consistently and positively associated with health knowledge[25,26]. In addition, individuals with different levels of education also differ in their attributions for the causes of health conditions and in their preferred sources for health information [27-29]. Finally, individuals with higher education may be better able or more motivated to effortfully process and understand health information than those with less education [30,31]. Indeed, those with more education consistently show higher levels of information seeking [26,32,33]. A question yet to be addressed is whether educational level might influence the value placed on pursuing genetically versus behaviorally-based health information. This is of particular importance as concerns have been raised that genomic advances may further exacerbate health disparities [34]. Differential access to and use of information serve as potential explanations for continued disparities [35].
Study Purpose
As part of the ongoing Multiplex Initiative (described in detail elsewhere) [36], a population-based survey of healthy adults (N = 1,959) was conducted that assessed self-reported behavioral risk factors, family history, beliefs about the relative contribution of behavior versus genetics as the cause of eight common health conditions and preferences for health information. We hypothesize that, among healthy young adults, awareness of family history alone will not prompt defensively biased interpretations of genetic risk information, whereas one's own risky behavioral habits (e.g., poor diet, physical inactivity) will prompt these biases. These biases will be seen in their causal attributions and information preferences. Specifically, we posed the following exploratory hypotheses that: (1) participants would place greater importance on learning about how genes affect risk for health conditions than about how health habits affect risk for health conditions; (2) reporting more behavioral risk factors and a family history of the condition would be positively associated with the tendency to hold causal attributions that favor genetic over behavioral explanations as the cause of health conditions; (3) reporting more behavioral risk factors, a positive family history, and holding causal beliefs favoring genetic explanations would be associated with expressing less interest in seeking information about how health habits influence risk but (4) more interest in seeking information about how genetics influence risk. We also explored the contribution of education and whether findings varied across conditions.
Methods
The methods of this study are reported in detail elsewhere [36,37]. Briefly, these data are drawn from the baseline assessment of the Multiplex Initiative, a multi-center prospective observational study. The sampling frame for the Multiplex Initiative was drawn from a pool of 350,000 members of a Midwestern health management organization. Sampling strategies have been described previously [37]. Briefly, the sample included members who, as identified in the Master Patient Index, were: enrolled two years or more, aged 25 to 40, and self-identified as either black or white race. We selected our age range to best capture young, healthy adults who would not currently be included in population-based screening for the diseases in question. Diagnoses codes from claims data were used to exclude members who had been diagnosed with the eight health conditions on the Multiplex Genetic Test (i.e., diabetes, osteoporosis, heart disease, high cholesterol, hypertension, lung, colon or skin cancers). The eight selected health conditions are adult onset and “preventable,” meaning that there are widely accepted evidence-based prevention recommendations for these conditions, and can impact men and women. A random sample of the members meeting these criteria was drawn, oversampling for: male gender, black race, and lower educational status based on census track information associated with their current address (“lower” being ≤ high school). Recruitment occurred from February 2007 to May 2008.
Members in this random sample were sent an advance letter explaining that they would be contacted by telephone to complete a survey about their health-related attitudes and beliefs about factors that contribute to health outcomes unless they called a toll free number to decline participation. 22 called to decline our initial phone contact. Telephone contact for baseline screening was attempted for all individuals who did not call to decline. Importantly, the intent of the telephone survey was described as to assess “what people believe to be the causes of common health conditions.” As reported previously [36], baseline surveys were attempted with 6,348 members of Henry Ford Health System. 1,292 refused the survey, 2,614 were unreachable despite repeated attempts, and 326 were ineligible. 2,116 completed the baseline. 157 of these were ineligible due to presence of a health condition, leaving 1,959 for analysis. (See Hensley-Alford et al. [37] for a more detailed discussion of the recruitment approach).
Measures
Family history was assessed with the item, To your knowledge, do any of the following diseases run in your family? and then queried for each condition with a yes/no response. Participants responded to family histories for cancers, heart disease, osteoporosis, hypertension, and high cholesterol.
Behavioral risk factors are described below. We assessed seven risk factors in a way that would determine present behavior and therefore, current risks that could be changed. For each of the behaviors, a variable was created to indicate whether the level reported constituted a risk factor (0 – no, 1 – yes) for one of the health conditions. The risk factors, seen in Table 1, were then summed to create a behavioral risk factor score for each disease.
Table 1.
Behavioral risk factors for eight health conditions.
| Physical activity | Diet | Sun exposure | Smoking | Risky drinking | No multivitamin | BMI >30 | BMI <18.5 | |
|---|---|---|---|---|---|---|---|---|
| Diabetes | X | X | X | X | ||||
| Osteoporosis | X | X | X | X | X | X | ||
| Heart disease | X | X | X | X | X | |||
| High | X | X | X | X | ||||
| cholesterol | ||||||||
| Hypertension | X | X | X | X | X | |||
| Lung cancer | X | |||||||
| Colon cancer | X | X | X | X | X | X | ||
| Skin cancer | X |
Physical activity was measured with an item used in previous clinical trials of physical activity [38,39], During the last 12 months, on a scale from 1 to 7 where 1 is Never and 7 is Daily, how many days per week did you do each of the following for at least 15 minutes at a time? (a) Walking for exercise; (b) Hiking; (c) Bicycling or exercycle; (d) Aerobic and calisthenics; (e) Swimming; (f) Water aerobics; (g) Weight training or strengthening; (h) Other exercise. Participants who reported being active fewer than 5 times per week were coded as having the behavioral risk factor [40].
Dietary risk was computed using a validated 7-item nutrition screener designed for primary care settings [41] (range = 0-14). Items include How many times in a typical week do you eat fast food meals or snacks? How many servings of fruit or vegetables do you usually eat per day? How many servings of regular sodas or sweet tea do you usually drink each day? How many times in a typical week do you eat beans (like pinto or black beans), chicken or fish? How many times in a typical week do you eat regular snack chips or crackers (not low-fat)? and How many times in a typical week do you eat desserts and other sweets?, with response options of 1, 2, or 3 or more times/week. In addition, respondents were asked How much margarine, butter or meat fat do you usually use to season vegetables or put on potatoes, bread, or corn? (Very little, some, a lot). The cutoff was the mean score for high risk patients (6.43) in the validation study.
Current smoking status was based on self-report of ever having smoked and having smoked in the last 7 days [42]. Current smokers were at-risk.
Alcohol consumption was assessed using three items. In the past 30 days, how often did you drink any alcoholic beverages (every day, almost every day, 3-4 time/week; once per week, 2-3 times/month, 1/month, never); In the past 30 days, on the days when you drank alcoholic beverages, how many drinks did you have each day, on the average? We also asked In the past 30 days, how many times did you have 5 or more drinks on any one occasion? Drinking more than an average of one (women) or two (men) alcoholic beverages a day or consuming more than 5 drinks in any one sitting was considered to indicate a risk factor [43,44].
Sun exposure was measured with one item: How many times in the last 12 months did you get a sunburn that blistered or peeled from the sun? Having one or more sunburns was considered to indicate a risk factor.
Multivitamin use was assessed using one item: On average, how many days a week do you take multivitamins or folic acid? Less frequently than four times per week was considered to indicate a risk factor [45].
Body mass index (BMI; BMI=kg/m2) was calculated from self-reported height and weight. BMI ≥ 30 (obese) was considered to indicate a risk factor for diabetes, high cholesterol, hypertension, and colon cancer [46]. BMI< 18.5 (underweight) was considered a risk factor for osteoporosis [47].
Educational status was based on a single item, What is the highest grade or year in school you completed? This was trichotomized as high school or less, some college/vocational school, and college degree or more.
Causal attributions related to behavioral risk factors were assessed using the item On a scale from 1 to 7 where 1 is Not at all and 7 is Completely, how much do you think health habits such as diet, exercise, and smoking determine whether or not a person will develop each of the following conditions? Attributions related to genetic make-up were assessed using the item On a scale from 1 to 7 where 1 is Not at all and 7 is Completely, how much do you think genetic make-up, that is characteristics that are passed from one generation to the next, determines whether or not a person will develop the following conditions?
These items were combined to compute a new variable ranging from 0 to 1 indicating a participant's perceptions of the relative contribution of genetic to behavior as causes for each of the health conditions. At the extreme value of 0, the person attributes the health condition entirely to behavior. At the extreme value of 1, the person attributes it entirely to genes. At the mid-point (0.50), the person attributes the condition equally to genes and behavior.
Information Preference Outcomes, based on genetic and behavioral risk factors, were assessed using two items. Preferences for behaviorally-based information was assessed using the item On a scale from 1 to 7 where 1 is Not at all important and 7 is Very important, how important is it to you to learn more about how your health habits affect your chance of getting a certain health condition? Preferences for information related to genetic make-up were assessed using the item On a scale from 1 to 7 where 1 is Not at all important and 7 is Very important, how important is it for you to learn more about how your genes, that is the characteristics that are passed from one generation to the next, affect your chance of getting certain health conditions? Due to the skewed distribution of these items, each was dichotomized (1-5 = Less important, 6-7 = More important).
Covariates, including age, gender, marital status (married/partnered v. other), and race (White, African American, Other) were assessed with standard items.
Data Analysis
We generated frequency distributions and descriptive statistics to determine the participants’ sociodemographics and to determine variable frequencies and distributions. We conducted bivariate analyses to determine the relationships between covariates and outcomes. Significant covariates were entered into our multivariate models.
Mediation analyses would have been the preferred method for understanding whether behavioral risk and family history impacted information outcomes through the causal attributions held by those at risk. We did not pursue specific mediation analyses given our cross-sectional data [48]. However, we did follow the steps of mediation analyses, without formally testing statistical significance of mediation. This approach enabled an orderly approach to assessing the interrelationships among our variables prior to entering predictors into our final multivariate models. These steps included testing the associations between (1) the predictors (behavioral risk and family history) and the outcome (information preferences), (2) the predictors and the mediator (attributions), and (3) the mediator and the outcome, as well as (4) the final model, which incorporates the predictors and the mediator to predict the outcome [49-51].
Hypothesis 1, that participants would place greater importance on learning about how genes affect risk for health conditions than about how health habits affect risk for health conditions, was tested by using McNemar's exact test to assess the difference between these two categorical variables.
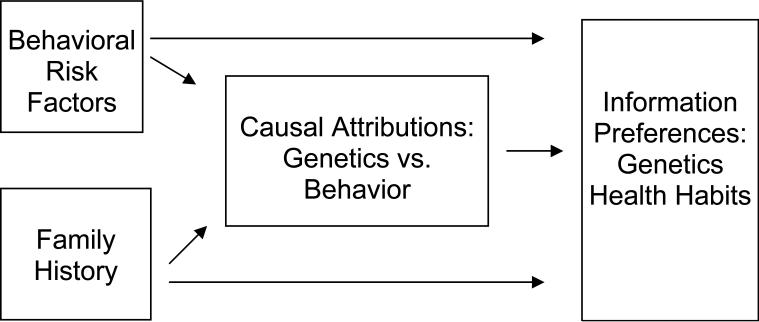
Hypothesis 2, that reporting more behavioral risk factors and a family history of the condition would be positively associated with the tendency to hold causal attributions that favor genetic over behavioral explanations as the cause of health conditions, was tested using bivariate correlations to examine the associations among behavioral risk factors, family history, and causal attributions (See Figure 1).
Figure 1.
Theoretical Model and Hypothesized Effects
We ran logistic regressions using PROC LOGISTIC from SAS 9.2 (SAS Institute, Inc., Cary, NC) to test hypotheses 3 and 4, that reporting more behavioral risk factors, a positive family history, and holding causal beliefs favoring genetic explanations would be associated with expressing less interest in seeking information about how health habits influence risk but more interest in seeking information about how genetics influence risk. Significance levels of p < .05 were used for all analyses.
Results
Participants, descriptive analyses and covariate analyses
As detailed in Table 2, 37% of the participants were college graduates and 63% were married. Fifty-three percent of participants were female and 37% were white; these demographics are roughly proportional to the population from which the sample was drawn. Approximately half of the participants reported a positive family history of each disease, with the exception of hypertension, which was much higher (83%), and osteoporosis, which was much lower (15%). 25% reported being current smokers, 25% of the sample reported being physically active < 5 days per week and 36% reported a BMI > 30. Attribution scores across the health conditions hovered just below the 0.5 threshold, indicating the tendency to equally attribute health conditions to genes and behavior; in all cases, the disease was attributed more to behavior than genetics. Education was not significantly associated with any causal attributions or for either information outcome. Gender (p < .05) and race (p < .001) were significantly correlated with the health seeking outcomes and were controlled for in multivariate analyses.
Table 2.
Participant demographics and model variables (n=1,959)
| Variable | Mean (SD) | |
|---|---|---|
| Demographics | ||
| Gender (% Female) | 53 | |
| Age (yrs) | 35 (4.18) | |
| Education | ||
| High school or less | 25 | |
| Some college | 38 | |
| College degree | 37 | |
| Ethnic group | ||
| Non-Hispanic white | 37 | |
| African American | 53 | |
| Other | 10 | |
| Marital status | ||
| Married / living as married | 63 | |
| Positive family history | ||
| Diabetes | 63 | |
| Osteoporosis | 15 | |
| Heart disease | 55 | |
| High cholesterol | 61 | |
| Hypertension | 83 | |
| Lung cancer | 52 | |
| Colon cancer | 52 | |
| Skin cancer | 52 | |
| Positive behavioral risk | ||
| Physical activity | 25 | |
| Diet | 40 | |
| Recent sun exposure | 3 | |
| Smoking | 25 | |
| Risky drinking | 22 | |
| No multivitamin | 61 | |
| BMI>30 | 36 | |
| BMI<18.5 | 1 | |
| Attributions1 | ||
| Diabetes | .44 (.18) | |
| Osteoporosis | .48 (.20) | |
| Heart disease | .45 (.12) | |
| High cholesterol | .40 (.17) | |
| Hypertension | .45 (.15) | |
| Lung cancer | .30 (.17) | |
| Colon Cancer | .44 (.18) | |
| Skin cancer | .36 (.20) | |
| Information preferences | ||
| Genetic information | ||
| Less important | 44 | |
| More important | 56 | |
| Health habit information | ||
| Less important | 33 | |
| More important | 67 |
range = 0-1
Preferences for Health Information
Our first hypothesis proposed that participants would place greater importance on learning about how genes affect risk for health conditions than about how health habits affect risk for health conditions. This was not supported. Though over half (56%) of the sample indicated that it would be very important to learn more about how their genes affect their chance of getting certain health conditions, significantly more indicated that it would be very important to learn more about how health habits affect their chance of getting certain health conditions (67%; X2 = 119. 04, p < .001).
Associations Among Behavioral Risk, Family History, Causal Attributions, and Information Preferences
Our second hypothesis proposed that the number of reported behavioral risk factors would be positively associated with attributions favoring genetic explanations of disease causation over behavioral explanations. This was partially confirmed for four of the eight diseases. As seen in Table 3, number of behavioral risk factors was positively associated with attributions favoring genetics, with those at greater behavioral risk favoring genetics as the cause for colon cancer (p < .001), skin cancer (p < .01), hypertension (p < .05) and lung cancer (p < .05). Having a family history also was positively associated with favoring genetics as the cause for diabetes (p < .001), heart disease (p < .001), high cholesterol (p < .001), hypertension (p < .001) and colon cancer (p < .05). Number of behavioral risk factors and having a family history of the condition were associated with each other for lung cancer (p < .05) and skin cancer (p < .05).
Table 3.
Correlations Among Behavioral Risk, Family History, Causal Attributions, and Information Preferences
| Causal Attributions for Eight Health Conditions2 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Diabetes | Osteoporosis | Heart disease | High cholesterol | Hypertension | Lung cancer | Colon cancer | Skin cancer | |
| Behavioral risks 1 | ||||||||
| Diabetes | .03 | |||||||
| Osteoporosis | .01 | |||||||
| Heart disease | .02 | |||||||
| High cholesterol | .00 | |||||||
| Hypertension | .05* | |||||||
| Lung cancer | .06* | |||||||
| Colon cancer | .08*** | |||||||
| Skin cancer | .06** | |||||||
| Family history | ||||||||
| Diabetes | .10*** | |||||||
| Osteoporosis | .00 | |||||||
| Heart disease | .12*** | |||||||
| High cholesterol | .15*** | |||||||
| Hypertension | .14*** | |||||||
| Cancer3 | .04 | .05* | .00 | |||||
Behavioral risks = the number of endorsed behavioral risk factors as detailed in Table 1. For example, the potential score range for behavioral risks for diabetes is 0-4. .03 indicates the correlation between this overall risk factor score and causal attributions for diabetes.
Causal attribution is scaled such that 0 = the health condition is attributed entirely to behavior and 1= the health condition is attributed entirely to genes.
Family history of cancer assessed using a single item for all cancers.
p < .05.
p < .01.
p < .001.
In keeping with our approach that parallels mediation analysis, we assessed interrelationships among behavioral risks, family history, and information outcomes, as well as between the eight attribution variables and our information outcomes. With the exception of lung cancer, behavioral risk factors were inversely associated with preferences for learning about how health habits affect disease risk (ps < .05). Behavioral risk factors were unrelated to preferences for learning about how genetics affect disease risk, as were causal attributions. Therefore, one key step for mediation, a significant association of the mediator and outcome, would have failed. However, given our cross-sectional data, we kept attribution in our final models.
Multivariate Analyses of Factors Associated with Importance Placed on Learning About How Health Habits Affect Disease Risk
Using logistic regression analysis, we examined whether reporting more behavioral risk factors, a positive family history, and holding causal beliefs favoring genetic explanations would be associated with lower odds of expressing interest in seeking information about how health habits influence risk. Our hypothesis was partially confirmed (Table 4). With the exception of lung cancer, as behavioral risks increased, importance on learning about how health habits impact disease risk decreased. For each behavioral risk factor for diabetes reported, the odds of valuing how health habits affect disease risk decreased by .86. Associations for family history were less consistent. Those with a family history of diabetes and hypertension placed greater importance on how health habits affect disease risk as compared to those without this risk factor. Attributions were unrelated to the importance of health habit information.
Table 4.
Logistic Regression Models (by Disease) Testing Associations with Importance Placed on Learning About How Health Habits Affect Disease Risk
| Diabetes OR (CI) | Osteoporosis OR (CI) | Heart Disease OR (CI) | High Cholesterol OR (CI) | |
|---|---|---|---|---|
| Male | .85 (.69-1.04) | .83 (.68-1.02) | .83 (.69-1.02) | .81 (.67-.99)* |
| Married/partnered | 1.15 (.93-1.42) | 1.15 (.93-1.42) | 1.15 (.93-1.42) | 1.16 (.94-1.44) |
| Race | ||||
| Other | 1.98 (1.39-2.81)*** | 2.02 (1.42-2.88)*** | 1.99 (1.41-2.84)*** | 2.01 (1.41-2.84)*** |
| African American | 3.38 (2.72-4.22)*** | 3.43 (2.74-4.28)*** | 3.46 (2.78-4.31)*** | 3.51 (2.82-4.37)*** |
| White | ||||
| Education | ||||
| ≤ High school | 1.01 (.78-1.32) | 1.02 (.78-1.33) | 1.04 (.80-1.35) | .99 (.77-1.29) |
| Some college | 1.09 (.86-1.38) | 1.10 (.87-1.38) | 1.11 (.88-1.39) | 1.08 (.86-1.36) |
| ≥ College | ||||
| Behavioral risk | .86 (.78-.96)** | .90 (.82-.99)* | .89 (.81-.97)* | .85 (.73-.98)* |
| Attribution | .76 (.43-1.36) | .63 (.38-1.05) | .55 (.24-1.27) | .78 (.42-1.43) |
| Family history | .30 (1.06-1.59)** | 1.09 (.82-1.46) | 1.11 (.91-1.37) | 1.19 (.97-1.46) |
| Hypertension OR (CI) | Lung Cancer OR (CI) | Colon Cancer OR (CI) | Skin Cancer OR (CI) | |
|---|---|---|---|---|
| Male | .83 (.68-1.01) | .80 (.65-.98)* | .84 (.68-1.02) | .84 (.68-1.03) |
| Married/partnered | 1.15 (.93-1.42) | 1.17 (.94-1.44) | 1.13 (.91-1.40) | 1.15 (.93-1.43) |
| Race | ||||
| Other | 1.90 (1.34-2.70)*** | 1.97 (1.38-2.80)*** | 2.00 (1.40-2.85)*** | 2.07 (1.45-2.94)*** |
| African American | 3.20 (2.57-3.98)*** | 3.28 (2.61-4.10)*** | 3.33 (2.67-4.15)*** | 3.53 (2.82-4.42)*** |
| White | ||||
| Education | ||||
| ≤ High school | 1.06 (.81-1.38) | 1.00 (.77-1.30) | 1.05 (.80-1.37) | 1.00 (.77-1.30) |
| Some college | 1.12 (.89-1.41) | 1.10 (.87-1.39) | 1.12 (.89-1.41) | 1.11 (.88-1.40) |
| ≥ College | ||||
| Behavioral risk | .84 (.74-.95)** | .86 (.69-1.08) | .89 (.82-.97)** | .78 (.64-.97)* |
| Attribution | 1.01 (.52-1.99) | .92 (.52-1.63) | .95 (.55-1.65) | .94 (.57-1.54) |
| Family history | 1.54 (1.21-1.97)*** | .94 (.77-1.15) | .93 (.76-1.14) | .94 (.76-1.15) |
p < .05.
p < .01.
p < .001.
Multivariate Analyses of Factors Associated with Importance Placed on Learning About How Genetics Affect Disease Risk
We performed a separate logistic regression analysis to examine whether reporting more behavioral risk factors, a positive family history, and causal beliefs favoring genetic over behavioral explanations would increase the odds of placing importance on information about how genetics impact disease risk. This hypothesis had limited support (Table 5). Except for our model for osteoporosis, number of risky behaviors was not associated with the value placed on learning about how genetics impact disease risk. Those reporting a family history of diabetes, osteoporosis and hypertension placed greater importance on learning about how genetics influences disease risk as compared to those without this risk factor. Finally, attributions favoring genetic explanations for diabetes, hypertension and colon cancer were associated with placing greater importance on learning about genetics. Education did not significantly contribute to any of our models. Gender and race were significant covariates, with men placing less importance on both sources of information than women (p < .05) and African-Americans and participants characterized as neither White nor African-American placing less importance on both sources of information than Whites (p < .001).
Table 5.
Logistic Regression Models (by Disease) Testing Associations with Importance Placed on Learning About How Genes Affect Disease Risk
| Diabetes OR (CI) | Osteoporosis OR (CI) | Heart Disease OR (CI) | High Cholesterol OR (CI) | |
|---|---|---|---|---|
| Male | .86 (.71-1.03) | .85 (.70-1.02) | .84 (.70-1.02) | .83 (.69-.99)* |
| Married/partnered | 1.10 (.90-1.34) | 1.09 (.90-1.33) | 1.07 (.88-1.30) | 1.09 (.90-1.33) |
| Race | ||||
| Other | 1.89 (1.35-2.65)*** | 2.05 (1.46-2.88)*** | 1.95 (1.39-2.72)*** | 1.95 (1.39-2.73)*** |
| African American | 2.14 (1.74-2.63)*** | 2.40 (1.94-2.96)*** | 2.34 (1.91-2.88)*** | 2.30 (1.87-2.82)*** |
| White | ||||
| Education | ||||
| ≤ High school | 1.19 (.93-1.52) | 1.23 (.96-1.58) | 1.26 (.98-1.61) | 1.24 (.97-1.57) |
| Some college | 1.10 (.89-1.37) | 1.13 (.91-1.40) | 1.14 (.92-1.42) | 1.14 (.92-1.41) |
| ≥ College | ||||
| Behavioral risk | .92 (.84-1.02) | .91 (.83-.99)* | .93 (.85-1.00) | .88 (.77-1.01) |
| Attribution | 1.79 (1.05-3.05)* | 1.30 (.81-2.10) | 1.82 (.86-3.87) | 1.20 (.68-2.10) |
| Family history | 1.30 (1.08-1.58)** | 1.33 (1.01-1.75)* | 1.08 (.89-1.31) | 1.11 (.92-1.34) |
| Hypertension OR (CI) | Lung Cancer OR (CI) | Colon Cancer OR (CI) | Skin Cancer OR (CI) | |
|---|---|---|---|---|
| Male | .85 (.70-1.02) | .84 (.69-1.01) | .85 (.70-1.03) | .82 (.68-.99)* |
| Married/partnered | 1.09 (.89-1.32) | 1.10 (.90-1.33) | 1.07 (.88-1.30) | 1.09 (.90-1.33) |
| Race | ||||
| Other | 1.90 (1.35-2.67)*** | 1.93 (1.38-2.71)*** | 1.90 (1.35-2.67)*** | 1.90 (1.35-2.65)*** |
| African American | 2.15 (1.76-2.64)*** | 2.29 (1.85-2.83)*** | 2.30 (1.87-2.82)*** | 2.28 (1.85-2.80)*** |
| White | ||||
| Education | ||||
| ≤ High school | 1.26 (.98-1.61) | 1.19 (.93-1.52) | 1.25 (.98-1.61) | 1.21 (.95-1.55) |
| Some college | 1.15 (.93-1.42) | 1.12 (.90-1.39) | 1.16 (.93-1.44) | 1.15 (.93-1.43) |
| ≥ College | ||||
| Behavioral risk | .91 (.81-1.02) | 1.01 (.81-1.25) | .93 (.86-1.01) | .92 (.76-1.12) |
| Attribution | 2.00 (1.06-3.76)* | 1.67 (.98-2.84) | 1.85 (1.11-3.10)* | 1.28 (.81-2.04) |
| Family history | 1.33 (1.05-1.69)* | 1.00 (.83-1.21) | .99 (.82-1.20) | 1.01 (.84-1.22) |
p < .05.
p < .01.
p < .001.
Discussion
Population-based data are needed to inform our understanding of whether genetic discovery may prompt the public to unduly favor genetics as a contributor to disease causation over behavioral and environmental factors. This report examined whether healthy adults’ pre-existing risk factors and education were associated with their explanations of the causes of common health conditions and the value given to health information about behaviors and genetics. Our findings suggest that as a whole, members of our large and diverse population-based sample, many with several behavioral risk factors, favored behavioral explanations for common diseases over genetic causes and valued information about health habits more than information about genetics. Indeed, in the case of many risk factors, such as BMI and current smoking status, our sample had similar or higher rates as compared to the general population [52]. These results are encouraging in that they do not suggest that this population gives genetic information inordinate precedence over behavioral information.
However, multivariate analyses suggest that these findings do not apply to all participants. Individuals with more behavioral risk factors tended to favor genetics to explain health conditions over behavior as a cause of disease, and placed less value on learning about how health habits affect disease risk. Individuals who had more risk factors may have responded defensively and endorsed genetics as a contributor to the health conditions and preferences for information consistent with these biases.
It is also possible that behaviorally at-risk participants who previously have sought and applied standard behavioral advice to reduce risk have not achieved success and in turn, see less value in behavioral information. Further, those at greater risk also may be experiencing message fatigue, a phenomenon occurring with long-term and repeated public health messages [53,54]. New methods might need to be considered to re-engage these individuals. The pattern of causal beliefs favoring genetics among those at higher behavioral risk might present a novel way to reengage those at risk with information they might not otherwise pursue. If those at higher behavioral risk are less interested in behavioral information due to defensive biases or fatigue, or some combination, novel information about the interrelationship between genetics, behavior and health outcomes might be more engaging.
Our findings did not support the hypothesis that those with family histories of five of the eight diseases favored genetic over behavioral explanations as causes of diseases. However, family history was associated consistently with interest in information about diabetes and heart disease. In a similar analog study of genetic testing for diabetes, heart disease, colon and lung cancer, having a family history of diabetes and heart disease was associated with these diseases being seen as less severe and worrisome than colon and lung cancer [55]. The broader genetic testing literature [16,56] suggests that direct experience with a disease impacts individual's perceptions of these diseases and their interest in information about how to reduce risk. Results also indicate that associations among risk factors, attributions, and information preferences varied by disease [55,57]. It is possible that defensive responses to risk information and its impact on information seeking preferences might vary by condition.
These data were collected in the context of a population-based study (i.e., with a known denominator) with a large, diverse population of healthy adults. Despite this strength, we note several limitations. First, we present cross-sectional data in this analysis. We can only speak to preferences for health information, not actual information-seeking. Also, given the sample size, many relatively small associations between psychosocial factors and primary outcomes were statistically significant, but may not be clinically meaningful. Further, our somewhat high refusal rate could potentially influence generalizability. Also, as is common in population-based research, our decision to reduce the participant burden and increase external validity resulted in the potential loss of measurement precision. For instance, our cancer family history measure was a global item for all cancers, not specific to each cancer. This may partially explain the lack of significant findings for our cancer models. Further, we acknowledge the lack of precision in assessing physical activity, dietary factors and alcohol consumption. For example, our physical activity measure assesses frequency each activity was performed for at least 15 minutes. This interval, as compared to 30 minute increments, may contribute to our high rates of physical activity (75%) as compared to the population (67%) [52]. Some participants also might have a poor understanding of some diseases or may not accurately self-report risk factors [58,59].
Despite these limitations, our findings contribute to the understanding of the extent to which healthy adults consider the contributions of genetics and health behaviors to disease causation and how these risk factors and attributions influence their preferences for seeking types of health information. As multiplex genetic susceptibility testing for common disease reaches the public, it is critical to understand how the public might respond to this information. Our results suggest that a diverse population of healthy adults do not, as a whole, over-ascribe common health conditions to genetics or hold defensively biased causal attributions that would inhibit needed behavior change. However, those with the greatest need for behavior change are at most risk for responding defensively and devaluing behavior change information. Future research should examine whether communication of the complex interaction of genetic and environmental influences on common conditions might increase the balance of participants’ interest in health information.
Acknowledgments
This work was supported by the Intramural Research Program of the National Human Genome Research Institute (NHGRI). However, the proposed research was made possible by collaboration with the Cancer Research Network funded by the National Cancer Institute (U19 CA 079689). Additional resources were provided by Group Health Research Institute and Henry Ford Hospital. Genotyping services were provided by the Center for Inherited Disease Research (CIDR). CIDR is fully funded through a federal contract from the National Institutes of Health to The Johns Hopkins University (HHSN268200782096C). We also thank the Multiplex steering committee (Drs. Colleen McBride, Lawrence Brody, Sharon Hensley Alford, Robert Reid, Eric Larson, Andreas Baxevanis and Sharon Kardia) who provided critical review of this report. Our thanks also go to the study participants who were all members of the Henry Ford Health System.
References
- 1.Feero WG, Guttmacher AE, Collins FS. The genome gets personal--almost. JAMA. 2008;299:1351–1352. doi: 10.1001/jama.299.11.1351. [DOI] [PubMed] [Google Scholar]
- 2.Goddard KA, Robitaille J, Dowling NF, et al. Health-related direct-to-consumer genetic tests: a public health assessment and analysis of practices related to Internet-based tests for risk of thrombosis. Public Health Genomics. 2009;12:92–104. doi: 10.1159/000176794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manolio TA, Collins FS, Cox NJ, et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–753. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gollust SE, Hull SC, Wilfond BS. Limitations of direct-to-consumer advertising for clinical genetic testing. JAMA. 2002;288:1762–1767. doi: 10.1001/jama.288.14.1762. [DOI] [PubMed] [Google Scholar]
- 5.Khoury MJ, Bradley LA. Why should genomic medicine become more evidence-based? Genomic Med. 2007;1:91–93. doi: 10.1007/s11568-007-9015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuehn BM. Risks and benefits of direct-to-consumer genetic testing remain unclear. JAMA. 2008;300:1503–1505. doi: 10.1001/jama.300.13.1503. [DOI] [PubMed] [Google Scholar]
- 7.Offit K. Genomic profiles for disease risk: predictive or premature? JAMA. 2008;299:1353–1355. doi: 10.1001/jama.299.11.1353. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention . Behavioral Risk Factor Surveillance System Survey Data. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, Georgia: 2008. [Google Scholar]
- 9.Carlsten C, Burke W. Potential for genetics to promote public health: genetics research on smoking suggests caution about expectations. JAMA. 2006;296:2480–2482. doi: 10.1001/jama.296.20.2480. [DOI] [PubMed] [Google Scholar]
- 10.Senior V, Marteau TM. Causal attributions for raised cholesterol and perceptions of effective risk-reduction: self-regulation strategies for an increased risk of coronary heart disease. Psychol Health. 2007;22:699–717. [Google Scholar]
- 11.Wright AJ, Weinman J, Marteau TM. The impact of learning of a genetic predisposition to nicotine dependence: an analogue study. Tob Control. 2003;12:227–230. doi: 10.1136/tc.12.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marteau TM, Weinman J. Self-regulation and the behavioural response to DNA risk information: a theoretical analysis and framework for future research. Soc Sci Med. 2006;62:1360–1368. doi: 10.1016/j.socscimed.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Griffin RJ, Dunwoody S, Neuwirth K. Proposed model of the relationship of risk information seeking and processing to the development of preventive behaviors. Environ Res. 1999;80:S230–S245. doi: 10.1006/enrs.1998.3940. [DOI] [PubMed] [Google Scholar]
- 14.Cameron LD, Leventhal H. The Self-Regulation of Health and Illness Behaviour. Routledge; New York: 2003. [Google Scholar]
- 15.Wiebe DJ, Korbel C. Defensive denial, affect, and the self-regulation of health threats. In: Cameron LD, Leventhal H, editors. The Self-Regulation of Health and Illness Behaviour. Routledge; New York: 2003. pp. 184–204. [Google Scholar]
- 16.Kelly KM, Andrews JE, Case DO, Allard SL, Johnson JD. Information seeking and intentions to have genetic testing for hereditary cancers in rural and Appalachian Kentuckians. J Rural Health. 2007;23:166–172. doi: 10.1111/j.1748-0361.2007.00085.x. [DOI] [PubMed] [Google Scholar]
- 17.Kunda Z. Motivated inference: self-serving generation and evaluation of causal theories. J Pers Soc Psychol. 1987;53:636–647. [Google Scholar]
- 18.Lieberman A, Chaiken S. Defensive processing of personally relevant health messages. Pers Soc Psychol Bull. 1992;18:669–679. [Google Scholar]
- 19.Sherman DK, Cohen GL. The psychology of self-defense: Self-affirmation theory. In: Zanna MP, editor. Advances in Experimental Social Psychology. Vol. 38. Academic Press; San Diego, CA: 2006. pp. 183–242. [Google Scholar]
- 20.van Koningsbruggen GM, Das E, Roskos-Ewoldsen DR. How self-affirmation reduces defensive processing of threatening health information: evidence at the implicit level. Health Psychol. 2009;28:563–568. doi: 10.1037/a0015610. [DOI] [PubMed] [Google Scholar]
- 21.Katapodi MC, Lee KA, Facione NC, Dodd MJ. Predictors of perceived breast cancer risk and the relation between perceived risk and breast cancer screening: a meta-analytic review. Prev Med. 2004;38:388–402. doi: 10.1016/j.ypmed.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Lipkus IM, Rimer BK, Lyna PR, Pradhan AA, Conaway M, Woods-Powell CT. Colorectal screening patterns and perceptions of risk among African-American users of a community health center. J Community Health. 1996;21:409–427. doi: 10.1007/BF01702602. [DOI] [PubMed] [Google Scholar]
- 23.Lipkus IM, Skinner CS, Green LS, Dement J, Samsa GP, Ransohoff D. Modifying attributions of colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2004;13:560–566. [PubMed] [Google Scholar]
- 24.Robb KA, Miles A, Wardle J. Perceived risk of colorectal cancer: sources of risk judgments. Cancer Epidemiol Biomarkers Prev. 2007;16:694–702. doi: 10.1158/1055-9965.EPI-06-0151. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez CD, Newby LK, McGuire DK, Hasselblad V, Feinglos MN, Ohman EM. Diabetes-related knowledge, atherosclerotic risk factor control, and outcomes in acute coronary syndromes. Am J Cardiol. 2005;95:1290–1294. doi: 10.1016/j.amjcard.2005.01.070. [DOI] [PubMed] [Google Scholar]
- 26.Shim M, Kelly B, Hornik R. Cancer information scanning and seeking behavior is associated with knowledge, lifestyle choices, and screening. J Health Commun. 2006;11(Suppl 1):157–172. doi: 10.1080/10810730600637475. [DOI] [PubMed] [Google Scholar]
- 27.Miller EA, West DM. Characteristics associated with use of public and private web sites as sources of health care information: results from a national survey. Med Care. 2007;45:245–251. doi: 10.1097/01.mlr.0000244509.60556.49. [DOI] [PubMed] [Google Scholar]
- 28.Quillin JM, Silberg J, Jones RM, et al. Tolerance for ambiguity could influence awareness of breast cancer genetic testing and inform health education. Cancer Causes Control. 2008;19:1227–1232. doi: 10.1007/s10552-008-9193-y. [DOI] [PubMed] [Google Scholar]
- 29.Singer E, Antonucci TC, Burmeister M, Couper MP, Raghunathan TE, Van Hoewyk J. Beliefs about genes and environment as determinants of behavioral characteristics. Int J Public Opin Res. 2007;19:331–353. [Google Scholar]
- 30.Dickerson S, Reinhart AM, Feeley TH, et al. Patient Internet use for health information at three urban primary care clinics. J Am Med Inform Assoc. 2004;11:499–504. doi: 10.1197/jamia.M1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petty RE, Cacioppo JT. Communication and Persuasion: Central and Peripheral Routes to Attitude Change. Springer-Verlag; New York: 1986. [Google Scholar]
- 32.Kaphingst KA, Lachance CR, Condit CM. Beliefs about heritability of cancer and health information seeking and preventive behaviors. J Cancer Educ. 2009;24:351–356. doi: 10.1080/08858190902876304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rutten LJ, Squiers L, Hesse B. Cancer-related information seeking: hints from the 2003 Health Information National Trends Survey (HINTS). J Health Commun. 2006;11(Suppl 1):147–156. doi: 10.1080/10810730600637574. [DOI] [PubMed] [Google Scholar]
- 34.Frank R. What to make of it? The (Re)emergence of a biological conceptualization of race in health disparities research. Soc Sci Med. 2007;64:1977–1983. doi: 10.1016/j.socscimed.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 35.Viswanath K. Science and society: the communications revolution and cancer control. Nat Rev Cancer. 2005;5:828–835. doi: 10.1038/nrc1718. [DOI] [PubMed] [Google Scholar]
- 36.McBride CM, Alford SH, Reid RJ, Larson EB, Baxevanis AD, Brody LC. Characteristics of users of online personalized genomic risk assessments: implications for physician-patient interactions. Genet Med. 2009;11:582–587. doi: 10.1097/GIM.0b013e3181b22c3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hensley Alford S, McBride CM, Reid RJ, Larson EB, Baxevanis AD, Brody LC. Participation in genetic testing research varies by social group. Public Health Genomics. 2010 doi: 10.1159/000294277. DOI: 10.1159/000294277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berke EM, Koepsell TD, Moudon AV, Hoskins RE, Larson EB. Association of the built environment with physical activity and obesity in older persons. Am J Public Health. 2007;97:486–492. doi: 10.2105/AJPH.2006.085837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Larson EB, Wang L, Bowen JD, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention [October 5, 2009];Physical activity for everyone. Available at http://www.cdc.gov/physicalactivity/everyone/guidelines/index.html.
- 41.Gaskins ND, Sloane PD, Mitchell CM, Ammerman A, Ickes SB, Williams CS. Poor nutritional habits: a modifiable predecessor of chronic illness? A North Carolina Family Medicine Research Network (NC-FM-RN) study. J Am Board Fam Med. 2007;20:124–134. doi: 10.3122/jabfm.2007.02.060151. [DOI] [PubMed] [Google Scholar]
- 42.Centers for Disease Control and Prevention [October 5, 2009];Smoking & tobacco use. Available at http://www.cdc.gov/tobacco/.
- 43.American Heart Association Nutrition Committee. Lichtenstein AH, Appel LJ, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 44.Kushi LH, Byers T, Doyle C, et al. American Cancer Society Guidelines on Nutrition and Physical Activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2006;56:254–281. doi: 10.3322/canjclin.56.5.254. [DOI] [PubMed] [Google Scholar]
- 45.Jacobs EJ, Connell CJ, Chao A, et al. Multivitamin use and colorectal cancer incidence in a US cohort: does timing matter? Am J Epidemiol. 2003;158:621–628. doi: 10.1093/aje/kwg190. [DOI] [PubMed] [Google Scholar]
- 46.National Institutes of Health. National Heart LaBI [November 4, 2009];Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. NIH publication no. 98-4083. Available at http://www.nhlbi.nih.gov/guidelines/obesity/ob_gdlns.htm.
- 47.Guthrie JR, Dennerstein L, Wark JD. Risk factors for osteoporosis: A review. Medscape Womens Health. 2000;5:E1. [PubMed] [Google Scholar]
- 48.Maxwell SE, Cole DA. Bias in cross-sectional analyses of longitudinal mediation. Psychol Methods. 2007;12:23–44. doi: 10.1037/1082-989X.12.1.23. [DOI] [PubMed] [Google Scholar]
- 49.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 50.Judd CM, Kenny DA. Process analysis: estimating mediation in treatment evaluations. Eval Rev. 1981;5:602–619. [Google Scholar]
- 51.MacKinnon DP. Analysis of mediating variables in prevention and intervention research. NIDA Res Monogr. 1994;139:127–153. [PubMed] [Google Scholar]
- 52.Schoenborn CA, Adams PF. Health behaviors of adults: United States, 2005-2007. National Center for Health Statistics. [April 26, 2010];Vital Health Stat. 2010 10(245) Available at http://cdc.gov/nchs/data/series/sr_10/sr10_245.pdf. [PubMed] [Google Scholar]
- 53.Goldberg JP. Nutrition and health communication: the message and the media over half a century. Nutr Rev. 1992;50:71–77. doi: 10.1111/j.1753-4887.1992.tb01272.x. [DOI] [PubMed] [Google Scholar]
- 54.Robinson T, Mayer J, Weaver F. Prevention message fatigue as an influence on condom use among urban MSM. Abstract #57839.. Paper presented at the 131st Annual Meeting (November 15-19, 2003) of the American Public Health Association (APHA); [December 12, 2009]. Available at http://apha.confex.com/apha/131am/techprogram/paper_57839.htm. [Google Scholar]
- 55.Cameron LD, Sherman KA, Marteau TM, Brown PM. Impact of genetic risk information and type of disease on perceived risk, anticipated affect, and expected consequences of genetic tests. Health Psychol. 2009;28:307–316. doi: 10.1037/a0013947. [DOI] [PubMed] [Google Scholar]
- 56.Braithwaite D, Emery J, Walter F, Prevost AT, Sutton S. Psychological impact of genetic counseling for familial cancer: a systematic review and meta-analysis. J Natl Cancer Inst. 2004;96:122–133. doi: 10.1093/jnci/djh017. [DOI] [PubMed] [Google Scholar]
- 57.Wang C, O'Neill SM, Rothrock N, et al. Comparison of risk perceptions and beliefs across common chronic diseases. Prev Med. 2009;48:197–202. doi: 10.1016/j.ypmed.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Merrill RM, Richardson JS. Validity of self-reported height, weight, and body mass index: findings from the National Health and Nutrition Examination Survey, 2001-2006. Prev Chronic Dis. 2009;6:A121. [PMC free article] [PubMed] [Google Scholar]
- 59.Moshfegh AJ, Rhodes DG, Baer DJ, et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am J Clin Nutr. 2008;88:324–332. doi: 10.1093/ajcn/88.2.324. [DOI] [PubMed] [Google Scholar]