Abstract
Developmental iron deficiency anemia (IDA) causes brain and behavioral deficits in rodent models, which cannot be reversed when treated at periods equivalent to later infancy in humans. This study sought to determine whether earlier iron treatment can normalize deficits of IDA in rats and what iron dose is optimal. The offspring of dams with IDA during gestation were cross-fostered at postnatal d (P) 8 to dams receiving diets with 1 of 3 iron concentrations until weaning (P21): 0.003–0.01 g/kg [totally iron deficient (TID)]; 0.04 g/kg [formerly iron deficient (FID-40)]; or 0.4 g/kg (FID-400). Always iron-sufficient control dams (CN-40) received a 0.04-g/kg iron diet. At P21, TID pups received a 0.01 g iron/kg diet; all others received a 0.04 g iron/kg diet. Hematocrit and brain iron and monoamine concentrations were assessed at P21 and P100. Pup growth, development, activity, object recognition, hesitancy, and watermaze performance were evaluated. Regional brain iron was restored by iron treatment. Regional monoamine and metabolite concentrations were elevated in FID-40 rats and reduced in FID-400 and TID rats compared with CN-40 rats. FID-40 offspring had motor delays similar to TID during lactation and FID-400 rats had elevated thigmotaxis similar to TID rats at P25 and P100 in the spatial watermaze. In conclusion, iron treatment at P8 in rats did not normalize all monoamine or behavioral measures after early IDA. Moderate iron treatment improved adult behavior, but higher iron treatment caused brain and behavioral patterns similar to TID in the short and long term.
Introduction
Iron is a cofactor in many central nervous system processes, including monoamine neurotransmitter production, NO metabolism, and oxidative phosphorylation (1). The need for brain iron is evident given that ∼20% of the body’s oxygen consumption consists of mitochondrial respiration in the brain. As such, iron is essential to support the activities of embryonic and dividing neurons, and considerable iron losses during brain development can have deleterious effects (2–6). In humans, iron deficiency (ID)9 and ID anemia (IDA) during infancy lead to developmental delays and poorer cognition, motor performance, and social-affective behavior (7–10). Many of these outcomes appear to persist throughout early childhood (7, 11, 12), adolescence (8), and into early adulthood (9) despite iron therapy. Overall, these studies provide evidence for the importance of identifying and treating ID and IDA in early life.
Similar to humans, pre/perinatal ID in rodents produces deficits in cognition, attention, and affect (3, 13, 14). Previous rodent studies differed with respect to the timing and duration of ID; however, initiation of ID during the early-fetal, mid-fetal, or lactational periods produced similar behavioral impairments. Animal models of early ID have indicated biochemical and structural abnormalities associated with the observed behavioral deficits, including altered monoamine metabolism (4, 15–19), myelin biology (20), hippocampal dendritic arborization (5, 21), and hippocampal and striatal metabolome (22, 23).
There is an ongoing effort to identify critical periods wherein iron treatment can prevent long-term deficits of ID. The literature supports the conclusion that iron repletion at postnatal day (P) 21 (weaning) is too late to correct the behavioral effects of earlier IDA in rats (3, 14, 24, 25). However, P21 iron intervention does restore brain and peripheral iron concentrations to iron-sufficient states despite earlier regional brain iron losses of 20–50%. These findings suggest that iron deprivation during the time of rapid cell differentiation and growth causes irreversible damage even though the brain iron concentration can be normalized postweaning. Iron treatment prior to P21 shows promise in rodent models. Rats with ID beginning at mid-gestation, and that received an iron intervention at P4, had only minor deficits in monoamine metabolism and no altered motor and exploratory behaviors in early adulthood (26). Failure of iron treatment at or after P8 (equivalent to term newborn period with respect to brain growth) (27) would suggest that efforts should concentrate on prenatal diagnosis and treatment, because the P4 time of treatment, a period of brain growth that likely approximates the third trimester of human pregnancy, provided benefit. However, because fetal ID is difficult to diagnose, it is important to test whether any dosing regimen delivered at P8 can restore brain iron and prevent long-term neurochemical and behavioral deficits.
To date, the timing and duration of ID and IDA and the doses of iron and methods used for treatment in rodents have varied considerably. In recent studies, IDA timing and duration varied from mid-gestation to early lactation (26) and from early gestation through lactation (3, 16). Most studies administered iron at 0.05–0.20 g/kg via weaning onto iron-adequate diets (4, 26, 28), transferring deficient pregnant and lactating dams to higher iron-containing diets (22, 29, 30), oral gavage (31), and cross-fostering (24). Regardless of treatment method and iron dose, persistent behavioral and brain deficits were shown with iron treatment beginning after P4. Moreover, treatment with high doses of iron in developing mice causes neurodegeneration and susceptibility to Parkinsonian-like behaviors (31). These studies show that both the dose and timing of iron treatment are crucial for outcomes following early IDA.
The current study focuses on narrowing the developmental window for iron treatment. The study determined whether treatment at P8 for rats that had ID throughout gestation can prevent the long-term behavioral and neurochemical outcomes, similar to what has been observed with iron intervention at P4. This study also considered the dose of iron that is sufficient but not toxic for repletion. We assessed moderate and higher iron treatment doses that are comparable with the range used to treat infants and children with IDA.
Materials and Methods
Determination of iron concentration in milk.
Milk samples were collected on P9, P12, and P15 from a separate group of dams from the main experiment. Pregnant dams were maintained on diets containing one of the following iron concentrations: 0.003 g/kg (n = 4), 0.08 g/kg (n = 4), 0.487 g/kg (n = 6), and 1.323 g/kg (n = 6) from gestational day (G) 5. Iron diets were prepared in our laboratory following the recipe of the AIN-93G diet with cornstarch as the source of carbohydrate and ferric citrate as the iron source (32). On P9, P12, and P15, dams were isolated from their pups and exposed to light anesthesia (3% isoflurane) for the duration of the procedure. Oxytocin, 20 kU/L solution, was s.c. injected in a dose of 300 μL to stimulate milk production. Milk was collected by manual expression and volumes were recorded. Milk iron content was determined by atomic absorption spectroscopy as described below. The estimated amount of iron consumed by pups at each time point was calculated based on the iron concentration of the milk and daily milk consumption at each age (33).
Diets, rats, and housing.
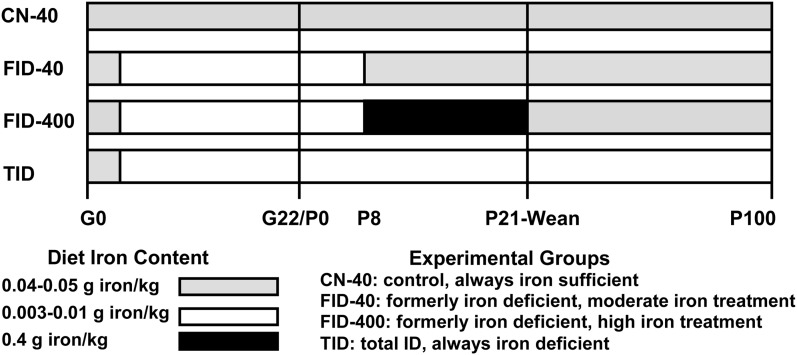
All purified diets (Harlan Teklad) are a modification of the AIN-76A diet (protein: 17.8% energy, carbohydrate: 70.4% energy, fat: 11.8% energy), with ferric citrate as the iron source where indicated (28, 32). The diet design is shown in Figure 1. Female Sprague Dawley rats (Harlan Laboratories), 8 wk of age, were fed a 0.04-g/kg iron diet (Harlan Teklad, TD89300) upon arrival. This concentration of dietary iron is adequate to maintain pregnancy outcomes (34) but not augment maternal iron stores. After 7–10 d, females were mated and conception was documented by vaginal plug presence. At G5, 62 females were randomized to diet group and fed iron diets containing 0.04 or 0.003 g/kg diet (Harlan Teklad, TD80396). Within 24 h of delivery (P1), pups were weighed and litters culled to 10–12 pups, retaining equal gender numbers as much as possible. Litter size ranged from 10 to 16 pups and did not differ between treatments. At P8, all litters were cross-fostered. For the control group (CN-40; n = 13 litters), pups from dams receiving 0.04 g/kg diet were cross-fostered to other CN-40 dams. Litters from dams fed the 0.003-g/kg diet were cross-fostered to dams receiving 1 of 3 iron diets: 0.04 g/kg to generate the formerly ID (FID) moderate iron treatment group (FID-40; n = 12 litters), 0.4 g/kg diet (Harlan Teklad, TD02545) to generate the FID higher iron treatment group (FID-400; n = 12 litters), or 0.003 g/kg diet to generate the totally ID group (TID; n = 11 litters). TID dams were fed a 0.01-g/kg diet (Harlan Teklad, TD01094) at P15 to maintain litter viability and TID offspring continued to be fed this diet after weaning (P21). All other offspring received the 0.04-g/kg diet at P21. Rats consumed the diet and deionized distilled water ad libitum and were housed in a temperature-controlled environment (22°C) on a 12-h-light/-dark cycle (lights on 0800 h). All experimental protocols were conducted in accordance with the NIH Animal Care guidelines and were approved by the University of Michigan Institutional Animal Care and Use Committee.
FIGURE 1.
Study design. CN-40, control; FID-40, formerly iron deficient fed 0.04-g/kg iron diet beginning at postnatal d 8; FID-400, formerly iron deficient fed 0.4-g/kg iron diet beginning at postnatal d 8; G, gestational day; P, postnatal day; TID, totally iron deficient.
Growth and hematology.
Dams were weighed at conception and at 5-d intervals until delivery. Offspring were weighed at delivery, P8, P15, P21, and P100. Brain and liver were weighed after killing (P21 or P100). Hematocrit (HCT) was determined as previously described for dams at delivery, during lactation, and at weaning and for offspring at the time they were killed (16).
Sampling.
For quantitative brain measurements, 1–2 male and female pups were sampled from each litter at P21 and P100. All rats assessed for brain measures were also tested for all behavioral measures. Where there was more than one litter-by-sex representative, outcome measures were averaged by sex within litter.
Brain dissection.
All rats were perfused as previously described (16). Brains were removed, hemi-sectioned, and one hemisphere was dissected on ice for frontal cortex, caudate putamen, ventral tegmentum-substantia nigra (ventral midbrain), hippocampus, and superficial cerebellum. Hemispheres designated for regional assessments (right or left) were equally balanced within diet groups. The regions were weighed and frozen at −80°C.
Iron determination.
Brain and milk samples were wet digested according to published procedures and analyzed for iron concentration by atomic absorption spectrometry (25). All standard curves exceeded r2 > 0.99.
Regional monoamine concentrations.
Regional monoamines and metabolites were analyzed by HPLC as previously described (4). Dopamine (DA), serotonin (5HT), 3,4-dihydroxyphenylacetic acid (DOPAC), and 5-hydroxyindoleacetic acid (5HIAA) peak areas were integrated using EZ Chrom Elite (Scientific Software) and quantified against known standards. All standard curves exceeded r2 > 0.98.
DA transporter autoradiography.
Radioligand binding for DA transporter (DAT) was performed using [125I]RTI-55 (Perkin Elmer Life Sciences) as previously reported (35).
Developmental and behavioral assessments
Developmental measures.
Physical and sensorimotor measures were assessed between 0900 and 1400 h at P9, P12, P15, P18, and P21 for all pups based on LaPointe and Nosal (36) and as described (4, 16, 23). Performance on the following measures was documented using a 2-point (0–1) or 6-point (0–5) scale (not present to fully present) or percent (i.e., successful limb placements of 10 trials) by a single observer unaware of the diet groups: 1) fur, eye, ear development; 2) auditory startle, unilateral and bilateral forelimb placing; and 3) bar holding, surface righting, and negative geotaxis.
Response to novelty (place or object).
Response to novelty was determined as follows: 1) open field (16, 36): behaviors coded from videotapes of 8- to 20-s trials in the open field (5–8 min inter-trial periods in home cage) at P21 or P75 included: time to leave center, number of rearings, freezing bouts, and sectors entered; 2) hesitancy (3): rats were individually assessed at P75 for time and number of entries into familiar and novel areas from videotapes as previously described; and 3) object and social-odor recognition. Four wooden balls were placed in the home cage overnight for the rats to become familiar with them and so they could take on the home-cage odor. The next day, the rats were removed then individually placed back into the home cage and 4 objects were presented, one at each corner. The objects varied for familiar and novel attributes: 1) shape, i.e., 2.5-cm wooden sphere (familiar) or block (novel); and 2) social odor, i.e., home cage bedding (familiar) or bedding from a different rat (novel). Time in contact with each object and number of snout touches during the testing at P30 (2 min) and P80 (5 min) were coded from videotapes. Different litter representatives were tested at each age.
Spatial watermaze.
Rats were tested at P25 or P80–100 as previously described (3) with a subset tested at both ages. Testing consisted of 8 trials/d, 3 d at P25 and up to 12 d (7 d minimum) in adulthood. Latency and path length to reach the platform, percent path in platform quadrant, and percent path thigmotaxis were measured for each trial and averaged for each individual rat daily. The criteria for learning were latency ≤15 s for ≥6 of 8 trials, percent of path within platform quadrant ≥40%, and percent path thigmotaxis ≤50%. At either 24 h after reaching criteria or 12 d of testing, rats were given a single, 1-min probe trial for percent path in the platform quadrant and thigmotaxis.
Statistics
All data were double-entered and checked for errors. The main independent variables were diet group and sex; for the watermaze, previous training at P25 was considered as a covariate. Brain iron and monoamines were assessed as repeated measures at P21 and P100 and analyzed using repeated-measures ANCOVA. Linear mixed models implemented using proc mixed in SAS were used to analyze these data at the individual rat level. A random effect for litter was included to account for the within-litter correlation. Hematology and weights were compared by diet and sex using ANOVA at each age. Body weight gain during lactation was also assessed by taking the mean of individual weights by litter and sex and comparing by diet group using proc mixed models. All behavioral outcomes for individuals were similarly averaged by sex to give one mean for male and female performance per litter at each age. Object recognition and hesitancy outcomes were compared by diet and sex using ANOVA. Behavioral tests with repeated assessments (developmental milestones, open field, watermaze) were analyzed using repeated measures controlling for sex to capture overall activity. Means were also compared by diet group at each age controlling for sex. For watermaze, ANCOVA was used controlling for sex and previous experience at P25. Only significant diet group or sex differences are reported for overall diet effects. Significance was set at P < 0.05 for all analysis unless specifically noted (e.g., trend levels). Unless otherwise stated, data presented in text and tables are mean ± SD. Data in figures are presented as mean ± SE. All analyses compare the means by sex of a minimum of 9–13 litters within each diet group unless otherwise stated.
Results
Milk iron
Milk iron concentrations increased with rising iron concentrations in the maternal diet until the iron concentration reached 0.487 g/kg diet (Table 1). There was no difference in milk iron from mothers receiving the 0.487- and 1.323-g/kg diets. Based on the mean daily milk consumption at the corresponding ages (33), these data translate to a daily iron delivery of 0.001 g/kg for the 0.003-g/kg diet, 0.002–0.003 g/kg for the 0.04-g/kg diet, and 0.006 g/kg for the 0.4-g/kg diet.
TABLE 1.
Effect of dietary iron on milk iron concentrations in rat dams1
| Dietary iron | Milk iron |
| g/kg | mg/L |
| 0.003 | 1.40 ± 0.16 c |
| 0.080 | 3.75 ± 0.41b |
| 0.487 | 8.24 ± 0.55 a |
| 1.323 | 7.38 ± 2.17 a |
Values are means ± SD, n = 4–6/diet group. Means without a common letter differ, P < 0.05.
Growth and hematology of dams and offspring
At G20, body weight was greater for the CN-40 dams (342.9 ± 21.4 g) than for the ID dams (320.2 ± 31.3 g) (P < 0.05). Weight gain during gestation (G20 to G1) was also greater for CN-40 dams (124.5 ± 23.9 g) than for the ID dams (100.7 ± 33.6 g) (P < 0.05). HCT was marginally higher for CN-40 (0.39 ± 0.04) than ID dams (0.37 ± 0.04) at delivery (P = 0.07) and higher for CN-40 (0.47 ± 0.03) than ID dams (0.38 ± 0.06) at P8 (P < 0.001).
For offspring body weights during lactation, there was an effect of day (P < 0.001), diet group (P < 0.005), and day × diet group interaction (P < 0.001). At P8, body weight was greater for the CN-40 pups (17.8 ± 4.1 g) than for the FID-40 pups (13.8 ± 3.5 g) (P < 0.01) and marginally greater than the FID-400 pups (15.3 ± 4.3 g) (P < 0.07). At P15, the body weight of CN-40 pups (28.6 ± 6.4 g) was greater than that of FID-40 (24.1 ± 4.8 g) (P < 0.01) and TID pups (22.6 ± 3.3 g) (P < 0.001). Also at P21, CN-40 rats (41.1 ± 7.7 g) had greater body weight than FID-40 (36.2 ± 7.5 g) (P < 0.01) and TID pups (31.5 ± 5.3 g) (P < 0.001).
At P21, there was an effect of diet (P < 0.001) and sex (P < 0.02) but no diet × sex interaction for whole brain weight (Table 2). Whole brain weight for the CN-40 rats was greater than for the TID rats (P < 0.05). Overall, male brains (1.32 ± 0.11 g) weighed more than female brains (1.26 ± 0.11 g). HCT was greater for all diet groups compared with TID (P < 0.05) (Table 2).
TABLE 2.
Brain weight, liver weight, and HCT of CN-40, FID-40, FID-400, and TID rats at P21 and P1001
| Age | CN-40 | FID-40 | FID-400 | TID | |
| P21 | Brain weight, g | 1.33 ± 0.15a | 1.28 ± 0.07a,b | 1.32 ± 0.10a | 1.21 ± 0.11b |
| Liver weight, g | 1.69 ± 0.53a,b | 1.36 ± 0.44a,b | 1.78 ± 0.51a | 1.28 ± 0.30b | |
| HCT | 0.37 ± 0.04a,b | 0.31 ± 0.05b | 0.39 ± 0.05a | 0.18 ± 0.01c | |
| P100 | Body weight, g | 322.4 ± 77.5a | 313.7 ± 74.1a,b | 321.4 ± 77.2a | 285.2 ± 54.1b |
| Brain weight, g | 1.81 ± 0.09a | 1.84 ± 0.10a | 1.85 ± 0.10a | 1.76 ± 0.08b | |
| Liver weight, g | 12.1 ± 0.7a | 12.0 ± 2.9a,b | 12.0 ± 3.4a.b | 11.0 ± 2.6b | |
| HCT | 0.48 ± 0.03a | 0.49 ± 0.02a | 0.47 ± 0.02a | 0.43 ± 0.05b |
Values are means (male and female) ± SD, n = 11–13 litters/diet group. Means in a row without a common letter differ, P < 0.05. CN-40, control; FID-40, formerly iron deficient fed 0.04-g/kg iron diet beginning at postnatal d 8; FID-400, formerly iron deficient fed 0.4-g/kg iron diet beginning at postnatal d 8; HCT, hematocrit; P, postnatal day; TID, totally iron deficient.
At P100, there was an effect of diet (P < 0.001), sex (P < 0.001), and a diet × sex interaction (P < 0.01) for body weight. CN-40 rats had a greater body weight than TID rats (P < 0.05) (Table 2). There was an effect of diet (P < 0.001) and sex (P < 0.001) but no diet × sex interaction for whole brain weight. Males had greater body and brain weights than females in all diet groups (P < 0.001). There was an effect of diet (P < 0.001) but not sex for HCT at P100; TID rat HCT was less than in all other diet groups (P < 0.05) (Table 2).
Brain iron
At both P21 and P100, there was an overall effect of diet group (P < 0.01). At P21, iron concentrations in TID rats were lower than those in CN-40, FID-40, and FID-400 rats in frontal cortex, striatum, and hippocampus (P < 0.05) (Table 3). At P100, iron concentrations in TID rats were lower than in CN-40 rats in striatum and midbrain; FID-40 rats in cerebellum, striatum, and midbrain; and FID-400 rats in striatum (P < 0.05) (Table 3).
TABLE 3.
Regional brain iron concentrations of CN-40, FID-40, FID-400, and TID rats at P21 and P1001
| Age | Brain region | CN-40 | FID-40 | FID-400 | TID |
| nmol/g tissue | |||||
| P21 | Cerebellum | 261 ± 55a | 243 ± 80a | 261 ± 91a | 160 ± 58b |
| Frontal cortex | 304 ± 96a | 286 ± 114a,b | 240 ± 70b | 149 ± 40c | |
| Striatum | 274 ± 51a | 279 ± 87a | 255 ± 90a | 153 ± 59b | |
| Hippocampus | 274 ± 97a | 264 ± 86a | 273 ± 107a | 159 ± 57b | |
| Midbrain | 285 ± 58a | 199 ± 88b | 227 ± 135b | 202 ± 135b | |
| P100 | Cerebellum | 328 ± 64ab | 385 ± 96a | 331 ± 45a | 269 ± 146b |
| Frontal cortex | 325 ± 77a | 335 ± 96a | 288 ± 54a | 342 ± 186a | |
| Striatum | 322 ± 66a | 326 ± 99a | 327 ± 54a | 272 ± 131a | |
| Hippocampus | 323 ± 84a | 377 ± 86a | 278 ± 76b | 253 ± 78b | |
| Midbrain | 300 ± 69b | 354 ± 94a | 290 ± 108b | 246 ± 98b | |
Values are means ± SD, n = 11–13 litters/diet group. Means in a row without a common letter differ, P < 0.05. CN-40, control; FID-40, formerly iron deficient fed 0.04-g/kg iron diet beginning at postnatal d 8; FID-400, formerly iron deficient fed 0.4-g/kg iron diet beginning at postnatal d 8; P, postnatal day; TID, totally iron deficient.
DAT concentration
At P21, there was a diet effect (P < 0.02) for DAT ligand concentration in striatum and nucleus accumbens. Striatal DAT levels were lower in TID rats (10.5 ± 1.8 fmol [125I]RTI-55/mg tissue (fmol/mg); n = 11) than in FID-400 (12.9 ± 2.4 fmol/mg; n = 15) (P < 0.05) and FID-40 rats (13.2 ± 0.7 fmol/mg; n = 6) (P < 0.07). In nucleus accumbens, TID DAT levels (8.1 ± 1.7 fmol/mg; n = 11) were reduced compared with FID-400 rats (10.6 ± 2.3 fmol/mg; n = 15) (P < 0.05). At P100, there was an effect of diet (P < 0.01) in striatum. TID DAT levels (16.4 ± 2.7 fmol/mg; n = 13) were lower than FID-400 rats (20.4 ± 3.7 fmol/mg; n = 13) (P < 0.02).
Monoamines and monoamine metabolites
Monoamine and monoamine metabolite patterns varied depending on region, treatment group, and age. In general, FID-40 rats had elevated concentrations compared with FID-400 rats (and CN-40 in some cases) and FID-400 rats were similar to TID rats.
At P21 (Table 4), DA concentrations were elevated in FID-40 rats compared with TID rats in all brain regions and compared with FID-400 rats in cerebellum, cortex, hippocampus, and midbrain (P < 0.05). Striatal DA concentrations were also greater in FID-40 rats relative to CN-40 rats (P < 0.05). Similarly, DOPAC concentrations were elevated in FID-40 compared with all other diet groups in cortex, striatum, and hippocampus. In almost all cases, DA and DOPAC concentrations did not differ between FID-400 and TID rats. 5HT concentrations were elevated in FID-40 rats compared with FID-400 and TID rats in all regions and with CN-40 rats in cortex, hippocampus, and midbrain (P < 0.05). Elevated 5-HIAA concentrations were observed in hippocampus for CN-40 rats compared with all other diet groups (P < 0.05).
TABLE 4.
Regional DA, DOPAC, 5HT, and 5HIAA concentrations of CN-40, FID-40, FID-400, and TID rats at P211
| Brain region | CN-40 | FID-40 | FID-400 | TID | |
| nmol/g tissue | |||||
| DA | Cerebellum | 2.02 ± 0.53a,b | 2.47 ± 0.95a | 0.14 ± 0.07b | 0.17 ± 0.05ab |
| Frontal cortex | 0.26 ± 0.34a,b | 2.41 ± 1.66a | 0.17 ± 0.10b | 0.15 ± 0.07b | |
| Striatum | 13.1 ± 5.36b | 20.4 ± 7.10a | 18.8 ± 5.04a | 7.6 ± 4.55c | |
| Hippocampus | 1.74 ± 1.79a,b | 3.02 ± 1.47a | 0.10 ± 0.06b | 0.12 ± 0.08b | |
| Midbrain | 2.13 ± 1.72a,b | 3.73 ± 3.25a | 1.34 ± 1.43b | 0.86 ± 0.94b | |
| DOPAC | Cerebellum | 5.17 ± 1.78a,b | 5.89 ± 2.18a | 4.50 ± 1.88a,b | 3.75 ± 1.40b |
| Frontal cortex | 4.85 ± 2.57b | 8.89 ± 3.50a | 5.62 ± 2.30b | 4.90 ± 1.36b | |
| Striatum | 14.8 ± 3.08b | 19.8 ± 5.55a | 11.0 ± 2.99c | 9.68 ± 2.88c | |
| Hippocampus | 5.87 ± 2.59b | 8.79 ± 4.16a | 5.18 ± 2.82b | 5.48 ± 2.23b | |
| Midbrain | 6.22 ± 3.34a,b | 7.29 ± 4.12a | 6.90 ± 2.47a,b | 4.69 ± 2.78b | |
| 5HT | Cerebellum | 0.49 ± 0.26a,b | 1.31 ± 0.48a | 0.37 ± 0.49b | 0.17 ± 0.09b |
| Frontal cortex | 1.38 ± 1.47b | 2.51 ± 1.73a | 0.45 ± 0.23b,c | 0.39 ± 0.15c | |
| Striatum | 1.67 ± 0.65a | 2.94 ± 0.97a | 0.69 ± 0.31b | 0.46 ± 0.23b | |
| Hippocampus | 1.64 ± 1.44b | 3.03 ± 1.97a | 0.86 ± 1.03c | 0.57 ± 0.33c | |
| Midbrain | 2.15 ± 1.27b | 3.22 ± 3.03a | 2.19 ± 2.35b | 1.79 ± 1.76b | |
| 5HIAA | Cerebellum | 3.01 ± 1.61a | 1.72 ± 0.61a | 1.52 ± 0.68a | 1.66 ± 0.60a |
| Frontal cortex | 2.75 ± 0.97a | 2.27 ± 0.94a | 1.61 ± 0.61a | 1.56 ± 0.54a | |
| Striatum | 4.92 ± 2.21a | 4.62 ± 1.40a | 2.77 ± 1.30a | 3.11 ± 2.47a | |
| Hippocampus | 11.2 ± 12.7a | 5.17 ± 7.72b,c | 6.00 ± 1.52b | 2.43 ± 1.00c | |
| Midbrain | 5.43 ± 2.29a | 4.77 ± 1.54a | 4.30 ± 2.62a | 2.46 ± 1.09a | |
Values are means ± SD, n = 11–13 litters/diet group. Means in a row without a common letter differ, P < 0.05. CN-40, control; DA, dopamine; DOPAC, 3,4 dihydroxyphenylacetic acid; FID-40, formerly iron deficient fed 0.04-g/kg iron diet beginning at postnatal d 8; FID-400, formerly iron deficient fed 0.4-g/kg iron diet beginning at postnatal d 8; 5HIAA, 5-hydroxyindoleacetic acid; 5HT, serotonin; P, postnatal day; TID, totally iron deficient.
At P100 (Table 5), striatal DA concentrations were greater in FID-40 compared with all other diet groups (P < 0.05). DOPAC concentrations remained elevated in FID-40 rats compared with all other groups in cerebellum, cortex, hippocampus, and striatum (P < 0.05). 5HT and 5HIAA concentrations also differed by diet group. FID-40 rats showed higher 5HT concentrations than all other diet groups in cortex, hippocampus, striatum, and midbrain (P < 0.05). Similarly, 5HIAA concentrations were elevated in CN-40 rats compared with the other diet groups in hippocampus and midbrain (P < 0.05).
TABLE 5.
Regional DA, DOPAC, 5HT, and 5HIAA concentrations of CN-40, FID-40, FID-400, and TID rats at P1001
| Brain region | CN-40 | FID-40 | FID-400 | TID | |
| nmol/g tissue | |||||
| DA | Cerebellum | 1.78 ± 0.55a | 2.09 ± 0.77a | 0.19 ± 0.15a | 0.21 ± 0.23a |
| Frontal cortex | 1.06 ± 0.94a | 2.63 ± 1.04a | 0.16 ± 0.09a | 0.42 ± 0.26a | |
| Striatum | 45.3 ± 11.7b | 61.9 ± 14.9a | 43.8 ± 15.2b | 44.9 ± 17.9b | |
| Hippocampus | 0.94 ± 1.04a | 2.55 ± 1.09a | 0.15 ± 0.19a | 0.24 ± 0.08a | |
| Midbrain | 1.66 ± 1.29a | 3.56 ± 2.02a | 0.49 ± 0.34a | 0.86 ± 0.83a | |
| DOPAC | Cerebellum | 7.13 ± 1.96b | 11.5 ± 2.73a | 5.50 ± 2.27b | 5.08 ± 1.59b |
| Frontal cortex | 7.40 ± 1.82b | 10.6 ± 2.70a | 5.81 ± 1.70b,c | 5.15 ± 2.00c | |
| Striatum | 15.5 ± 3.72b,c | 21.1 ± 4.98a | 13.8 ± 6.20c | 16.5 ± 5.28b | |
| Hippocampus | 6.79 ± 3.14b | 10.5 ± 2.93a | 5.07 ± 1.71b,c | 3.85 ± 1.84c | |
| Midbrain | 6.85 ± 3.51b | 11.2 ± 2.30a | 5.41 ± 2.22b | 5.25 ± 1.18b | |
| 5HT | Cerebellum | 1.85 ± 3.01a | 1.22 ± 0.38a,b | 0.16 ± 0.16b | 0.21 ± 0.15a,b |
| Frontal cortex | 3.06 ± 1.50b | 6.26 ± 3.19a | 1.57 ± 0.66c | 1.50 ± 0.71c | |
| Striatum | 4.21 ± 2.48b | 7.19 ± 2.53a | 1.87 ± 1.58c | 1.43 ± 0.85c | |
| Hippocampus | 2.45 ± 1.60b | 4.78 ± 2.38a | 0.76 ± 0.29c | 1.14 ± 0.50b,c | |
| Midbrain | 5.85 ± 2.99b | 9.37 ± 3.26a | 2.12 ± 2.84c | 2.46 ± 1.41c | |
| 5HIAA | Cerebellum | 1.11 ± 0.20a | 0.90 ± 0.77a | 0.38 ± 0.18a | 0.63 ± 0.39a |
| Frontal cortex | 2.55 ± 1.05a | 2.97 ± 0.78a | 1.27 ± 0.50a | 1.44 ± 0.59a | |
| Striatum | 6.36 ± 2.72a,b | 8.10 ± 2.64a | 2.70 ± 1.33c | 3.26 ± 2.37b,c | |
| Hippocampus | 11.1 ± 16.0a | 3.92 ± 5.39b | 0.95 ± 0.37b | .28 ± 0.84b | |
| Midbrain | 11.3 ± 13.6a | 6.35 ± 9.08b | 1.86 ± 1.15c | 1.52 ± 1.16c | |
Values are means ± SD, n = 11–13 litters/diet group. Means in a row without a common letter differ, P < 0.05. CN-40, control; DA, dopamine; DOPAC, 3,4 dihydroxyphenylacetic acid; FID-40, formerly iron deficient fed 0.04-g/kg iron diet beginning at postnatal d 8; FID-400, formerly iron deficient fed 0.4-g/kg iron diet beginning at postnatal d 8; 5HIAA, 5-hydroxyindoleacetic acid; 5HT, serotonin; P, postnatal day; TID, totally iron deficient.
Developmental and behavioral assessments
Developmental measures.
Physical measures.
There were no significant diet group differences for fur, ear, or eye development.
Auditory startle and forelimb placing.
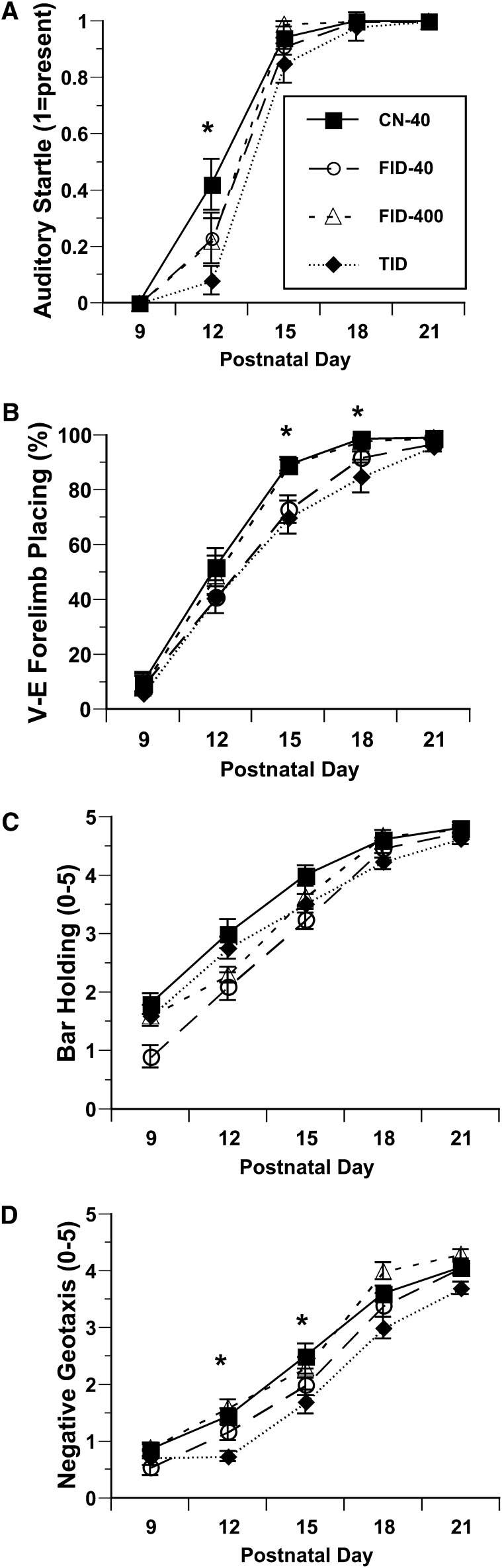
For auditory startle, there was a diet effect (P < 0.03) and CN-40 rats had a greater response than TID rats over time (P < 0.05). CN40 rats had a greater startle response at P12 (P < 0.05) (Fig. 2A). For vibrissae-elicited forelimb placing, there was also an effect of diet (P < 0.01) and CN-40 rats were greater in this measure than TID rats over time (P < 0.05). CN40 rats had a greater percent placement on P15 and P18 than TID rats (P < 0.05) (Fig. 2B). For bilateral forelimb placing, there was a diet effect (P < 0.01) and CN-40 rats had better bilateral forelimb placing than TID rats (P < 0.06). Bilateral forelimb placing was greater for CN40 than for TID rats at P9 (P < 0.05) (data not shown). By P21, there were no differences in auditory startle and forelimb placing between diet groups.
FIGURE 2.
Auditory startle (A), vibrissae-elicited forelimb placing (B), bar holding (C), and negative geotaxis (D) in CN-40, FID-40, FID-400, and TID rats during lactation. Values are means ± SE, n = 11–13 litters/group. *P < 0.05: CN-40 vs. TID at the specified postnatal day. CN-40, control; FID-40, formerly iron deficient fed 0.04-g/kg iron diet beginning at postnatal d 8; FID-400, formerly iron deficient fed 0.4-g/kg iron diet beginning at postnatal d 8; TID, totally iron deficient.
Bar holding, surface righting, and negative geotaxis.
Overall, there was an effect of diet for bar holding (P < 0.01) (Fig. 2C) but no diet group differences on post hoc comparisons. For negative geotaxis, there was an effect of diet overall (P < 0.02) (Fig. 2D) and a post hoc difference for CN40 compared with FID-40 rats over time (P < 0.05). CN-40 rats performed better than FID-40 rats in the bar-holding task at P9, P12, and P15 and on the negative geotaxis task and they also performed better than TID rats on P12 and P15. CN40 rats performed better than FID-40 rats for surface righting (data not shown) overall (P < 0.05), for which there was an effect of diet (P < 0.01); however, no significant diet group differences were observed at specific time points. By P21, there were no significant diet group differences.
Response to novelty (place and object).
Open field.
At P21, there was an effect of diet (P < 0.01) for time to leave the center over all 8 trials (data not shown) that was greater in TID rats than in FID-400 (P < 0.01) and CN-40 rats (P < 0.09). TID rats had a greater mean of all 8 trials for time to leave center (11.0 ± 5.1 s) than FID-400 (5.3 ± 3.1 s) (P < 0.01), FID-40 (5.0 ± 1.1 s) (P < 0.06), and CN-40 rats (4.0 ± 0.9 s) (P < 0.08). When time to leave center was considered, there were no significant diet group differences for total sectors entered. Taking the mean of the 8 trials, fewer freezing bouts were observed in TID rats (3.4 ± 0.7) than in CN-40 (4.1 ± 0.5) and FID-40 rats (4.1 ± 0.7) (P < 0.05 for both). There were no significant group differences in rearing.
At P75, there was an effect of diet (P < 0.05), a trend for sex (P < 0.08), and a diet × sex interaction (P < 0.03) for time to leave the center at the first trial (data not shown). FID-400 rats (7.6 ± 5.1 s) took marginally longer than CN-40 (4.0 ± 3.4 s) (P < 0.09) and FID-40 rats (3.7 ± 2.9 s) (P < 0.08) to leave the center of the open field. There were no other significant group differences for time to leave center, total sectors entered, rearing, or freezing.
Hesitancy test.
There were no significant diet group differences for latency to first entry or total entries into the novel or familiar areas. Overall, TID rats spent more time in the novel area (182.5 ± 27.3 s) than CN-40 (151.7 ± 24.0 s) (P < 0.03) or FID-40 (149.5 ± 26.8 s) (P < 0.02) rats. FID-400 rats did not differ significantly from rats in the other diet groups (170.8 ± 31.8 s).
Object recognition.
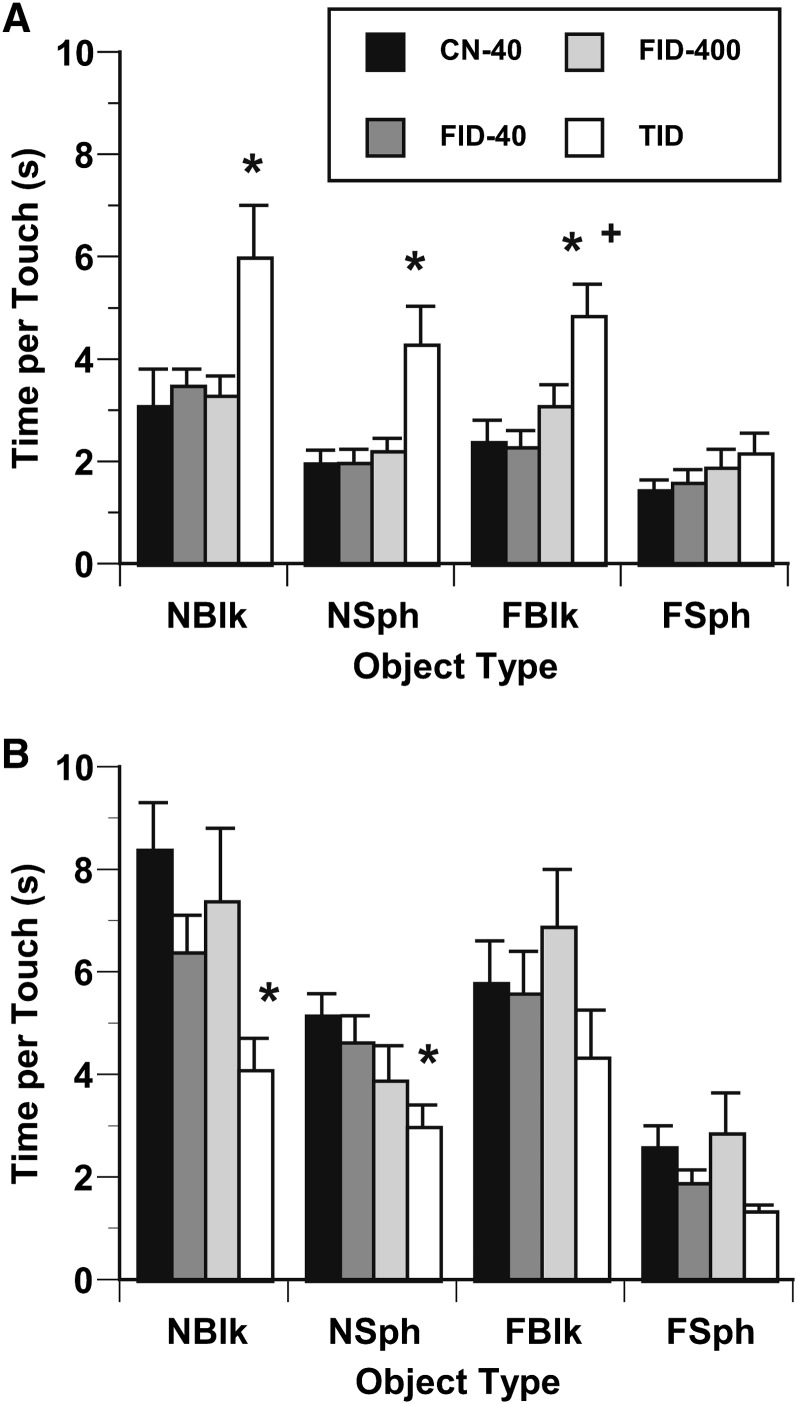
At P30, the number of touches on objects did not differ between CN40 rats and the other diet groups (P > 0.15). There was a significant effect of diet group for time per touch (Fig. 3A). Time per touch was significantly greater for TID than CN-40 rats for objects that were novel for smell and shape (novel smelling block) and objects with one novel attribute (novel-smelling sphere, familiar-smelling block) (P < 0.05). FID-40 rats also spent less time per touch than TID rats on the familiar-smelling block (P < 0.05). There were no significant diet group differences for the familiar-smelling sphere (i.e., no novel attributes).
FIGURE 3.
Time per touch in object recognition task for CN-40, FID-40, FID-400, and TID rats at P30 (A) and P80 (B). *P < 0.05: CN-40 vs. TID; +P < 0.05: FID-40 vs. TID. Values are means ± SE, n = 11–13 litters/group. CN-40, control; FID-40, formerly iron deficient fed 0.04-g/kg iron diet beginning at postnatal d 8; FID-400, formerly iron deficient fed 0.4-g/kg iron diet beginning at postnatal d 8; FBlk, familiar-smelling block, FSph, familiar-smelling sphere, NBlk, novel-smelling block, NSph, novel-smelling sphere; P, postnatal day; TID, totally iron deficient.
At P80, there was a diet group effect for total number of object touches (P < 0.01). The total number of touches was greater for TID rats (25.3 ± 10.6) than for CN-40 (16.1 ± 5.9) (P < 0.01), FID-40 (16.6 ± 6.5) (P < 0.01), and FID-400 (17.0 ± 6.7) (P < 0.07) rats. Time per touch for TID rats was also less than for CN-40 rats on the novel block and novel sphere (P < 0.05) (Fig. 3B). There were no significant group differences for the familiar block or sphere.
Spatial watermaze.
P25.
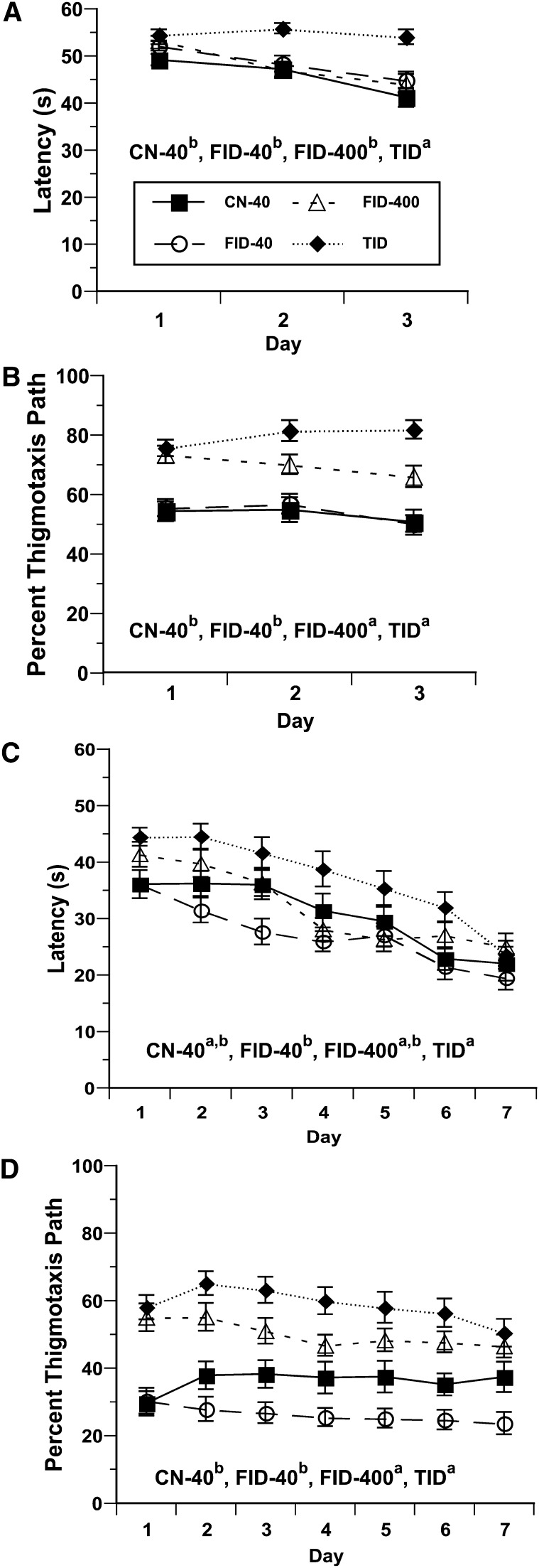
Overall, there was a significant effect of diet group for latency to reach the platform (Fig. 4A). Latency to reach the platform was greater for TID rats than all other groups (P < 0.02). There was also a significant diet effect for percent of path length that was thigmotaxic (Fig. 4B). The path lengths for TID and FID-400 rats were greater than for the CN-40 and FID-40 rats (P < 0.01). There was a diet effect for percent path in the platform quadrant (P < 0.02). The mean percent path in the platform quadrant (d 1–3) for TID rats (30.4 ± 5.0%) was less than that for CN-40 rats (35.0 ± 5.0%) (P < 0.03); FID-40 (34.5 ± 4.1%) and FID-400 (33.9 ± 5.1%) rats did not differ significantly from any other group.
FIGURE 4.
Latency (s) to reach the platform at P25 (A), percent thigmotaxis at P25 (B), latency (s) to reach the platform at P100 (C), and percent thigmotaxis path at P100 (D) in CN-40, FID-40, FID-400, and TID rats. Diet groups without a common letter differ, P < 0.05. Values are means ± SE, n = 11–13 litters/group. CN-40, control; FID-40, formerly iron deficient fed 0.04-g/kg iron diet beginning at postnatal d 8; FID-400, formerly iron deficient fed 0.4-g/kg iron diet beginning at postnatal d 8; P, postnatal day; TID, totally iron deficient.
P100.
Overall, there was an effect of diet group (P < 0.04) and previous experience at P25 (P < 0.01) but no diet × previous experience interaction for latency to reach the platform at P100 (Fig. 4C). Overall, TID rats had longer latencies than FID-40 rats. For the path length to reach the platform, there were effects of group (P < 0.001), sex (P < 0.02), and previous experience at P25 (P < 0.001), but no diet group × sex or previous experience interaction. The mean difference for path length from d 1 to 7 for CN-40 rats (2.4 ± 3.2 cm) was shorter than for TID (4.7 ± 3.4 cm) (P < 0.08) and FID-400 (4.5 ± 2.7 cm) (P < 0.08) rats; the FID-40 rats (3.6 ± 3.1 cm) did not differ from CN-40 rats. For percent path thigmotaxis, there was a significant effect of diet (P < 0.001) and previous experience (P < 0.01) but no effect of sex (Fig. 4D). Over 7 trial days, FID-400 (P < 0.03) and TID rats (P < 0.001) had a greater percent path thigmotaxis than both CN-40 and FID-40 rats. There were no significant differences between diet groups for percent path in the platform quadrant.
In the 1-min probe trial, there was an effect of diet group for percent path thigmotaxis (P < 0.01) but no effect of sex or previous experience. Percent path thigmotaxis was greater in TID rats (57.2 ± 28.4%) than in FID-40 (34.7 ± 27.4%) (P < 0.02) and CN-40 (37.8 ± 20.2%) (P < 0.06) rats. FID-400 rats (50.3 ± 21.1%) did not significantly differ from the other groups. There were no significant diet group differences for percent path in the platform quadrant.
Discussion
This study focused on determining the dose of iron, administered at a brain growth period similar to term human birth, that would best ameliorate the long-term neurochemical and behavioral outcomes associated with early ID. Our previous studies on gestational ID in rats demonstrated that a moderate iron dose beginning at P4 was largely successful in treating monoamine abnormalities, but treatment at P21 was too late to recover from early iron losses (3, 16, 24–26). In terms of whole brain growth, the P4 time period for rodent brain development is similar to third trimester timing in humans, suggesting that treatment in humans should be focused on the mother rather than the offspring (27). However, fetal ID is difficult to diagnose and thus, the identification of successful iron treatment strategies at birth or later time points is an important issue. Therefore, we used a treatment model where iron was administered at P8, which is the peak of the brain growth spurt during rat development and is equivalent to the growth trajectory of the brain at human birth (27). Thus, this time point represents a period of rapid brain growth, which suggests that the impact of exposure to environmental changes (i.e., diet) may be enhanced.
Although rat studies have indicated that IDA extending through lactation (P21) can cause permanent developmental changes, only moderate dietary iron doses were used to treat ID in this study (3, 16). This raises the question of whether higher iron doses would be more effective in treating the brain and behavioral deficits. We therefore administered iron to ID rat pups at P8 at either moderate [0.04 g/kg diet, equivalent to 2–3 mg/(kg · d)] or higher [0.4 g/kg, equivalent to 6 mg/(kg · d)] doses. Importantly, both treatments fall within the range of iron used to treat infants and children with IDA. Iron treatment beginning at P8 did restore brain iron, but short- and long-term neurochemical and behavioral changes persisted, with outcome patterns differing by iron dose.
Dietary ID in rodents has long been known to deplete brain iron (2, 15, 37), and the TID group concentrations in the current study are similar to previous investigations. Iron treatment at moderate (0.04 g/kg) or higher (0.4 g/kg) doses generally normalized iron concentrations at P21 and P100, confirming that the treatments were successful in correcting overall brain iron. Importantly, the high-iron treatment did not elevate brain iron concentrations above the control, which parallels previous studies (38). Restrictions in iron uptake at the blood brain barrier are thought to prevent a potential iron toxicity in these feeding paradigms (39). However, we did not investigate earlier time points during lactation or iron localization within specific cell types, leaving open the possibility that iron regulation at the cellular level remains altered despite treatment.
Whereas P4 iron treatment mainly resolved monoamine alterations, P8 treatment appears ineffective at preventing monoamine changes. However, in the previous study, the ID time frame was G15 to P4 compared with G5 to P8 in the current study, which represents a much shorter time frame of ID. Therefore, successful repletion at P4 relative to P8 may be related to the duration and severity of ID experienced by the rats early in life. After P8 treatment, the moderate- and high-iron–treated rats had opposing changes in monoamine concentrations relative to CN-40 rats. With moderate iron treatment, DA, 5HT, and DOPAC were elevated in frontal cortex, striatum, and hippocampus at P21 and P100. Rats receiving the higher concentration of dietary iron at P8 had reductions, most notably in 5HT, in these measures in adulthood. It is unclear what mechanisms underlie or explain these opposing findings in a paradigm where factors leading up to the treatment were similar. However, it appears that the ID brain responds differently to the dose of iron available and that its response varies depending on the monoamine and region analyzed. As noted by others (40, 41), there are regional developmental differences in metabolic demand that may help to explain the monoamine changes observed in the present study.
The decline in neurotransmitter concentrations in rats receiving the high-iron treatment was comparable with that observed with ID (26). The similar pattern of monoamine losses in TID and FID-400 rats relative to CN-40 rats suggests that treatment with relatively high iron concentrations can be potentially as harmful as insufficient iron. Although regional brain iron concentrations in the high-iron treatment group were similar to CN40 rats in adolescence and adulthood, as stated earlier, we have no indication of how treatment with a higher iron diet following ID may alter the localization or function of iron in the brain. Nonetheless, any impairment in brain iron metabolism can have a significant downstream impact on monoamine metabolism, myelin content, and neuronal architecture and function (2, 5, 22, 23). In mice, treatment with high doses of iron causes neurodegeneration and a susceptibility to Parkinsonian-like behaviors (31). The current study revealed monoamine differences and potential dysfunctions that could be consistent with neurodegeneration. Several of the functional behavioral findings in this study also support such an interpretation.
Differences between rats treated with moderate- and high-iron doses extended to a subset of the behavioral outcomes. In the present study, patterns of developmental milestone acquisition were similar between FID-40 and TID rats; however, both treatment groups did achieve all milestones by P21. Moreover, in young adulthood, FID-40 rats performed similarly to CN-40 rats despite elevated DA and 5HT concentrations in the FID-40 group. Behavioral assessments, including pharmacological and longer term aging studies, could reveal deficits that may be correlated with these brain monoamine changes. However, the present findings suggest that moderate iron treatment of ID at P8 results in behavioral patterns that are largely similar to controls, at least at the level we assessed them.
Of particular concern is the overlap in performance between the TID and FID-400 rats. Although their acquisition of developmental milestones was similar to CN-40 rats, the performance of the FID-400 group on the spatial watermaze mirrored that of the TID group in adolescence and adulthood. Previous watermaze experience improved but did not eliminate the pattern of higher percent path thigmotaxis. This pattern also appeared to persist in the probe trial, although it did not reach significance. Thigmotaxis path describes the normal prepotent response of many animals in a novel environment. However, its persistence has been suggested to reflect greater anxiousness leading to less cognitive flexibility (42). Previous studies have shown that thigmotaxis can be modulated by changes in 5HT neurotransmission (43), suggesting that the deficits in 5HT at P21 and P100 in FID-400 and TID rats may help to explain this behavioral outcome.
A limitation of this study is that the focus on timing and dose of iron treatment precluded our ability to more fully determine the neurochemical and behavioral mechanisms that underlie the findings. However, both timing and dose of iron are important initial questions to address for pediatric practice, because ID and IDA remain common problems during early human development that have long-term neurodevelopmental implications. It has been assumed that the persistence of behavioral and learning deficits despite iron treatment argued for earlier and more aggressive treatment. Yet, the current study suggests that such treatment regimens with doses commonly used in clinical practice do not result in optimal neurologic outcomes. Whether this is due to the rapid developmental time line of the rat resulting in a more prolonged period of brain ID relative to the human or whether there is toxicity of higher dose iron remains to be determined. Clinically, this is an important distinction, because the former would suggest earlier or more rapid repletion, whereas the latter would support a more gradual strategy.
In summary, the P8 moderate-iron treatment appeared to afford the best behavioral outcome in the longer term for early ID rats despite remaining differences in monoamine concentrations. The higher iron treatment, using an iron dose that is within the range used to treat IDA in children, showed monoamine deficits and some behavioral patterns that were similar to TID rats. This study raises important questions about iron-trafficking mechanisms that could lead to such different monoamine and behavioral patterns in animals where the only difference in treatment was the iron dose that the ID brain received.
Acknowledgments
E.L.U. and B.F. designed and conducted the research, wrote the paper, and had primary responsibility for content; A.R.H. conducted the research; M.K.G., T.S., R.R., J.R.C., and B.L. designed the research and edited the manuscript; and N.K. performed the statistical analysis. All authors read and approved the final manuscript.
Footnotes
Supported by grant P01 HD39386, Brain and Behavior in Early Iron Deficiency from the NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations used: CN-40, control rats; DA, dopamine; DAT, dopamine transporter; DOPAC, 3,4 dihydroxyphenylacetic acid; FID-40, formerly iron deficient fed 0.04-g/kg iron diet beginning at postnatal d 8; FID-400, formerly iron deficient fed 0.4-g/kg iron diet beginning at postnatal d 8; G, gestational day; HCT, hematocrit; ID, iron deficiency; IDA, iron deficiency anemia; P, postnatal day; TID, totally iron-deficient rat; 5HIAA, 5-hydroxyindoleacetic acid; 5HT, serotonin.
Literature Cited
- 1.Lozoff B, Beard J, Connor J, Felt B, Georgieff M, Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev. 2006;64:S34–43; discussion S72–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beard J, Erikson KM, Jones BC. Neonatal iron deficiency results in irreversible changes in dopamine function in rats. J Nutr. 2003;133:1174–9 [DOI] [PubMed] [Google Scholar]
- 3.Felt BT, Beard J, Schallert T, Shao J, Aldridge JW, Connor JR, Georgieff MK, Lozoff B. Persistent neurochemical and behavioral abnormalities in adulthood despite early iron supplementation for perinatal iron deficiency anemia in rats. Behav Brain Res. 2006;171:261–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Unger EL, Paul T, Murray-Kolb LE, Felt B, Jones BC, Beard JL. Early iron deficiency alters sensorimotor development and brain monoamines in rats. J Nutr. 2007;137:118–24 [DOI] [PubMed] [Google Scholar]
- 5.Jorgenson LA, Sun M, O'Connor M, Georgieff MK. Fetal iron deficiency disrupts the maturation of synaptic function and efficacy in area CA1 of the developing rat hippocampus. Hippocampus. 2005;15:1094–102 [DOI] [PubMed] [Google Scholar]
- 6.Carlson ES, Tkac I, Magid R, O'Connor MB, Andrews NC, Schallert T, Gunshin H, Georgieff MK, Petryk A. Iron is essential for neuron development and memory function in mouse hippocampus. J Nutr. 2009;139:672–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lozoff B, Corapci F, Burden MJ, Kaciroti N, Angulo-Barroso R, Sazawal S, Black M. Preschool-aged children with iron deficiency anemia show altered affect and behavior. J Nutr. 2007;137:683–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lozoff B, Jimenez E, Hagen J, Mollen E, Wolf AW. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics. 2000;105:E51. [DOI] [PubMed] [Google Scholar]
- 9.Lukowski AF, Koss M, Burden MJ, Jonides J, Nelson CA, Kaciroti N, Jimenez E, Lozoff B. Iron deficiency in infancy and neurocognitive functioning at 19 years: evidence of long-term deficits in executive function and recognition memory. Nutr Neurosci. 2010;13:54–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peirano PD, Algarin CR, Chamorro R, Reyes S, Garrido MI, Duran S, Lozoff B. Sleep and neurofunctions throughout child development: lasting effects of early iron deficiency. J Pediatr Gastroenterol Nutr. 2009;48 Suppl 1:S8–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lozoff B, Klein NK, Nelson EC, McClish DK, Manuel M, Chacon ME. Behavior of infants with iron-deficiency anemia. Child Dev. 1998;69:24–36 [PubMed] [Google Scholar]
- 12.Shafir T, Angulo-Barroso R, Su J, Jacobson SW, Lozoff B. Iron deficiency anemia in infancy and reach and grasp development. Infant Behav Dev. 2009;32:366–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlson ES, Stead JD, Neal CR, Petryk A, Georgieff MK. Perinatal iron deficiency results in altered developmental expression of genes mediating energy metabolism and neuronal morphogenesis in hippocampus. Hippocampus. 2007;17:679–91 [DOI] [PubMed] [Google Scholar]
- 14.Mohamed WM, Unger EL, Kambhampati SK, Jones BC. Methylphenidate improves cognitive deficits produced by infantile iron deficiency in rats. Behav Brain Res. 2011;216:146–52 [DOI] [PubMed] [Google Scholar]
- 15.Beard JL, Chen Q, Connor J, Jones BC. Altered monamine metabolism in caudate-putamen of iron-deficient rats. Pharmacol Biochem Behav. 1994;48:621–4 [DOI] [PubMed] [Google Scholar]
- 16.Beard JL, Felt B, Schallert T, Burhans M, Connor JR, Georgieff MK. Moderate iron deficiency in infancy: biology and behavior in young rats. Behav Brain Res. 2006;170:224–32 [DOI] [PubMed] [Google Scholar]
- 17.Erikson KM, Jones BC, Beard JL. Iron deficiency alters dopamine transporter functioning in rat striatum. J Nutr. 2000;130:2831–7 [DOI] [PubMed] [Google Scholar]
- 18.Erikson KM, Jones BC, Hess EJ, Zhang Q, Beard JL. Iron deficiency decreases dopamine D1 and D2 receptors in rat brain. Pharmacol Biochem Behav. 2001;69:409–18 [DOI] [PubMed] [Google Scholar]
- 19.Unger EL, Wiesinger JA, Hao L, Beard JL. Dopamine D2 receptor expression is altered by changes in cellular iron levels in PC12 cells and rat brain tissue. J Nutr. 2008;138:2487–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beard JL, Wiesinger JA, Connor JR. Pre- and postweaning iron deficiency alters myelination in Sprague-Dawley rats. Dev Neurosci. 2003;25:308–15 [DOI] [PubMed] [Google Scholar]
- 21.Brunette KE, Tran PV, Wobken JD, Carlson ES, Georgieff MK. Gestational and neonatal iron deficiency alters apical dendrite structure of CA1 pyramidal neurons in adult rat hippocampus. Dev Neurosci. 2010;32:238–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rao R, Tkac I, Townsend EL, Gruetter R, Georgieff MK. Perinatal iron deficiency alters the neurochemical profile of the developing rat hippocampus. J Nutr. 2003;133:3215–21 [DOI] [PubMed] [Google Scholar]
- 23.Ward KL, Tkac I, Jing Y, Felt B, Beard J, Connor J, Schallert T, Georgieff MK, Rao R. Gestational and lactational iron deficiency alters the developing striatal metabolome and associated behaviors in young rats. J Nutr. 2007;137:1043–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piñero D, Jones B, Beard J. Variations in dietary iron alter behavior in developing rats. J Nutr. 2001;131:311–8 [DOI] [PubMed] [Google Scholar]
- 25.Piñero DJ, Li NQ, Connor JR, Beard JL. Variations in dietary iron alter brain iron metabolism in developing rats. J Nutr. 2000;130:254–63 [DOI] [PubMed] [Google Scholar]
- 26.Beard JL, Unger EL, Bianco LE, Paul T, Rundle SE, Jones BC. Early postnatal iron repletion overcomes lasting effects of gestational iron deficiency in rats. J Nutr. 2007;137:1176–82 [DOI] [PubMed] [Google Scholar]
- 27.Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83 [DOI] [PubMed] [Google Scholar]
- 28.Felt BT, Lozoff B. Brain iron and behavior of rats are not normalized by treatment of iron deficiency anemia during early development. J Nutr. 1996;126:693–701 [DOI] [PubMed] [Google Scholar]
- 29.Rao R, Tkac I, Townsend EL, Ennis K, Gruetter R, Georgieff MK. Perinatal iron deficiency predisposes the developing rat hippocampus to greater injury from mild to moderate hypoxia-ischemia. J Cereb Blood Flow Metab. 2007;27:729–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt AT, Ladwig EK, Wobken JD, Grove WM, Georgieff MK. Delayed alternation performance in rats following recovery from early iron deficiency. Physiol Behav. 2010;101:503–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaur D, Peng J, Chinta SJ, Rajagopalan S, Di Monte DA, Cherny RA, Andersen JK. Increased murine neonatal iron intake results in Parkinson-like neurodegeneration with age. Neurobiol Aging. 2007;907–13 [DOI] [PubMed] [Google Scholar]
- 32.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–51 [DOI] [PubMed] [Google Scholar]
- 33.O'Connor DL, Picciano MF, Sherman AR. Impact of maternal iron deficiency on quality and quantity of milk ingested by neonatal rats. Br J Nutr. 1988;60:477–85 [DOI] [PubMed] [Google Scholar]
- 34.Lin W, Kirksey A. Effects of different levels of dietary iron on pregnancy superimposed upon growth in the rat. J Nutr. 1976;106:543–54 [DOI] [PubMed] [Google Scholar]
- 35.Burhans MS, Dailey C, Beard Z, Wiesinger J, Murray-Kolb L, Jones BC, Beard JL. Iron deficiency: differential effects on monoamine transporters. Nutr Neurosci. 2005;8:31–8 [DOI] [PubMed] [Google Scholar]
- 36.Lapointe G, Nosal G. A rat model of neurobehavioral development. Experientia. 1979;35:205–7 [DOI] [PubMed] [Google Scholar]
- 37.Youdim MB, Sills MA, Heydorn WE, Creed GJ, Jacobowitz DM. Iron deficiency alters discrete proteins in rat caudate nucleus and nucleus accumbens. J Neurochem. 1986;47:794–9 [DOI] [PubMed] [Google Scholar]
- 38.Unger EL, Beard JL, Jones BC. Iron regulation in C57BLI6 and DBA/2 mice subjected to iron overload. Nutr Neurosci. 2007;10:89–95 [DOI] [PubMed] [Google Scholar]
- 39.Taylor EM, Crowe A, Morgan EH. Transferrin and iron uptake by the brain: effects of altered iron status. J Neurochem. 1991;57:1584–92 [DOI] [PubMed] [Google Scholar]
- 40.Kretchmer N, Beard JL, Carlson S. The role of nutrition in the development of normal cognition. Am J Clin Nutr. 1996;63:S997–1001 [DOI] [PubMed] [Google Scholar]
- 41.de Deungria M, Rao R, Wobken JD, Luciana M, Nelson CA, Georgieff MK. Perinatal iron deficiency decreases cytochrome c oxidase (CytOx) activity in selected regions of neonatal rat brain. Pediatr Res. 2000;48:169–76 [DOI] [PubMed] [Google Scholar]
- 42.Choi KH, Kwon JH. Social cognition enhancement training for schizophrenia: a preliminary randomized controlled trial. Community Ment Health J. 2006;42:177–87 [DOI] [PubMed] [Google Scholar]
- 43.Kalueff AV, Jensen CL, Murphy DL. Locomotory patterns, spatiotemporal organization of exploration and spatial memory in serotonin transporter knockout mice. Brain Res. 2007;1169:87–97 [DOI] [PubMed] [Google Scholar]