Abstract
Stable hydrogen isotope methodology is used in nutrition studies to measure growth, breast milk intake, and energy requirement. Isotope ratio MS is the best instrumentation to measure the stable hydrogen isotope ratios in physiological fluids. Conventional methods to convert physiological fluids to hydrogen gas (H2) for mass spectrometric analysis are labor intensive, require special reagent, and involve memory effect and potential isotope fractionation. The objective of this study was to determine the accuracy and precision of a platinum catalyzed H2-water equilibration method for stable hydrogen isotope ratio measurements. Time to reach isotopic equilibrium, day-to-day and week-to-week reproducibility, accuracy, and precision of stable hydrogen isotope ratio measurements by the H2-water equilibration method were assessed using a Thermo DELTA V Advantage continuous-flow isotope ratio mass spectrometer. It took 3 h to reach isotopic equilibrium. The day-to-day and week-to-week measurements on water and urine samples with natural abundance and enriched levels of deuterium were highly reproducible. The method was accurate to within 2.8 o/oo and reproducible to within 4.0 o/oo based on analysis of international references. All the outcome variables, whether in urine samples collected in 10 doubly labeled water studies or plasma samples collected in 26 body water studies, did not differ from those obtained using the reference zinc reduction method. The method produced highly accurate estimation on ad libitum energy intakes, body composition, and water turnover rates. The method greatly reduces the analytical cost and could easily be adopted by laboratories equipped with a continuous-flow isotope ratio mass spectrometer.
Introduction
The stable hydrogen isotope method has been used in nutrition studies to measure growth (1), breast milk intake (2, 3), lipid metabolism (4), and free-living energy expenditure (EE) (5–9). With obesity reaching epidemic proportions in the United States, the use of the stable hydrogen isotope method to measure growth and EE under free-living conditions is of particular importance, because the tracer, deuterium (2H)3 oxide, is nonradioactive and the heavier isotope, 2H, is present in body tissues and in foods and beverages that are being consumed by humans every day. Therefore, the stable hydrogen isotope method can be used in all age groups, including premature infants, newborns, toddlers, children, and pregnant and lactating women with no known adverse effects.
Isotope ratio MS is considered to be the best instrumentation for the accurate and precise measurements of 2H in physiological samples (10, 11). However, stable hydrogen isotope ratio measurement is considered most difficult, because the process to reduce water in the physiological fluids to hydrogen gas (H2) for isotope ratio measurement is labor intensive, involves a potential huge isotope fractionation effect, and requires correction for memory effect.
In the current study, we sought to determine whether a platinum catalyzed H2-water equilibration method, without the requirement to reduce the physiological fluid to H2, could produce accurate and reproducible stable hydrogen isotope ratio measurements to support nutrition studies using the stable hydrogen isotope methodology.
Methods
Mass spectrometer system
A Thermo Delta V Advantage continuous-flow isotope ratio mass spectrometer system equipped with a Finnigan GasBench II (Thermo Electron North America) was used to assess the accuracy and reproducibility of the platinum catalyzed H2-water equilibration method for stable hydrogen isotope ratio measurements. Briefly, the mass spectrometer system was set to H mode with high voltage at 3 kV and the magnet at 1579 steps. The reference H2 (Air Liquide Healthcare) was set at 449 kPa and the carrier gas, helium (at 99.999%, Air Liquide Healthcare) was set at 414 kPa. All stable hydrogen isotope ratio measurements are expressed in δ per mil unit (o/oo) vs. the international reference materials, Standard Mean Ocean Water (SMOW), and Standard Light Antarctic Precipitation (SLAP) (12, 13) as follows:
where (2H/1H)sample and (2H/1H)reference represent the 2H:1H ratio of the sample or the reference H2, respectively. A change in 1 δ2HSMOW/SLAP unit is equivalent to a change of 65 μmol/L in 2H content.
Sample preparation procedure
After uncapping a 12-mL Exetainer (Labco International), ∼5 mg of activated charcoal (Fisher Scientific) and ∼200 mg of copper powder (Fisher Scientific) were introduced into the Exetanier followed with a platinum catalytic rod (ThermoScientific). The activated charcoal and copper powder were added to remove any potential contaminants in the samples that might poison the catalyst. After putting 0.2 mL of sample into the Exetainer, the Exetainer was recapped and placed into the GasBench II and flushed with 2% H2 in helium at 483 kPa (M0001710-P-44 at 99.999% H2 and 99.996% helium, Air Liquide Healthcare) for 7 min. The sample was allowed to equilibrate with the H2 at room temperature. At the end of the equilibration, an aliquot of the H2 in the Exetainer was injected into the Thermo Delta V Advantage mass spectrometer system for stable hydrogen isotope ratio measurement against the reference H2.
Equilibration time
The time to reach isotopic equilibrium was assessed by allowing 18 Exetainers containing a water sample with a δ2HSMOW/SLAP value of +963% to equilibrate between 0.7 and 8.8 h. At the end of each equilibration period, an aliquot of the H2 in the Exetainers was injected into the mass spectrometer system for stable hydrogen isotope ratio measurement.
Day-to-day and week-to-week reproducibility
Two water samples, one at natural abundance (δ2HSMOW/SLAP = −14.9 o/oo) and one at an enriched level of 2H (δ2HSMOW/SLAP = +1096.8 o/oo) as well as 2 urine samples, one at natural abundance (δ2HSMOW/SLAP = −37.9 o/oo) and one at an enriched level of 2H (δ2HSMOW/SLAP = +1077.3 o/oo), were analyzed once per day for 8 d and once per week for 5 wk.
Accuracy and precision of stable hydrogen isotope ratio measurements
The accuracy and precision of the H2-water equilibration method for stable hydrogen isotope ratio measurements were assessed using 3 international reference materials: Greenland Ice Sheet Precipitation (GISP) (12, 13), International Atomic Energy Agency (IAEA) 302A, and IAEA 302B (14). Three separate aliquots of the GISP were analyzed and each analysis represented 10 measurements. The IAEA reference materials were analyzed during a period of 4 d and each analysis again represented 10 measurements.
Accuracy of the H2-water equilibration method in doubly labeled water and body water studies
To further assess the accuracy of the platinum catalyzed H2-water equilibration method, urine samples collected from 10 doubly labeled water (DLW) studies with EE values ranging between 1943 and 3006 kcal/d were analyzed using the platinum catalyzed H2-water equilibration method following the stable hydrogen isotope ratio measurements using the reference zinc reduction method (15) and the stable oxygen isotope ratio measurements using the reference H2O-CO2 equilibration method (11). The DLW outcome parameters: fractional turnover rate of 2H (kH), isotope dilution space of 2H (NH), ratio between the isotope dilution spaces of 2H and oxygen-18 (NH:NO), and EE derived from the stable hydrogen isotope ratio measurements using the H2-water equilibration method, were compared with those obtained using the reference methods (11, 15). To determine whether the equilibration method was able to accurately measure the stable hydrogen isotope content in serum samples, serum samples collected in 26 body water studies with NH ranging between 46 and 86 kg were analyzed. The NH obtained from the equilibration method was compared with those obtained using the reference zinc reduction method (15).
Nutrition applications of the H2-water equilibration method
Ad libitum energy intake.
In a clinical trial (Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy) to determine the effect of energy restriction on human physiology, the ad libitum energy intake of 221 normal-weight study participants was determined using the DLW method. In the DLW method, after the collection of 2 baseline urine samples, each participant ingested 1.7 g/kg body weight of DLW. Two urine samples were collected 4–5 h postdose. Four more urine samples, 2 on each day, were collected 7 and 14 d postdose. Because these participants were weight stable, the ad libitum energy intake was equal to EE. To demonstrate the accuracy and precision of the H2-water equilibration method to estimate ad libitum energy intake, urine samples collected from 10 study participants in this clinical trial were analyzed using the traditional zinc reduction method and again using the H2-water equilibration method. The ad libitum energy intakes were then compared between the 2 analytical methods.
Body fat measurements.
In a companion animal study to look at the effect of diet on body composition, the 2H dilution spaces of house cats (Felis catus) at various stages of obesity were measured by the 2H oxide dilution method. After collection of a baseline serum sample, each cat was given orally 60 mg/kg body weight of 2H oxide. Another serum sample was collected 3 h postdose. The serum samples collected from 10 cats were analyzed for 2H content using both the traditional zinc reduction method and the H2-water equilibration method. Total body water was calculated by dividing the 2H dilution spaces by 1.04, because 2H dilution is known to overestimate total body water by 4%. Lean body mass was calculated by dividing total body water by 0.73, the hydration constant of lean body mass in mature cats. Body fat was calculated as the difference between body weight and lean body mass. The body fat obtained in these 10 studies was compared between the 2 analytical methods.
Water turnover rates.
Water turnover rates can be estimated using the 2H dilution method. The information can be used to estimate breast milk production in lactating women or fluid requirement under challenging field conditions. After collection of a baseline urine sample, the study participants ingested 100 mg/kg body weight of 2H oxide. Six more postdose urine samples were collected on d 1, 7, and 14. The urine samples from 10 soldiers in a training field study were analyzed using the traditional zinc reduction method and again using the H2-water equilibration method. The 2H dilution spaces were calculated as described in the body fat measurements. The fractional turnover rates of 2H were calculated from the monoexponential disappearing curves of 2H over time in the urine samples. Water turnover rates were calculated as the product between the 2H dilution spaces and the fractional turnover rates. The water turnover rates were compared between the 2 analytical methods.
All human and animal protocols described in this manuscript have been approved by the Institutional Review Board for Human and Animal Studies at the study institutions (Baylor College of Medicine, Pennington Biomedical Research Institute, and Nestle Purina PetCare).
Statistical analysis
The Bland and Altman pairwise comparison analysis (16) was used to evaluate the accuracy of the platinum catalyzed H2-water equilibration method for the measurements of kH, NH, NH:NO, and/or EE against those generated using the reference zinc reduction method (11, 15). The paired samples t test was used to compare the results obtained in the studies to measure ad libitum energy intake, body fat, and water turnover rate. An α value of 0.05 was used in all statistical analyses and all values in the text are mean ± SD.
Results
It took ∼3 h for the stable hydrogen isotopes to reach isotopic equilibrium at room temperature. The stable hydrogen isotope ratios remained stable for up to at least 9 h.
The day-to-day and week-to-week measurements of a water material at natural abundance of 2H (δ2HSMOW/SLAP = −14.9 o/oo) were reproducible to within 1.5 and 1.4 o/oo, respectively. For a water material at the enriched level of 2H (δ2HSMOW/SLAP = +1096.8 o/oo), the day-to-day and week-to-week measurements were reproducible to within 4.6 and 4.6 o/oo, respectively.
For a urine material at natural abundance of 2H (δ2HSMOW/SLAP = −37.9 o/oo), the day-to-day and week-to-week measurements were reproducible to within 1.5 and 1.7 o/oo, respectively. At the enriched level of 2H (δ2HSMOW/SLAP = +1,077.3 o/oo), the day-to-day and week-to-week measurements were reproducible to within 3.8 and 4.1 o/oo, respectively.
Table 1 summarized the accuracy and precision of the stable hydrogen isotope ratio measurements of the equilibration method. Three aliquots of GISP were analyzed and measured. The δ2HSMOW/SLAP values for GISP were accurate to +0.7 o/oo compared with the accepted δ2HSMOW/SLAP value for GISP (12, 13). The overall precision of the GISP measurement was determined to be 1.5 o/oo. With the IAEA 302A reference material at a δ2HSMOW/SLAP value of +506.2 o/oo, the measured δ2HSMOW/SLAP values over a period of 4 d were accurate to −1.4 o/oo and reproducible to within 4.0 o/oo. With the IAEA 302B reference material at a δ2HSMOW/SLAP value of +992.3 o/oo, the measured δ2HSMOW/SLAP values over a period of 4 d were accurate to +2.8 o/oo and reproducible to within 2.7 o/oo.
TABLE 1.
Accuracy and precision of stable hydrogen isotope ratio measurements by the platinum catalyzed H2-water equilibration method using international water reference materials1
| References | Accepted δ2H value | Measured δ2H value2 | Accuracy2 | Mean accuracy | Overall precision3 |
| o/oo | |||||
| GISP | −189.7 (−190.2 to −189.1) | −189.7 ± 1.3 | −0.0 | +0.7 | 1.5 |
| −190.1 ± 1.7 | −0.4 | ||||
| −188.8 ± 1.5 | +0.9 | ||||
| IAEA 302A | 506.2 (504.0–508.4) | 504.8 ± 2.4 | −1.4 | −1.4 | 4.0 |
| 507.9 ± 3.4 | +1.7 | ||||
| 503.0 ± 4.4 | −3.2 | ||||
| 503.5 ± 5.4 | −2.7 | ||||
| IAEA 302B | 992.3 (985.6–999.0) | 991.6 ± 2.4 | −0.7 | +2.8 | 2.7 |
| 995.4 ± 3.0 | +3.1 | ||||
| 998.7 ± 3.2 | +6.4 | ||||
| 994.8 ± 2.1 | +2.5 | ||||
Values are mean ± SD or accepted values (95%CI), n = 10. GISP, Greenland Ice Sheet Precipitation; 2H, deuterium; IAEA 302A, International Atomic Energy Agency 2H enriched reference water material 302A; IAEA 302B, International Atomic Energy Agency 2H enriched reference water material 302B.
Accuracy (o/oo) = measured δ2H value (o/oo) – accepted δ2H value (o/oo).
Overall precision (o/oo) = 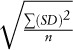 , where SD is the internal precision or SD of each set of measurements and n is the total number of sets of δ2H values used in the evaluation.
, where SD is the internal precision or SD of each set of measurements and n is the total number of sets of δ2H values used in the evaluation.
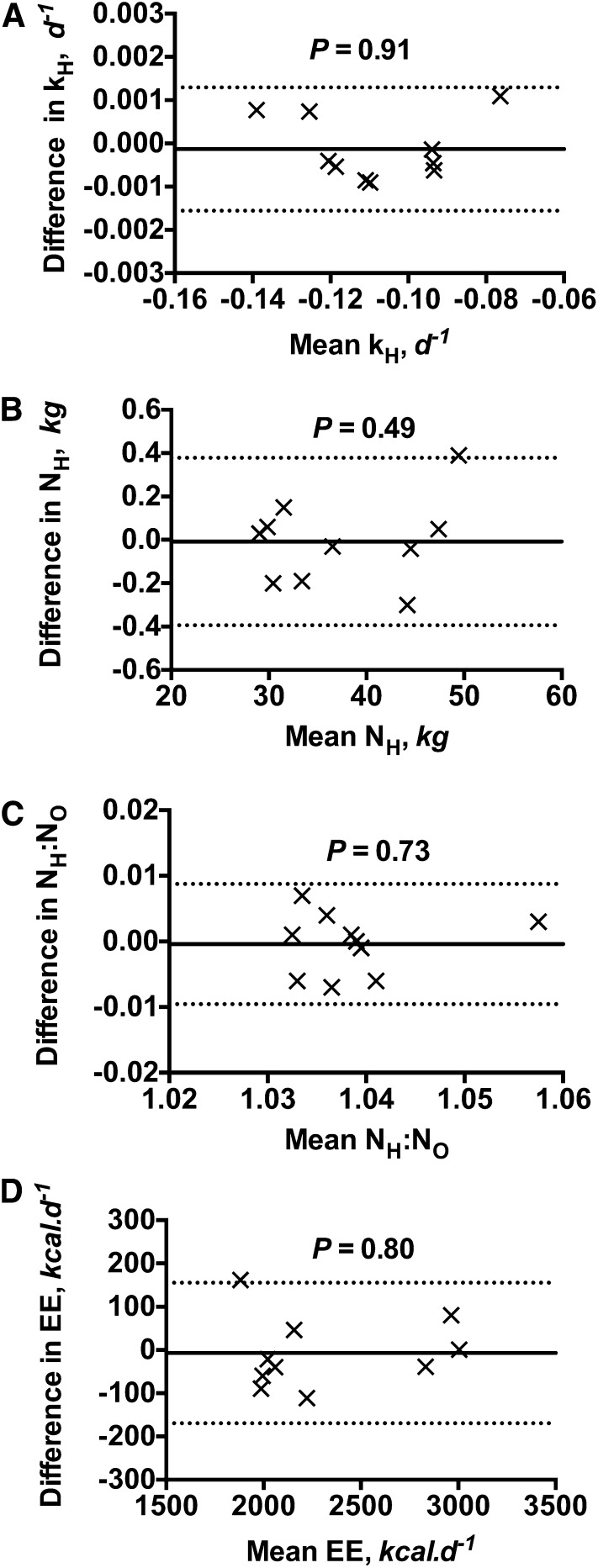
Figure 1 compared the kH, NH, NH:NO, and EE measurements by the H2-water equilibration method with those obtained using the reference zinc reduction method. The kH values were found to be similar between the 2 methods (Fig. 1A), with bias calculated to be: −0.00013 d−1 and the lower and upper limits of agreement to be −0.00156 and 0.00129 d−1, respectively. For NH (Fig. 1B), the bias was −0.01 kg and the lower and upper limits of agreement were −0.39 and 0.38 kg, respectively. The NH:NO ratio (Fig. 1C) also was very similar between the 2 methods (bias: −0.0004; lower and upper limits of agreement: −0.0096 and 0.0088, respectively). Measurements of EE (Fig. 1D) also were highly reproducible between the 2 methods (bias: −7.1 kcal/d; lower and upper limits of agreement: −169.5 and 155.4 kcal/d, respectively). As shown by the P values in each figure, no significant relationship was detected between the differences and the mean values.
FIGURE 1.
Bland and Altman pairwise comparison of the DLW outcome variables: kH (A), NH (B), NH:NO (C), and EE (D) generated from urine samples collected in 10 DLW human studies by the platinum catalyzed H2-water equilibration method and the reference zinc reduction method. The y-axis represents the differences between the 2 measurements. The x-axis represents the means between the 2 measurements. The solid lines represent the bias and the dashed lines represent the lower and upper limits of agreement. The P value in each figure represents the significant value by linear regression analysis between the differences and the mean values. DLW, doubly labeled water; EE, energy expenditure; H2, hydrogen gas; kH, fractional turnover rate of deuterium; NH, isotope dilution space of deuterium; NH:NO, ratio between the isotope dilution spaces of deuterium and oxygen-18.
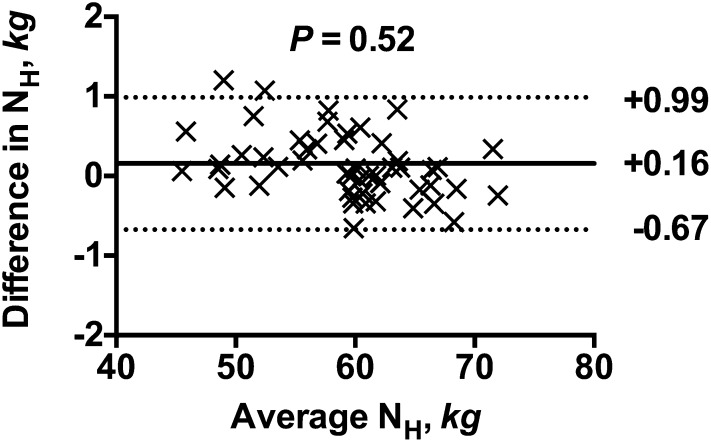
The accuracy of the equilibration method to measure the stable hydrogen isotope content in serum samples is demonstrated in Figure 2. As shown in the figure, the NH values measured by the equilibration method were only 0.16 kg (bias) higher than that obtained using the zinc reduction method, with lower and upper limits of agreement at −0.67 and 0.99 kg, respectively.
FIGURE 2.
Bland and Altman pairwise comparison of the isotope dilution spaces of 2H generated from serum samples collected in 26 body water studies in common house cats between the platinum catalyzed H2-water equilibration method and the reference zinc reduction method. The y-axis represents the differences between the 2 measurements. The x-axis represents the means between the 2 measurements. The solid lines represent the bias and the dashed lines represent the lower and upper limits of agreement. The P value represents the significant value by linear regression analysis between the differences and the mean values. H2, hydrogen gas; 2H, deuterium.
Table 2 summarizes the comparison of ad libitum energy intakes, body fat, and water turnover rates between the 2 analytical methods. As shown in the table, ad libitum energy intakes were accurate to 8 ± 81 kcal/d between the 2 methods. Body fat was accurate to 0.01 ± 0.01 kg between the 2 methods. Water turnover rates were not different (0.00 ± 0.01 kg/d) between the 2 methods. Essentially, the nutritional outcome values measured using the H2-water equilibration method were not significantly different from those measured using the reference zinc reduction method. In fact, the ad libitum energy intakes, body fat, and water turnover rates were highly correlated (r = 0.98) between the 2 analytical methods.
TABLE 2.
Accuracy and precision of the platinum catalyzed H2-water equilibration method in a caloric restriction study in normal-weight adults, in an obesity study on Felis catus, and in a combat field study against the reference zinc reduction method1
| Nutrition applications | Values by reference zinc reduction method | Values by H2-water equilibration method | Accuracy2 | Precision3 | P4 |
| Ad libitum energy intake, kcal/d | 2309 ± 4474 | 2317 ± 438 | 8 | 81 | 0.77 |
| Body fat, kg | 1.71 ± 0.84 | 1.72 ± 0.85 | 0.01 | 0.01 | 0.21 |
| Water turnover rate, kg/d | 4.0 ± 0.6 | 4.0 ± 0.6 | 0.0 | 0.01 | 0.77 |
Values are expressed as mean ± SD, n = 10. H2, hydrogen gas.
Accuracy = values by H2-water equilibration method – values by reference zinc reduction method.
Precision = SD of the differences between the 2 methods.
P values level by paired samples t test.
Discussion
Our results showed that the platinum catalyzed H2-water equilibration method, using a small sample size of 200 μL, produced highly accurate and reproducible stable hydrogen isotope ratios in natural waters, international reference materials, and physiological fluids at both natural abundance and enriched levels of 2H commonly encountered in the DLW and body water studies using the stable hydrogen isotope methodology. The method is very straightforward, because it has minimal sample pretreatment and does not require the reduction of water samples into H2 prior to mass spectrometric analysis. The equilibration method also does not require correction for memory effect commonly associated with the in-line reduction method.
As shown in Table 2, the H2-water equilibration method can be used in many nutrition studies, including studies requiring information on ad libitum energy intakes, body fat, and water turnover rates. In fact, several NIH-funded projects are using the DLW method to evaluate the accuracy and precision of various instruments for gathering energy intakes in free-living subjects as well as to determine the energy requirements in toddlers. The DLW method also has been used to monitor study compliance in participants undergoing a weight-loss program or to look at changes in thermogenesis with diets. The H2-water equilibration method will definitely facilitate the sample processing in these studies.
The H2-water equilibration method for stable hydrogen isotope ratio measurements was first described by Horita (17, 18) with a precision of 1.5 o/oo on natural waters. The method was subsequently adapted by Coplen (19), showing a precision of 1.3 o/oo on natural water samples. He reported the use of copper granules to prevent the sulfide in the water samples to poison the platinum catalyst. Coplen (20) subsequently tested the H2-water equilibration method on stable hydrogen isotope ratio measurements on urine samples collected from 2 diabetic patients. The δ2HSMOW/SLAP values of the urine samples ranged between −51 and +890 o/oo. Compared to the reference zinc reduction method, the authors reported that the accuracy varied between −211 and +71 o/oo. The authors attributed the huge variation in accuracy to potential incomplete reduction of the water to H2 or the inherent poor precision of the zinc reduction method. This is a possibility, because their zinc reduction procedure put the urine samples in direct contact with the zinc reagent, thus providing an opportunity for compounds in the urine samples to poison the zinc reagent. However, when the sample is not allowed to come in contact with the zinc reagent, as we described in our zinc reduction procedure (15), we have not observed such an effect.
Scrimgeour (21) subsequently tested the H2-equilibration method on 3 tap water, 3 urine samples, and 3 plasma samples at natural abundance of 2H as well as urine samples collected in one body water study and urine samples collected in 3 DLW studies. Using a sample size of 400 μL, the results were highly reproducible but took 3 d to reach isotopic equilibrium. In the DLW studies, the kH values were found to be similar to those obtained using the zinc reduction method. The study lacked the evaluation of the equilibration method on the day-to-day and week-to-week reproducibility of the measurements as well as the accuracy of the measurements against international reference materials. The study also did not evaluate the equilibration method with a wide range of body water or EE values as we performed in our study. The long equilibration time reported possibly could be due to the limited surface contact between the H2, the catalyst and the sample, because the catalyst (platinum-on-alumina) was placed inside a glass vial (32 mm × 5 mm o.d.) in this study. Our study showed that it took only 3 h to reach isotopic equilibrium using a smaller sample size of 200 μL.
The platinum catalyzed H2-water equilibration method has several advantages over the conventional methods. The classical method to use uranium turnings to reduce water into H2 requires the use of a dedicated vacuum line and a mercury Topler pump to transfer the H2 into a sample bulb, because H2 is not condensable at liquid nitrogen temperature. The procedure obviously has memory issue and is labor intensive. The development of an in-line uranium reduction method coupled with an isotope ratio mass spectrometer (22) eliminated the requirement of a separate sample preparation vacuum line but has huge memory effect. In both cases, replacement of the uranium turnings required shutting down the vacuum line or the sample inlet.
The zinc reduction method to convert water or water in physiological fluids to H2 has no memory effect but is labor intensive and required a special zinc reagent to ensure complete reduction (11, 15).
With the development of the continuous-flow isotope ratio MS, manufacturers have been able to include an in-line chromium reactor to reduce water into H2 for stable hydrogen isotope ratio measurements. The method is well accepted by most users, because it requires a very small amount of sample and is relatively quick. The method, however, involves some memory effect and the standard chromium reactor is good for only ∼200 injections. Replacement of the chromium reactor also requires cooling down the reactor and shutting down the inlet system. The samples also need to be pretreated with activated charcoal followed by the removal of the activated charcoal from the treated sample through a 0.2-μm filter. The filtration process requires at least 1–5 mL of sample to avoid possible contamination from moisture adhered to the barrel of the syringe, plunger, and filter.
In conclusion, our manuscript represents the most detailed evaluation of a platinum catalyzed H2-water equilibration method for stable hydrogen isotope ratio measurements and its potential application in many nutrition studies. The method is simple, highly reproducible, accurate, and without memory effect. Therefore, any laboratories equipped with a continuous-flow isotope ratio mass spectrometer system should be able to adopt the method to support their nutrition studies using the stable hydrogen isotope methodology.
Acknowledgments
L.L.C. and W.W.W. designed research; L.L.C. conducted research; and W.W.W. wrote the manuscript and analyzed data. Both authors read and approved the final manuscript.
Footnotes
Supported by the NIH National Center for Research Resources Shared Instrumentation Grant (1S10RR026764-01) and USDA/Agricultural Research Service grant no. 6250-51000-053. The contents of this publication do not necessarily reflect the views or policies of the USDA or the NIH, nor does mention of trade names, commercial products, or organizations imply endorsement.
Abbreviations used: DLW, doubly labeled water; EE, energy expenditure; H2, hydrogen gas; 2H, deuterium; GISP, Greenland Ice Sheet Precipitation; IAEA, International Atomic Energy Agency; kH, fractional turnover rate of deuterium; NH, isotope dilution space of deuterium; NH:NO, ratio between the isotope dilution spaces of deuterium and oxygen-18.
Literature Cited
- 1.Butte NF, Hopkinson JM, Wong WW, Smith EO, Ellis KJ. Body composition during the first 2 years of life: an updated reference. Pediatr Res. 2000;47:578–85 [DOI] [PubMed] [Google Scholar]
- 2.Butte NF, Villalpando S, Wong WW, Flores-Huerta S, Hernandez-Beltran M, Smith EO, Garza C. Human milk intake and growth faltering of rural Mesoamerindian infants. Am J Clin Nutr. 1992;55:1109–16 [DOI] [PubMed] [Google Scholar]
- 3.Galpin L, Thakwalakwa C, Phuka J, Ashorn P, Maleta K, Wong WW, Manary MJ. Breast milk intake is not reduced more by the introduction of energy dense complementary food than by typical infant porridge. J Nutr. 2007;137:1828–33 [DOI] [PubMed] [Google Scholar]
- 4.Wong WW, Hachey DL, Insull W, Opekun AR, Klein PD. Effect of dietary cholesterol on cholesterol synthesis in breast-fed and formula-fed infants. J Lipid Res. 1993;34:1403–11 [PubMed] [Google Scholar]
- 5.Jensen CL, Butte NF, Wong WW, Moon JK. Determining energy expenditure in preterm infants: comparison of 2H218O method and indirect calorimetry. Am J Physiol. 1992;263:R685–92 [DOI] [PubMed] [Google Scholar]
- 6.Roberts SB, Coward WA, Schlossman SF, Schlingensseipen KH, Nohria V, Lucas A. Comparison of the doubly labeled water (2H2–18O) method with indirect calorimetry and a nutrient balance study for simultaneous determination of energy expenditure, water intake, and metabolizable energy intake in preterm infants. Am J Clin Nutr. 1986;44:315–22 [DOI] [PubMed] [Google Scholar]
- 7.Butte NF, Wong WW, Ferlic L, Smith EO, Klein PD, Garza C. Energy expenditure and deposition of breast-fed and formula-fed infants during early infancy. Pediatr Res. 1990;28:631–40 [DOI] [PubMed] [Google Scholar]
- 8.Treuth MS, Butte NF, Wong WW. Effects of familial predisposition to obesity on energy expenditure in multiethnic prepubertal girls. Am J Clin Nutr. 2000;71:893–900 [DOI] [PubMed] [Google Scholar]
- 9.Wong WW, Butte NF, Ellis KJ, Hergenroeder AC, Hill RB, Stuff JE, Smith EO. Pubertal African-American girls expended less energy at rest and during physical activity than Caucasian girls. J Clin Endocrinol Metab. 1999;84:906–11 [DOI] [PubMed] [Google Scholar]
- 10.Wong WW, Klein PD. A review of techniques for the preparation of biological samples for mass-spectrometric measurements of hydrogen-2/hydrogen-1 and oxygen-18/oxygen-16 isotope ratios. Mass Spectrom Rev. 1986;5:313–42 [Google Scholar]
- 11.Wong WW, Lee LS, Klein PD. Deuterium and oxygen-18 measurements on microliter samples of urine, plasma, saliva, and human milk. Am J Clin Nutr. 1987;45:905–13 [DOI] [PubMed] [Google Scholar]
- 12.Gonfiantini R, Rozanski K, Stichler W. Intercalibration of environmental isotope measurements: the program of the International Atomic Energy Agency. Radiocarbon. 1990;32:369–74 [Google Scholar]
- 13.Gonfiantini R. Advisory group meeting on stable isotope reference samples for geochemical and hydrological investigations. Vienna: International Atomic Energy Agency; 1984 [Google Scholar]
- 14.Wong WW, Klein PD, Parr RM, Clements SA. Interlaboratory analysis of reference water samples enriched with deuterium and oxygen-18. Int J Appl Radiat Isot. 1993;44:561–6 [Google Scholar]
- 15.Wong WW, Clarke LL, Llaurador M, Klein PD. A new zinc product for the reduction of water in physiological fluids to hydrogen gas for 2H/1H isotope ratio measurements. Eur J Clin Nutr. 1992;46:69–71 [PubMed] [Google Scholar]
- 16.Altman DG, Bland JM. Measurement in medicine: the analysis of method comparison studies. 1983:307–17 [Google Scholar]
- 17.Horita J. Hydrogen isotope analysis of natural waters using an H2-water equilibration method: a special implication to brines. Chem Geol. 1988;72:89–94 [Google Scholar]
- 18.Horita J, Ueda A, Mizukami K, Takatori I. Automatic 2D and 18O analyses of multi-water samples using H2-and CO2-water equilibration methods with a common equilibration set-up. Appl Radiat Isot. 1989;40:801–5 [Google Scholar]
- 19.Coplen TB, Wildman JD, Chen J. Improvements in the gaseous hydrogen-water equilibration technique for hydrogen isotope ratio analysis. Anal Chem. 1991;63:910–2 [Google Scholar]
- 20.Coplen TB, Harper IT. An improved technique for the 2H/1H analysis of urines from diabetic volunteers. Biol Mass Spectrom. 1994;23:437–9 [DOI] [PubMed] [Google Scholar]
- 21.Scrimgeour CM, Rollo MM, Mudambo SM, Handley LL, Prosser SJ. A simplified method for deuterium/hydrogen isotope ratio measurements on water samples of biological origin. Biol Mass Spectrom. 1993;22:383–7 [DOI] [PubMed] [Google Scholar]
- 22.Wong WW, Cabrera MP, Klein PD. Evaluation of a dual mass spectrometer system for rapid simultaneous determination of hydrogen-2/hydrogen-1 and oxygen-18/oxygen-16 ratios in aqueous samples. Anal Chem. 1984;56:1852–8 [DOI] [PubMed] [Google Scholar]