Abstract
Evidence from the Seychelles Child Development Nutrition Study suggests that maternal nutritional status can modulate the relationship between prenatal methylmercury (MeHg) exposure and developmental outcomes in children. The aim of this study was to investigate whether maternal PUFA status was a confounding factor in any possible associations between prenatal MeHg exposure and developmental outcomes at 5 y of age in the Republic of Seychelles. Maternal status of (n-3) and (n-6) PUFA were measured in serum collected at 28 wk gestation and delivery. Prenatal MeHg exposure was determined in maternal hair collected at delivery. At 5 y of age, the children completed a comprehensive range of sensitive developmental assessments. Complete data from 225 mothers and their children were available for analysis. Multiple linear regression analyses revealed Preschool Language Scale scores of the children improved with increasing maternal serum DHA [22:6(n-3)] concentrations and decreased with increasing arachidonic acid [20:4(n-6)] concentrations, albeit verbal intelligence improved with increasing (n-6) PUFA concentrations in maternal serum. There were no adverse associations between MeHg exposure and developmental outcomes. These findings suggest that higher fish consumption, resulting in higher maternal (n-3) PUFA status, during pregnancy is associated with beneficial developmental effects rather than detrimental effects resulting from the higher concomitant exposures of the fetus to MeHg. The association of maternal (n-3) PUFA status with improved child language development may partially explain the authors’ previous finding of improving language scores, as prenatal MeHg exposure increased in an earlier mother-child cohort in the Seychelles where maternal PUFA status was not measured.
Introduction
The potential of nutrients to modify metal toxicity is an area of growing scientific interest. Fish is the primary dietary source of long-chain PUFA, which are critical for normal brain development, and is also the main mode of human exposure to methylmercury (MeHg)7, a neurotoxicant at high exposures (1). The amount of MeHg found within ocean fish varies widely by species and whether there are adverse neurodevelopmental effects at lower exposure levels is unclear. Research on fish consumption during pregnancy indicates that allowing for PUFA present in fish in statistical analysis may influence whether or not neurocognitive associations with MeHg are found (2, 3). Other studies have found direct beneficial associations between fish consumption and child development (4, 5). The safety of fish consumption is an important issue when as many as 3 billion people worldwide depend on fish as a daily dietary source of protein (6). The US FDA and the US Environmental Protection Agency jointly issued a fish consumption advisory cautioning women of child-bearing age to limit fish consumption because of possible MeHg toxicity (7). However, the advisory did not address the benefits of fish consumption or consider the importance of PUFA also present in fish. Subsequent to its issuance, the already-limited fish consumption among American women of childbearing age dropped further (8, 9). Fish is the primary dietary source of the (n-3) PUFA DHA, which is essential during neurodevelopment; therefore, limiting fish consumption during pregnancy could adversely affect child development. Consequently, a recent joint report from the FAO/WHO recommended that the neurodevelopmental benefits of consuming fish be explicated (10).
The Seychelles Child Development Nutrition Study (SCDNS) is addressing this issue in a longitudinal, prospective cohort study examining the development of offspring of mothers who consumed a diet high in fish (mean ± SD of 527 ± 327 g/wk) during pregnancy. The first reports from this cohort (2, 3) indicated that maternal (n-3) PUFA status, including DHA, was associated with an improved psychomotor development index as measured by the Bayley Scales of Infant Development II at 9 mo of age. At 30 mo of age on the same outcome, the authors found an adverse association with maternal MeHg, but only when it had been adjusted for maternal serum (n-3) PUFA concentrations. The authors also reported that as (n-6) PUFA, including arachidonic acid [AA; 20:4(n-6)] increased, there was decreasing performance on the 30-mo Bayley Scales of Infant Development II psychomotor development index.
These results are consistent with the growing literature on the benefits of consuming a diet high in fish (4, 5, 11–18). The longitudinal design of the present study allows examination of the children at older ages to determine if associations present early in life persist. The authors previously showed that scores at 66 mo of age on the Preschool Language Scale (PLS) slightly improved with increasing maternal MeHg exposure in a different cohort in the Seychelles Child Development Study (SCDS) and in which maternal PUFA status was not measured (19). The aim of the current study of SCDNS children at 5 y of age was to confirm earlier findings and to determine if confounding by maternal PUFA status could potentially explain the positive associations between certain developmental outcomes and prenatal MeHg exposure previously found in an earlier mother-child cohort in the Seychelles.
Participants and Methods
Setting.
The study was conducted in the Republic of Seychelles, an Indian Ocean archipelago with ∼85,000 inhabitants largely of African descent. Health care, education, and social services are free, readily available, and modeled on the British system. The local diet is high in fish (3); MeHg concentrations in local fish are similar to those of fish commercially available around the world (20), and sea mammals are not consumed. The population is not exposed to significant levels of other environmental pollutants, such as polychlorinated biphenyls, pesticides, or lead (21). The study was reviewed and approved by the Seychelles Ethics Board and the Research Subjects Review Board at the University of Rochester.
Participants.
In 2001, all women making a first visit to their local Antenatal Clinic were invited to participate if they were Seychellois and at least 16 y of age. Enrollment was completed when 300 volunteers had given informed consent. Maternal age ranged from 16 to 43 y and gestational age ranged from 14 to 24 wk. At 5 y of age, there were 275 eligible children of whom 256 completed the 5-y evaluation. However, only 225 children had complete covariate data (114 males and 111 females) and were available for the primary analysis.
Design and procedure.
The study was double-blinded; neither the observers nor the participants knew the MeHg exposure measures. The test battery included the following specific developmental tests; finger-tapping (FT) for the dominant and nondominant hand to measure dexterity and speed; the Preschool Language Scale-Revised Edition, yielding a total language score (PLS-TL) and scores for verbal ability (PLS-VA) and auditory comprehension (PLS-AC); the Woodcock-Johnson Scholastic Achievement Test, second edition, measuring letter-word recognition and applied problems; and the Child Behavior Checklist. The authors used the Kaufman Brief Intelligence Test to estimate both maternal and child IQ, comprising the subtests verbal knowledge (KBIT-VK) and matrices (KBIT-M), which were treated as separate outcomes for each child. One subtest of riddles was omitted, because riddles are an uncommon means of communication in the Seychelles. Only the KBIT-M subtest was used to estimate maternal IQ, because mothers in the Seychelles are less familiar than their offspring with reading the English language.
A team of experienced maternal and child health nurses were specifically trained in Rochester to administer all the tests. All tests were translated to Creole, the native language spoken in the Republic of Seychelles.
Exposure measures.
The authors measured (n-3) and (n-6) PUFA as total lipids (including phospholipids) in maternal serum samples taken at 28 wk gestation and delivery as described earlier (3). The primary PUFA focus was on DHA and AA, the most important PUFA for normal brain growth and development (22). EPA was measured[20:5(n-3)] in addition to DHA to represent the major (n-3) PUFA found in fish. α-Linolenic acid [18:3(n-3)] was measured to calculate total (n-3) PUFA. Linoleic acid was measured [18:2(n-6)] in addition to AA to give a measure of total (n-6) PUFA, which may have antagonistic associations with developmental outcomes. The geometric mean of the PUFA values measured at 28 wk of gestation and delivery was used because the transfer of PUFA from the mother to the fetus occurs mainly during the third trimester (23). The geometric mean averages the logarithm of the 2 pertinent values, before exponentiating to put the final value on the original scale. Missing PUFA values (8% at 28 wk and 20% at delivery) were imputed as previously described (2). Briefly, a missing PUFA value at one time period was imputed as the predicted value based on the observed value at the other time period and an assumed bivariate lognormal distribution at the 2 time periods.
The authors used mean maternal hair mercury (Hg) during pregnancy as the biomarker for prenatal MeHg exposure. Hair samples were cut at delivery and the longest available segment of maternal hair growing during gestation was analyzed, assuming a hair growth rate of 1.1 cm/mo. Total and inorganic Hg was measured by cold vapor atomic absorption as previously described (24).
Statistical analysis.
Covariate-adjusted linear regression models were run to test the associations between each of the 10 outcomes and different combinations of PUFA (see below). Twelve models (6 ways of coding PUFA, each with and without adjustment for prenatal MeHg) were fitted for each outcome. Analysis was also conducted to investigate associations between prenatal MeHg exposure and developmental outcomes without adjusting for maternal PUFA status. Model assumptions, including linearity, constant variance, and normally distributed residuals, were checked using standard methods (25). If model assumptions were violated, the relevant outcome variable was transformed to better satisfy these model assumptions. Models were also examined for outliers, based on studentized residuals, and influential points, based on Cook’s distances (25). No model had unduly influential observations (all Cook’s distances were < 0.5). Unless otherwise noted, model results include all observations. If an overall model F-test was not significant at the 0.05 level, further results for the model were not reported. Within each significant model, the authors used a 2-sided α level of P ≤ 0.05 to determine the significance of independent variable effects.
The authors tested for associations between outcomes and the following combinations of PUFA: model 1: DHA, AA; model 2: the sum of DHA+EPA, AA; model 3: DHA, the sum of AA+linoleic acid [(n-6) PUFA]; model 4: the sum of DHA+EPA+α-linolenic acid [(n-3) PUFA], (n-6) PUFA; model 5: AA:DHA ratio; and model 6: (n-6):(n-3) PUFA ratio. These models were the result of a priori decisions based on our primary hypothesis that maternal PUFA status might confound the possible association between MeHg and developmental outcomes. Model 1 was chosen based on DHA and AA being the most important PUFA in neurodevelopment. Model 2 included AA and also focused on the major (n-3) PUFA found in fish. Models 3 and 4 were designed to test possible antagonistic effects of (n-6) PUFA on the (n-3) PUFA, and models 5 and 6 investigated the possible antagonistic effects of (n-6) PUFA on (n-3) PUFA as a ratio.
All models were run twice, first including prenatal MeHg exposure and again without correcting for MeHg. All models were adjusted for covariates selected a priori for their reported associations with neurodevelopmental outcomes, including birth weight, child age at testing, socioeconomic status [the Hollingshead Four-Factor Socioeconomic Status modified for use in the Seychelles (19)], home environment (the Pediatric Review of Children’s Environmental Support and Stimulation), maternal IQ (the KBIT-M subtest), sex of the child, family status (the number of immediate family members living with the child, recorded as either < 2 or ≥ 2), and maternal age.
Results
A total of 225 mother-child pairs had complete data for model covariates and outcomes. Table 1 shows the mean, SD, and range for each predictor variable (prenatal MeHg exposure and maternal serum PUFA status) and developmental outcome score. The mean prenatal MeHg exposure was 28.5 nmol/g maternal hair, ∼5 nmol/g lower than that found in the SCDS Main Cohort, enrolled in 1989–1990 (19), but 12 times higher than the U.S. mean of 2.3 nmol/g (26).
TABLE 1.
Maternal concentrations of hair MeHg, serum PUFA, and developmental outcome scores of 5-y-old children1
| Variable | |
| Prenatal hair MeHg, nmol Hg/g | 28.5 ± 18.4 (0.9, 92.2) |
| Maternal serum DHA, mmol/L | 0.08 ± 0.02 (0.03, 0.2) |
| Maternal serum EPA + DHA, mmol/L | 0.09 ± 0.03 (0.04, 0.2) |
| Maternal serum AA, mmol/L | 0.3 ± 0.1 (0.2, 0.6) |
| Maternal serum (n-3) PUFA, mmol/L | 0.1 ± 0.03 (0.04, 0.2) |
| Maternal serum (n-6) PUFA, mmol/L | 4.3 ± 0.7 (2.3, 6.1) |
| Maternal serum AA:DHA ratio | 3.9 ± 0.9 (1.9, 7.7) |
| Maternal serum (n-6):(n-3) PUFA ratio | 46.0 ± 13.5 (15.1,103) |
| FT, dominant hand, total taps in 10 s | 23.4 ± 5.7 (5.4, 39.6) |
| FT, nondominant hand, total taps in 10 s | 21.3 ± 4.8 (8.6, 34.8) |
| PLS-TL | 119 ± 5.4 (100, 128) |
| PLS-AC | 55.6 ± 2.7 (47.0, 60.0) |
| PLS-VA | 63.1 ± 3.2 (51.0, 68.0) |
| WJ-AP | 15.1 ± 4.1 (2.0, 24.0) |
| WJ-LW | 10.9 ± 6.0 (1.0, 24.0) |
| CBCL, total t score | 59.3 ± 8.7 (25.0, 77.0) |
| KBIT-VK | 11.8 ± 2.8 (6.0, 18.0) |
| KBIT-M | 7.7 ± 1.2 (2.0, 9.0) |
Values are mean ± SD (range), n = 225. AA, arachidonic acid; ALA, α-linolenic acid; CBCL, Child Behavior Checklist; FT, finger-tapping; KBIT-VK, Kaufman Brief Intelligence Test-verbal knowledge; KBIT-M, Kaufman Brief Intelligence Test-matrices; MeHg, methylmercury; PLS-AC, Preschool Language Scale-auditory comprehension; PLS-TL, Preschool Language Scale-total language score; PLS-VA, Preschool Language Scale- verbal ability; WJ-AP, Woodcock-Johnson Scholastic Achievement Test-applied problems; WJ-LW, Woodcock-Johnson Scholastic Achievement Test-letter-word recognition.
All models were significant for all outcomes except some FT models. For the FT dominant hand, models 1 and 2, which included prenatal MeHg, were not significant. For the FT nondominant hand, no models were significant. Analysis conducted between prenatal MeHg and the 10 outcomes revealed one significant positive association (with PLS-TL; β = 0.04; P = 0.04). However, prenatal MeHg was not significantly associated with any outcome in any model. In contrast, maternal PUFA status was significantly associated with 4 developmental outcomes: PLS-TL, PLS-AC, PLS-VA, and the KBIT-VK scores (Table 2). The results for these 4 outcomes in models that adjusted for prenatal MeHg are shown in Table 2. The PLS-TL score increased with increasing DHA with and without adjusting for prenatal MeHg and covariates (model 1). The PLS-TL score increased with increasing DHA plus EPA (model 2), but this relationship was significant only without adjustment for prenatal MeHg. There was a significant inverse association between AA and the PLS-TL score in models 1 and 2, both with and without adjustment for prenatal MeHg. AA also had a significant inverse association with the PLS-AC score (models 1 and 2), and the PLS-VA score (model 1). For the PLS-AC and PLS-TL scores, there was an inverse association with the AA:DHA ratio (model 5), but it was present only without adjustment for MeHg exposure.
TABLE 2.
Associations among maternal serum PUFA, prenatal hair MeHg, and developmental outcomes of 5-y-old children1
| PLS-TL |
PLS-AC |
PLS-VA |
KBIT-VK |
|||||||||
| Variable | Coefficient | SE | P value | Coefficient | SE | P value | Coefficient | SE | P value | Coefficient | SE | P value |
| Model 1 | ||||||||||||
| Maternal serum DHA, mmol/L | 41.3 | 19.3 | 0.03* | 16.7 | 9.7 | 0.1 | 24.6 | 12.2 | 0.04* | 3.3 | 10.5 | 0.8 |
| Maternal serum AA, mmol/L | −15.8 | 6.5 | 0.02* | −7.5 | 3.2 | 0.02* | −8.3 | 4.1 | 0.04* | 1.2 | 3.5 | 0.7 |
| Prenatal hair MeHg, nmol/g | 0.03 | 0.02 | 0.2 | 0.01 | 0.01 | 0.2 | 0.01 | 0.01 | 0.3 | 0.0 | 0.01 | 0.7 |
| Model 2 | ||||||||||||
| Maternal serum DHA + EPA, mmol/L | 31.7 | 16.6 | 0.1 | 13.1 | 8.3 | 0.1 | 18.6 | 10.5 | 0.1 | 0.01 | 8.9 | 1.0 |
| Maternal serum AA, mmol/L | −14.7 | 6.4 | 0.02* | −7.1 | 3.2 | 0.03* | −7.6 | 4.0 | 0.1 | 1.9 | 3.5 | 0.6 |
| Prenatal hair MeHg, nmol/g | 0.03 | 0.02 | 0.2 | 0.01 | 0.01 | 0.2 | 0.01 | 0.01 | 0.3 | 0.01 | 0.01 | 0.6 |
| Model 3 | ||||||||||||
| Maternal serum DHA, mmol/L | 14.4 | 16.4 | 0.4 | 3.7 | 8.2 | 0.7 | 10.7 | 10.3 | 0.3 | −1.1 | 8.7 | 0.9 |
| Maternal serum (n-6) PUFA, mmol/L | −0.2 | 0.5 | 0.7 | −0.1 | 0.3 | 0.8 | −0.1 | 0.3 | 0.7 | 0.6 | 0.3 | 0.02* |
| Prenatal hair MeHg, nmol/g | 0.03 | 0.02 | 0.1 | 0.02 | 0.01 | 0.1 | 0.02 | 0.01 | 0.2 | 0.0 | 0.01 | 0.7 |
| Model 4 | ||||||||||||
| Maternal serum (n-3) PUFA, mmol/L | 10.0 | 13.7 | 0.5 | 2.7 | 6.8 | 0.7 | 7.4 | 8.6 | 0.4 | −1.5 | 7.2 | 0.8 |
| Maternal serum (n-6) PUFA, mmol/L | −0.1 | 0.5 | 0.8 | −0.1 | 0.2 | 0.8 | −0.1 | 0.3 | 0.8 | 0.6 | 0.3 | 0.02* |
| Prenatal hair MeHg, nmol/g | 0.03 | 0.02 | 0.1 | 0.02 | 0.01 | 0.1 | 0.02 | 0.01 | 0.2 | 0.0 | 0.01 | 0.7 |
| Model 5 | ||||||||||||
| Maternal serum AA:DHA ratio | −0.6 | 0.4 | 0.1 | −0.3 | 0.2 | 0.1 | −0.4 | 0.2 | 0.1 | −0.0 | 0.2 | 0.9 |
| Prenatal hair MeHg, nmol/g | 0.03 | 0.02 | 0.1 | 0.01 | 0.01 | 0.2 | 0.02 | 0.01 | 0.2 | 0.01 | 0.01 | 0.6 |
Significant results defined by *P < 0.05, from fully adjusted multiple linear regression models. AA, arachidonic acid; KBIT-VK, Kaufman Brief Intelligence Test-verbal knowledge; MeHg, methylmercury; PLS-AC, Preschool Language Scale-auditory comprehension; PLS-TL, Preschool Language Scale-total language score; PLS-VA, Preschool Language Scale-verbal ability.
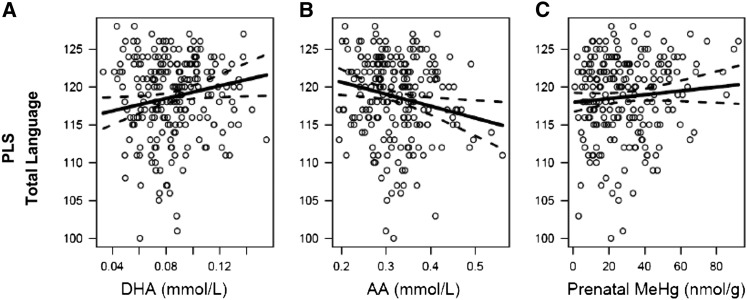
For the KBIT-VK subtest, scores were estimated to increase with increasing (n-6) PUFA concentrations in models 3 and 4 (Table 2). These associations were significant with and without adjustment for MeHg. The association of maternal PUFA status and prenatal MeHg with the PLS-TL score present in model 1 is shown graphically in Figure 1. There was a positive association with DHA (Fig. 1A), an inverse association with AA (Fig. 1B), and no association with prenatal MeHg (Fig. 1C).
FIGURE 1.
Associations between the 5-y PLS-TL score and maternal serum DHA (A; P = 0.03), AA (B; P = 0.02), and prenatal hair MeHg (C; P = 0.20). Circles represent individual data points and the solid line is the fully adjusted regression line using all available observations (n = 225). Dotted lines are the confidence limits for the adjusted regression. AA, arachidonic acid; MeHg, methylmercury; PLS-TL, Preschool Language Scale-total language.
The coefficients, SE, and P values with respect to the covariates from model 1 with prenatal MeHg are shown in Table 3. The coefficients for covariates from the other models were very similar to these results and are therefore not depicted. Older children performed significantly better than younger children even though the tests were normed, and girls had higher scores than boys on some but not all tests. The scores on the Woodcock-Johnson Scholastic Achievement Test-applied problems and the Child Behavior Checklist were significantly influenced by maternal IQ.
TABLE 3.
Associations between maternal and child covariates and developmental outcomes of 5-y-old children1
| PLS-TL |
PLS-AC |
PLS-VA |
KBIT-VK |
|||||||||
| Variable | Coefficient | SE | P value | Coefficient | SE | P value | Coefficient | SE | P value | Coefficient | SE | P value |
| Sex (girl) | 1.4 | 0.7 | 0.05* | 0.3 | 0.3 | 0.4 | 1.1 | 0.4 | 0.01* | 0.8 | 0.4 | 0.02* |
| Family status | 0.3 | 0.7 | 0.6 | 0.4 | 0.4 | 0.2 | −0.1 | 0.4 | 0.8 | 0.3 | 0.4 | 0.5 |
| Maternal age | 0.1 | 0.1 | 0.2 | 0.1 | 0.03 | 0.1 | 0.03 | 0.04 | 0.4 | 0.03 | 0.03 | 0.4 |
| Maternal IQ2 | 0.03 | 0.02 | 0.3 | 0.02 | 0.01 | 0.1 | 0.0 | 0.02 | 0.8 | 0.01 | 0.01 | 0.4 |
| SES | 0.03 | 0.03 | 0.4 | 0.02 | 0.02 | 0.3 | 0.01 | 0.02 | 0.6 | 0.03 | 0.02 | 0.1 |
| Home environment3 | 0.03 | 0.02 | 0.2 | 0.01 | 0.01 | 0.5 | 0.03 | 0.02 | 0.1 | −0.0 | 0.01 | 0.9 |
| Child age at testing | 5.9 | 1.2 | 0.01** | 3.5 | 0.6 | 0.01** | 2.4 | 0.7 | 0.01** | 2.9 | 0.6 | 0.01** |
| Birth weight | −0.0 | 0.0 | 0.6 | 0.0 | 0.0 | 0.9 | −0.0 | 0.0 | 0.4 | 0.0 | 0.0 | 0.2 |
Significant results as defined by *P < 0.05, **P < 0.01 (linear regression models). KBIT-M, Kaufman Brief Intelligence Test-matrices; KBIT-VK, Kaufman Brief Intelligence Test -verbal knowledge; PLS-AC, Preschool Language Scale-auditory comprehension; PLS-TL, Preschool Language Scale-total language score; PLS-VA, Preschool Language Scale-verbal ability; SES, Hollingshead Four-Factor Socioeconomic Status.
Assessed by the KBIT-M subtest.
Assessed by the Pediatric Review of Children’s Environmental Support and Stimulation.
Discussion
The authors found significant positive associations between maternal (n-3) PUFA status and test scores on several measures of child neurodevelopment. Maternal status of DHA was a significant positive predictor of the PLS-TL and PLS-VA outcomes. As maternal DHA concentrations rose, test scores on the PLS increased. These associations agree with experimental data that indicate an important role for DHA in brain development and function (14). Fish is the primary dietary source of EPA and DHA, and DHA is particularly important for brain development. Some, but not all, studies have shown positive effects of maternal DHA or fish oil supplementation on mental and motor development measured in their offspring during early childhood (27–29). Findings from these supplementation studies are supported by observational studies showing the benefits of maternal fish intake on neurodevelopmental outcomes in childhood (4, 30). Moreover, our longitudinal data also suggest that the positive associations of maternal (n-3) PUFA status with outcomes found in this cohort at age 9 (3) and 30 mo of age persist to 5 y of age (31).
The authors found no associations between prenatal MeHg exposure from frequent fish consumption and 10 developmental outcomes in models adjusting for maternal PUFA status. The absence of associations with prenatal MeHg exposure when analyses are corrected for maternal nutritional status provides important insights into our previously reported findings from the SCDS main cohort at 5 y of age (19). In the earlier study, maternal nutritional status was not measured, but it was found that the PLS-TL score was among several outcomes that had higher scores with increasing prenatal MeHg exposure in the range being studied. The authors postulated that those associations might be explained by important nutrients derived from maternal fish consumption and which could be driving the positive association found between MeHg (a proxy measure of fish consumption) and developmental outcomes. This positive association between prenatal MeHg and the PLS-TL score was observed, in the absence of adjustment for PUFA status, in the analysis of this cohort of 5-y-old children and confirmed the previous suggestion that maternal PUFA status was driving this association. Aside from language functions, other developmental domains were measured, including cognitive ability, motor speed, and problem behaviors, in this study. In past studies in which maternal PUFA status was not measured, only language functions were positively related to MeHg exposure (19). The lack of associations between domains other than language functions and either MeHg exposure or PUFA status in the current study is consistent with these earlier findings. It is possible that associations with domains other than language function will emerge with further examinations at older ages.
The negative associations between developmental outcomes and (n-6) PUFA, first found in this cohort at 9 mo of age (3), are still present with respect to the (n-6) PUFA, AA, and language ability at 5 y of age. Total (n-6) PUFA, however, was a significant positive predictor of child KBIT-VK. The (n-3) and (n-6) PUFA are precursors for a number of eicosanoids and other metabolites that have various and opposing physiological functions in humans (32). For example, eicosanoids derived from DHA and EPA have antiinflammatory properties. Consequently, these PUFA, for which fish is the best dietary source, could play a critical role in protecting against early oxidative or inflammatory insults such as those that may be induced by MeHg toxicity (33). In contrast, eicosanoids derived from the (n-6) PUFA AA have proinflammatory effects. Although AA may be important for general brain growth, it would not protect against oxidative or inflammatory insults (31). It is also possible that the contrasting effects of the (n-3) and (n-6) PUFA on mitigating inflammatory processes may be important only at higher levels of MeHg exposure with correspondingly higher inflammatory insults. The mechanisms underlying the interrelationships between MeHg and PUFA from fish consumption and the overall diet appear to be complex and need further clarification.
In these analyses, associations between maternal PUFA status and some developmental outcomes weakened after adjusting for prenatal MeHg exposure. These findings differ from our earlier results where correcting for prenatal MeHg strengthened the associations between PUFA and cognitive outcomes (3). This finding suggests that with increasing age, maternal PUFA status may become more important for developmental outcomes than prenatal MeHg exposure.
Postnatal exposure was not included in these analyses, because the primary concern for MeHg exposure from fish consumption has been prenatal exposure. In addition, the SCDNS was originally designed to investigate prospectively the association of MeHg exposure and nutrients from fish consumption during pregnancy with child developmental outcomes. The paradigm followed in both nutrition and toxicology is that prenatal exposures, if important for longer term development, should be able to be demonstrated through associations that are robust even in the face of differing postnatal exposures.
The authors examined the (n-3) PUFA as a group, because these fatty acids are known to have antiinflammatory effects that in turn might protect against inflammatory insults of MeHg on brain tissue. Although the total (n-3) PUFA in maternal serum was almost entirely comprised of DHA (Table 1), the authors’ a priori analysis plan included EPA as the goal was to investigate (n-3) PUFA from fish intake (2). The authors do not have a good explanation for why the maternal serum (n-6):(n-3) PUFA ratio was so high. There are a large number of factors, including diet, metabolism, and possibly genetics, that affect the biological status of PUFA and it is difficult to compare these values among studies (34–36). Fatty acid metabolism is known to be altered in pregnant women (37). It is therefore possible that greater amounts of DHA are being selectively transferred to the fetus via the placenta and such a mechanism could account for lower (n-3) PUFA and higher (n-6) PUFA found in the maternal circulation in this cohort. Another possibility is that, even though every effort was made to prevent any deterioration of the serum samples prior to processing, there may have been selective oxidation of the more susceptible PUFA among a random subset of the serum samples. If true, this possibility would result in nondifferential measurement error of these PUFA and in the current study the observed associations would be closer to the null hypothesis than the true associations.
This study has a number of strengths. Mothers consumed large quantities of fish and had hair MeHg levels ∼12 times higher than in U.S. women. The participants were enrolled early in pregnancy and PUFA status was measured both during pregnancy and at delivery. The children’s test battery incorporated 10 sensitive and specific developmental outcomes as measures of preschool cognitive function. These tests were similar to those recommended by Cheatham et al. (38) for the study of (n-3) PUFA and cognitive development. The study also has some limitations. It was not designed to evaluate interactions between PUFA and MeHg. Therefore, the authors were not able to determine whether the positive associations between maternal PUFA status and developmental outcomes reflect a direct influence of nutritional status on development or a modification of possible MeHg neurotoxicity. Current efforts are ongoing to examine interactions between MeHg and nutrients in a newly recruited SCDNS cohort with a sample size sufficiently large to evaluate such interactions. Inherent to the overall aim and design of the SCDNS, multiple measures of exposures and cognitive outcomes and multiple comparisons that may be of concern were included. The authors chose not to correct for multiple testing for 3 reasons. First, the statistical models are not independent but instead address the same underlying hypothesis. Second, the interpretation of the results is strongly guided by prior data reported in a different SCDS cohort at 66 mo of age. And third, simple correction of multiple testing (e.g., Bonferroni adjustment) is overly conservative and suffers from numerous limitations (39, 40).
This study adds to previous findings from the SCDS that fish consumption has benefits for child neurodevelopment and highlights the importance of adjusting for maternal nutritional status in studies investigating associations between MeHg exposure and outcomes. The findings do not support the hypothesis that there is a risk from prenatal MeHg exposure and it has been conducted in a population with prenatal MeHg exposure > 12 times that of the US and consuming > 10 times more fish than in the US. These findings are highly pertinent in light of the recent report from the FAO/WHO that highlighted the benefits of fish consumption during pregnancy (10).
Supplementary Material
Acknowledgments
J.J.S. and P.W.D. had full access to all data in the study, with the exception of Hg data, and take responsibility for the integrity of the data and accuracy of the data analysis. G.E.W. takes responsibility for the integrity of the Hg data. J.J.S., P.W.D., M.S.M., A.J.M., C.F.S., G.E.W., D.A.C-S., J.M.W.W., E.M.M., M.P.B., T.W.C., and G.J.M. were involved in study concept and design. P.W.D., C.F.S., D.A.C.-S., J.H., and J.S.-R. were involved in acquisition of the data. J.J.S, P.W.D., S.W.T., D.H., M.S.M., A.J.M., E.v.W., G.E.W., G.Z., M.L., J.M.W.W., E.M.M., M.P.B., A.S.-R., J.J., and G.J.M. were involved in statistical analysis and data interpretation. P.W.D., E.v.W., J.S.-R., J.H., J.J., and R.W. provided administrative, technical, or material support. J.J.S., P.W.D., E.v.W., C.F.S., and G.J.M provided overall study supervision. This body of work would have not been possible without the contributions of our late colleagues Octavie Choisy and Julie Wallace who are missed by all of the authors. All authors read and approved the final manuscript.
Footnotes
Supported by grants 5-RO1-ES010219, 5-R01-ES-015578, P30-ES01247, and T32-ES007271 from the U.S. National Institute of Environmental Health Sciences, NIH, and by the Government of the Republic of Seychelles. Participation of investigators from the Republic of Seychelles and the University of Ulster was partially supported by the EU through its Sixth Framework Programme for RTD (contract no. FOOD-CT-2006- 016253). This manuscript reflects only the authors’ views. The Community is not liable for any use that may be made of the information contained therein. Sponsors provided financial support and facilities for the conduct of the research. No sponsor had any role in design of the study, analysis and interpretation of results, or in preparation of this report.
Abbreviations used: AA, arachidonic acid; ALA, α-linolenic acid; FT, finger-tapping; KBIT-M, Kaufman Brief Intelligence Test-matrices; KBIT-VK, Kaufman Brief Intelligence Test-verbal knowledge; MeHg, methylmercury; PLS-AC, Preschool Language Scale-auditory comprehension; PLS-TL, Preschool Language Scale-total language score; PLS-VA, Preschool Language Scale-verbal ability; SCDNS, Seychelles Child Development Nutrition Study; SCDS, Seychelles Child Development Study.
Literature Cited
- 1.Clarkson TW, Strain JJ. Nutritional factors may modify the toxic action of methyl mercury in fish-eating populations. J Nutr. 2003;133:S1539–43 [DOI] [PubMed] [Google Scholar]
- 2.Strain JJ, Davidson PW, Bonham MP, Duffy EM, Stokes-Riner A, Thurston SW, Wallace JM, Robson PJ, Shamlaye CF, Georger LA, et al. Associations of maternal long-chain polyunsaturated fatty acids, methyl mercury, and infant development in the Seychelles Child Development Nutrition Study. Neurotoxicology. 2008;29:776–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonham MP, Duffy EM, Wallace JM, Robson PJ, Myers GJ, Davidson PW, Clarkson TW, Shamlaye CF, Strain JJ. Habitual fish consumption does not prevent a decrease in LCPUFA status in pregnant women (The Seychelles Child Development Nutrition Study). Prostaglandins Leukot Essent Fatty Acids. 2008;78:343–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hibbeln JR, Davis JM, Steer C, Emmett P, Rogers I, Williams C, Golding J. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC Study): an observational cohort study. Lancet. 2007;369:578–85 [DOI] [PubMed] [Google Scholar]
- 5.Oken E, Radesky JS, Wright RO, Bellinger DC, Amarasiriwardena CJ, Kleinman KP, Hu H, Gillman MW. Maternal fish intake during pregnancy, blood mercury levels, and child cognition at age 3 years in a US cohort. Am J Epidemiol. 2008;167:1171–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.FAO of the United Nations. The state of world fisheries and aquaculture. Rome: FAO; 2008
- 7.United States FDA. What you need to know about mercury in fish and shellfish; 2004 [cited 2012 Apr]. http://www.fda.gov/food/resourcesforyou/consumers/ucm110591.htm.
- 8.Oken E, Kleinman KP, Berland WE, Simon SR, Rich-Edwards JW, Gillman MW. Decline in fish consumption among pregnant women after a national mercury advisory. Obstet Gynecol. 2003;102:346–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bloomingdale A, Guthrie LB, Price S, Wright RO, Platek D, Haines J, Oken E. A qualitative study of fish consumption during pregnancy. Am J Clin Nutr. 2010;92:1234–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.FAO/WHO. Joint FAO/WHO expert consultation on the risks and benefits of fish consumption. Rome: FAO; 2010
- 11.Clarkson TW, Strain JJ. Methyl mercury: loaves versus fishes. Seychelles Med Dent J. 2004;7:61–5 [DOI] [PubMed] [Google Scholar]
- 12.Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. 2006;296:1885–99 [DOI] [PubMed] [Google Scholar]
- 13.Escolano-Margarit MV, Ramos R, Beyer J, Csabi G, Parrilla-Roure M, Cruz F, Perez-Garcia M, Hadders-Algra M, Gil A, Decsi T, et al. Prenatal DHA status and neurological outcome in children at age 5.5 years are positively associated. J Nutr. 2011;141:1216–23 [DOI] [PubMed] [Google Scholar]
- 14.Innis SM. Dietary (n-3) fatty acids and brain development. J Nutr. 2007;137:855–9 [DOI] [PubMed] [Google Scholar]
- 15.Boucher O, Burden MJ, Muckle G, Saint-Amour D, Ayotte P, Dewailly E, Nelson CA, Jacobson SW, Jacobson JL. Neurophysiologic and neurobehavioral evidence of beneficial effects of prenatal omega-3 fatty acid intake on memory function at school age. Am J Clin Nutr. 2011;93:1025–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen JT, Bellinger DC, Shaywitz BA. A quantitative analysis of prenatal methyl mercury exposure and cognitive development. Am J Prev Med. 2005;29:353–65 [DOI] [PubMed] [Google Scholar]
- 17.Hadders-Algra M. Prenatal and early postnatal supplementation with long-chain polyunsaturated fatty acids: neurodevelopmental considerations. Am J Clin Nutr. 2011;94:S1874–9 [DOI] [PubMed] [Google Scholar]
- 18.Jacobson JL, Jacobson SW, Muckle G, Kaplan-Estrin M, Ayotte P, Dewailly E. Beneficial effects of a polyunsaturated fatty acid on infant development: evidence from the Inuit of Arctic Quebec. J Pediatr. 2008;152:356–64 [DOI] [PubMed] [Google Scholar]
- 19.Davidson PW, Myers GJ, Cox C, Axtell C, Shamlaye C, Sloane-Reeves J, Cernichiari E, Needham L, Choi A, Wang Y, et al. Effects of prenatal and postnatal methylmercury exposure from fish consumption on neurodevelopment: outcomes at 66 months of age in the Seychelles Child Development Study. JAMA. 1998;280:701–7 [DOI] [PubMed] [Google Scholar]
- 20.Robinson J, Schroff J. The fishing sector in Seychelles: an overview, with an emphasis on artisanal fisheries. Seychelles Med Dent J. 2004;7:52–6 [DOI] [PubMed] [Google Scholar]
- 21.Shamlaye C, Davidson PW, Myers GJ. The Seychelles Child Development Study: two decades of collaboration. Seychelles Med Dent J. 2004;7:92–9 [DOI] [PubMed] [Google Scholar]
- 22.Marszalek JR, Lodish HF. Docosahexaenoic acid, fatty acid-interacting proteins, and neuronal function: breastmilk and fish are good for you. Annu Rev Cell Dev Biol. 2005;21:633–57 [DOI] [PubMed] [Google Scholar]
- 23.Montgomery C, Speake BK, Cameron A, Sattar N, Weaver LT. Maternal docosahexaenoic acid supplementation and fetal accretion. Br J Nutr. 2003;90:135–45 [DOI] [PubMed] [Google Scholar]
- 24.Cernichiari E, Brewer R, Myers GJ, Marsh DO, Lapham LW, Cox C, Shamlaye CF, Berlin M, Davidson PW, Clarkson TW. Monitoring methylmercury during pregnancy: maternal hair predicts fetal brain exposure. Neurotoxicology. 1995;16:705–10 [PubMed] [Google Scholar]
- 25.Weisberg S. Applied linear regression. New York: Wiley Interscience; 2005 [Google Scholar]
- 26.McDowell MA, Dillon CF, Osterloh J, Bolger PM, Pellizzari E, Fernando R, Montes de Oca R, Schober SE, Sinks T, Jones RL, et al. Hair mercury levels in U.S. children and women of childbearing age: reference range data from NHANES 1999–2000. Environ Health Perspect. 2004;112:1165–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunstan JA, Simmer K, Dixon G, Prescott SL. Cognitive assessment of children at age 21/2 years after maternal fish oil supplementation in pregnancy: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2008;93:F45–50 [DOI] [PubMed] [Google Scholar]
- 28.Helland IB, Smith L, Saarem K, Saugstad OD, Drevon CA. Maternal supplementation with very-long-chain n-3 fatty acids during pregnancy and lactation augments children's IQ at 4 years of age. Pediatrics. 2003;111:e39–44 [DOI] [PubMed] [Google Scholar]
- 29.Jensen CL, Voigt RG, Prager TC, Zou YL, Fraley JK, Rozelle JC, Turcich MR, Llorente AM, Anderson RE, Heird WC. Effects of maternal docosahexaenoic acid intake on visual function and neurodevelopment in breastfed term infants. Am J Clin Nutr. 2005;82:125–32 [DOI] [PubMed] [Google Scholar]
- 30.Oken E, Wright RO, Kleinman KP, Bellinger D, Amarasiriwardena CJ, Hu H, Rich-Edwards JW, Gillman MW. Maternal fish consumption, hair mercury, and infant cognition in a U.S. Cohort. Environ Health Perspect. 2005;113:1376–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stokes-Riner A, Thurston SW, Myers GJ, Duffy EM, Wallace J, Bonham M, Robson P, Shamlaye CF, Strain JJ, Watson G, et al. A longitudinal analysis of prenatal exposure to methylmercury and fatty acids in the Seychelles. Neurotoxicol Teratol. 2011;33:325–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calder PC. Polyunsaturated fatty acids, inflammatory processes and inflammatory bowel diseases. Mol Nutr Food Res. 2008;52:885–97 [DOI] [PubMed] [Google Scholar]
- 33.Chapkin RS, Kim W, Lupton JR, McMurray DN. Dietary docosahexaenoic and eicosapentaenoic acid: emerging mediators of inflammation. Prostaglandins Leukot Essent Fatty Acids. 2009;81:187–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hodge AM, Simpson JA, Gibson RA, Sinclair AJ, Makrides M, O'Dea K, English DR, Giles GG. Plasma phospholipid fatty acid composition as a biomarker of habitual dietary fat intake in an ethnically diverse cohort. Nutr Metab Cardiovasc Dis. 2007;17:415–26 [DOI] [PubMed] [Google Scholar]
- 35.Arab L. Biomarkers of fat and fatty acid intake. J Nutr. 2003;133 Suppl 3:S925–32 [DOI] [PubMed] [Google Scholar]
- 36.Glaser C, Lattka E, Rzehak P, Steer C, Koletzko B. Genetic variation in polyunsaturated fatty acid metabolism and its potential relevance for human development and health. Matern Child Nutr. 2011;7 Suppl 2:27–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haggarty P. Fatty acid supply to the human fetus. Annu Rev Nutr. 2010;30:237–55 [DOI] [PubMed] [Google Scholar]
- 38.Cheatham CL, Colombo J, Carlson SE. n-3 fatty acids and cognitive and visual acuity development: methodologic and conceptual considerations. Am J Clin Nutr. 2006;83:S1458–66 [DOI] [PubMed] [Google Scholar]
- 39.Glantz SA. A primer of biostatistics. New York: McGraw-Hill; 2002 [Google Scholar]
- 40.Perneger TV. What’s wrong with Bonferroni adjustments. BMJ. 1998;316:1236–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.