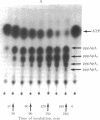
Abstract
2',5'-Oligoadenylate synthetase has been purified from a rabbit reticulocyte lysate to a high degree of purity. The enzyme contained no detectable interfering activities that could degrade the nucleoside triphosphate substrate or the oligomeric products. Two basic properties of this enzyme have been examined: the elongation mechanism for the synthesis of oligoadenylates and the substrate specificity for nucleotides. Kinetic studies on the formation of different oligomeric intermediates show that the dimer pppA2'p5'A is the first product to accumulate in predominant proportion during the first period of reaction; the trimer and other longer oligomers appear after a lag phase. The amount of the trimer increases at the expense of the dimer. Preformed dimers and trimers added to the incubation mixture were readily incorporated into higher oligomers, suggesting the free access of these dimers and trimers to the active center after the onset of polymerization of ATP. The results indicate clearly that the enzyme catalyzes the de novo synthesis of the oligonucleotide from ATP and that the mechanism of elongation of the 2',5'-oligonucleotides catalyzed by the enzyme is not processive. Polymerization of a mixture of ATP and another nucleoside triphosphate shows that the enzyme is not only an ATP polymerase. The 2',5'-oligoadenylate synthetase is in fact a 2',5'-nucleotidyltransferase that catalyzes the formation of co-oligonucleotides. However, the purified reticulocyte enzyme catalyzed only the addition of one unit of GMP, UMP, CMP, 2'-dAMP, 3'-dAMP, dCMP, dGMP, or TMP to the 2'-OH end of a preformed oligoadenylate. A procedure for the separation of 2',5'-oligonucleotides with or without the 5'triphosphate end also is described.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORSOOK H., DEASY C. L., HAAGENSMIT A. J., KEIGHLEY G., LOWY P. H. Incorporation in vitro of labeled amino acids into proteins of rabbit reticulocytes. J Biol Chem. 1952 May;196(2):669–694. [PubMed] [Google Scholar]
- Baglioni C., Minks M. A., Maroney P. A. Interferon action may be mediated by activation of a nuclease by pppA2'p5'A2'p5'A. Nature. 1978 Jun 22;273(5664):684–687. doi: 10.1038/273684a0. [DOI] [PubMed] [Google Scholar]
- Ball L. A. Induction of 2'5'-oligoadenylate synthetase activity and a new protein by chick interferon. Virology. 1979 Apr 30;94(2):282–296. doi: 10.1016/0042-6822(79)90462-8. [DOI] [PubMed] [Google Scholar]
- Ball L. A., White C. N. Nuclease activation by double-stranded RNA and by 2',5'-oligoadenylate in extracts of interferon-treated chick cells. Virology. 1979 Mar;93(2):348–356. doi: 10.1016/0042-6822(79)90239-3. [DOI] [PubMed] [Google Scholar]
- Farrell P. J., Sen G. C., Dubois M. F., Ratner L., Slattery E., Lengyel P. Interferon action: two distinct pathways for inhibition of protein synthesis by double-stranded RNA. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5893–5897. doi: 10.1073/pnas.75.12.5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godefroy T. Kinetics of polymerization and phosphorolysis reactions of Escherichia coli polynucleotide phosphorylase. Evidence for multiple binding of polynucleotide in phosphorolysis. Eur J Biochem. 1970 Jun;14(2):222–231. doi: 10.1111/j.1432-1033.1970.tb00281.x. [DOI] [PubMed] [Google Scholar]
- Gupta R. C., Randerath K. Use of specific endonuclease cleavage in RNA sequencing. Nucleic Acids Res. 1977 Jun;4(6):1957–1978. doi: 10.1093/nar/4.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovanessian A. G., Kerr I. M. Synthesis of an oligonucleotide inhibitor of protein synthesis in rabbit reticulocyte lysates analogous to that formed in extracts from interferon-treated cells. Eur J Biochem. 1978 Mar;84(1):149–159. doi: 10.1111/j.1432-1033.1978.tb12151.x. [DOI] [PubMed] [Google Scholar]
- Hovanessian A. G., Wood J., Meurs E., Montagnier L. Increased nuclease activity in cells treated with pppA2'p5'A2'p5' A. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3261–3265. doi: 10.1073/pnas.76.7.3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis A. P., White C., Ball A., Gupta S. L., Ratner L., Sen G. C., Colby C. Interferon-associated, dsRNA-dependent enzyme activities in a mutant 3T6 cell engaged in the semiconstitutive synthesis of interferon. Cell. 1978 Aug;14(4):879–887. doi: 10.1016/0092-8674(78)90343-4. [DOI] [PubMed] [Google Scholar]
- Kerr I. M., Brown R. E. pppA2'p5'A2'p5'A: an inhibitor of protein synthesis synthesized with an enzyme fraction from interferon-treated cells. Proc Natl Acad Sci U S A. 1978 Jan;75(1):256–260. doi: 10.1073/pnas.75.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E. M., Birdsall N. J., Brown R. E., Kerr I. M. Enzymic synthesis, characterisation and nuclear-magnetic-resonance spectra of pppA2'p5'A2'p5'A and related oligonucleotides: comparison with chemically synthesised material. Eur J Biochem. 1979 Apr 2;95(2):295–307. doi: 10.1111/j.1432-1033.1979.tb12965.x. [DOI] [PubMed] [Google Scholar]
- Minks M. A., Benvin S., Maroney P. A., Baglioni C. Metabolic stability of 2' 5'oligo (A) and activity of 2' 5'oligo (A)-dependent endonuclease in extracts of interferon-treated and control HeLa cells. Nucleic Acids Res. 1979 Feb;6(2):767–780. doi: 10.1093/nar/6.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minks M. A., Benvin S., Maroney P. A., Baglioni C. Synthesis of 2'5'-oligo(A) in extracts of interferon-treated HeLa cells. J Biol Chem. 1979 Jun 25;254(12):5058–5064. [PubMed] [Google Scholar]
- Randerath E., Randerath K. Ion-exchange thin-layer chromatography. XVI. Techniques for preparation and analysis of oligonucleotides. J Chromatogr. 1967 Dec;31(2):485–499. doi: 10.1016/s0021-9673(01)86099-4. [DOI] [PubMed] [Google Scholar]
- Ratner L., Wiegand R. C., Farrell P. J., Sen G. C., Cabrer B., Lengyel P. Interferon, double-stranded RNA and RNA degradation. Fractionation of the endonucleaseINT system into two macromolecular components; role of a small molecule in nuclease activation. Biochem Biophys Res Commun. 1978 Apr 14;81(3):947–954. doi: 10.1016/0006-291x(78)91443-2. [DOI] [PubMed] [Google Scholar]
- Schmidt A., Zilberstein A., Shulman L., Federman P., Berissi H., Revel M. Interferon action: isolation of nuclease F, a translation inhibitor activated by interferon-induced (2'-5') oligo-isoadenylate. FEBS Lett. 1978 Nov 15;95(2):257–264. doi: 10.1016/0014-5793(78)81006-0. [DOI] [PubMed] [Google Scholar]
- Stark G. R., Dower W. J., Schimke R. T., Brown R. E., Kerr I. M. 2-5A synthetase: assay, distribution and variation with growth or hormone status. Nature. 1979 Mar 29;278(5703):471–473. doi: 10.1038/278471a0. [DOI] [PubMed] [Google Scholar]
- Thang M. N., Harvey R. A., Grunberg-Manago M. Model for the elongation of polynucleotide chains by polynucleotide phosphorylase. J Mol Biol. 1970 Oct 28;53(2):261–280. doi: 10.1016/0022-2836(70)90299-8. [DOI] [PubMed] [Google Scholar]
- Vaquero C. M., Clemens M. J. Inhibition of protein synthesis and activation of nuclease in rabbit reticulocyte lysates by the unusual oligonucleotide pppA2'p5'A2'p5'A. Eur J Biochem. 1979 Jul;98(1):245–252. doi: 10.1111/j.1432-1033.1979.tb13182.x. [DOI] [PubMed] [Google Scholar]
- Williams B. R., Gilbert C. S., Kerr I. M. The respective roles of the protein kinase and pppA2' p5' A2' p5 A-activated endonuclease in the inhibition of protein synthesis by double stranded RNA in rabbit reticulocyte lysates. Nucleic Acids Res. 1979 Apr;6(4):1335–1350. doi: 10.1093/nar/6.4.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B. R., Golgher R. R., Kerr I. M. Activation of a nuclease by pppA2'p5'A2'p5'A in intact cells. FEBS Lett. 1979 Sep 1;105(1):47–52. doi: 10.1016/0014-5793(79)80885-6. [DOI] [PubMed] [Google Scholar]
- Wood J. N., Hovanessian A. G. Interferon enhances 2-5A synthetase in embryonal carcinoma cells. Nature. 1979 Nov 1;282(5734):74–76. doi: 10.1038/282074a0. [DOI] [PubMed] [Google Scholar]
- Zilberstein A., Kimchi A., Schmidt A., Revel M. Isolation of two interferon-induced translational inhibitors: a protein kinase and an oligo-isoadenylate synthetase. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4734–4738. doi: 10.1073/pnas.75.10.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]