Abstract
Background and Aims
Recent observational and genetic studies suggest that some hyperplastic polyps may be associated with increased risk of colorectal cancer. Prospective information on the risk of adenoma recurrence associated with hyperplastic polyps is limited. We sought to investigate whether the coexistence of hyperplastic polyps with adenomatous polyps increases the risk of adenoma recurrence.
Methods
We used multiple unconditional logistic regression models to examine the association between baseline hyperplastic polyps and subsequent adenoma recurrence during a three-year follow up, among 1,637 participants in the Polyp Prevention Trial.
Results
A total of 437 participants (26.7%) had hyperplastic polyps coexisting with adenomas at baseline. Of these, 132 (30.2%) had at least one hyperplastic polyp in the proximal colon while 305 (69.8%) had only distal hyperplastic polyps. When compared with subjects without any hyperplastic polyps at baseline, there was no statistically significant association between presence of baseline hyperplastic polyps and recurrence of any adenoma, OR 1.19 (95% CI: 0.94–1.51) or advanced adenoma, OR 1.25 (95% CI: 0.78–2.03). Also, there was no association between hyperplastic polyp location and adenoma recurrence: OR 1.01 (95% CI: 0.69–1.48) for any proximal hyperplastic polyp and OR 1.26 (95% CI: 0.96–1.65) for distal hyperplastic polyps.
Conclusions
The coexistence of hyperplastic polyps with adenomas, irrespective of location, does not confer an increased risk of adenoma recurrence beyond that of adenomatous polyps alone within three years of follow-up. Prospective long-term studies on adenoma recurrence risk associated with hyperplastic polyps in screening populations are needed.
Keywords: Hyperplastic polyps, adenomatous polyps, colonoscopy, adenoma recurrence, surveillance guidelines
INTRODUCTION
Hyperplastic polyps are frequently encountered in the colon and rectum but have long been regarded as not having malignant potential.1, 2 Studies that have evaluated distal hyperplastic polyps as predictors of synchronous proximal adenomatous polyps have yielded inconsistent results with some reporting an association 3–8 while others did not. 9–14 Few studies have evaluated hyperplastic polyps as predictors of subsequent metachronous adenomatous polyps. Small, retrospective case-control studies, with follow-up ranging from approximately 1–4 years have shown a positive association 15–17 but a prospective study utilizing data from two large chemoprevention trials did not find an association after three years of follow-up.18
Surveys of primary care providers 19 and gastrointestinal endoscopists 20 have shown that care providers recommend early colonoscopic surveillance for subjects with hyperplastic polyps, in contrast to postpolypectomy clinical guidelines which do not. The US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society advise that subjects with only hyperplastic polyps do not require repeat screening colonoscopy for 10 years.21 The American Society for Gastrointestinal Endoscopy (ASGE) and the American College of Gastroenterology (ACG) “Quality indicators for colonoscopy” state that “the findings of small distal hyperplastic polyps should not alter the recommended interval for surveillance” 22, reiterating the general acceptance that distal hyperplastic polyps are benign findings. However, these two guidelines’ recommendations referred to situations when hyperplastic polyps are the primary findings at colonoscopy.
Recently, studies have suggested that hyperplastic polyps may acquire genetic mutations such as Kras, and evolve into adenomas (mixed polyps) with the concept of a “fusion” pathway. Hyperplastic polyps with BRAF mutation, which predisposes to malignant transformation, have been suggested to have a predilection for the proximal colon.23–25 However, prospective information on the risk of adenoma recurrence associated with coexistence of hyperplastic polyps with adenomatous polyps is limited.
In this study, we sought to investigate whether the presence of hyperplastic polyps and their location increases the risk of adenoma recurrence beyond that conferred by adenomatous polyps alone among participants in the Polyp Prevention Trial (PPT).
METHODS
Study Population
The details of the rationale, design, and results of the PPT have been described in previous publications.26–28 In brief, the PPT was a 4-year multicenter, randomized, controlled trial to assess the effect of a low-fat, high-fiber, fruit and vegetable diet on the risk of recurrence of adenomatous polyps. The study involved 2,079 participants who were at least 35 years old and had one or more histologically confirmed adenomatous polyps removed within 6 months prior to randomization. Exclusion criteria were previous history of surgical resection of adenomatous polyps, bowel resection, colorectal carcinoma, polyposis syndrome, or inflammatory bowel disease. Participants were also required to weigh less than 150% of their ideal body weight and could not be taking any lipid-lowering medications. The clinical trial was approved by the Institutional Review Boards of the National Cancer Institute, and each of the participating clinical centers. All subjects gave written informed consents. The subjects were randomized to adopt a dietary pattern of low-fat, high-fiber, fruits and vegetable diet or their usual diet. A total of 1,905 (91.6%) participants completed the trial by undergoing the final colonoscopy, which determined the end-point of the trial. The trial did not show any effect of the dietary intervention on adenoma recurrence.28
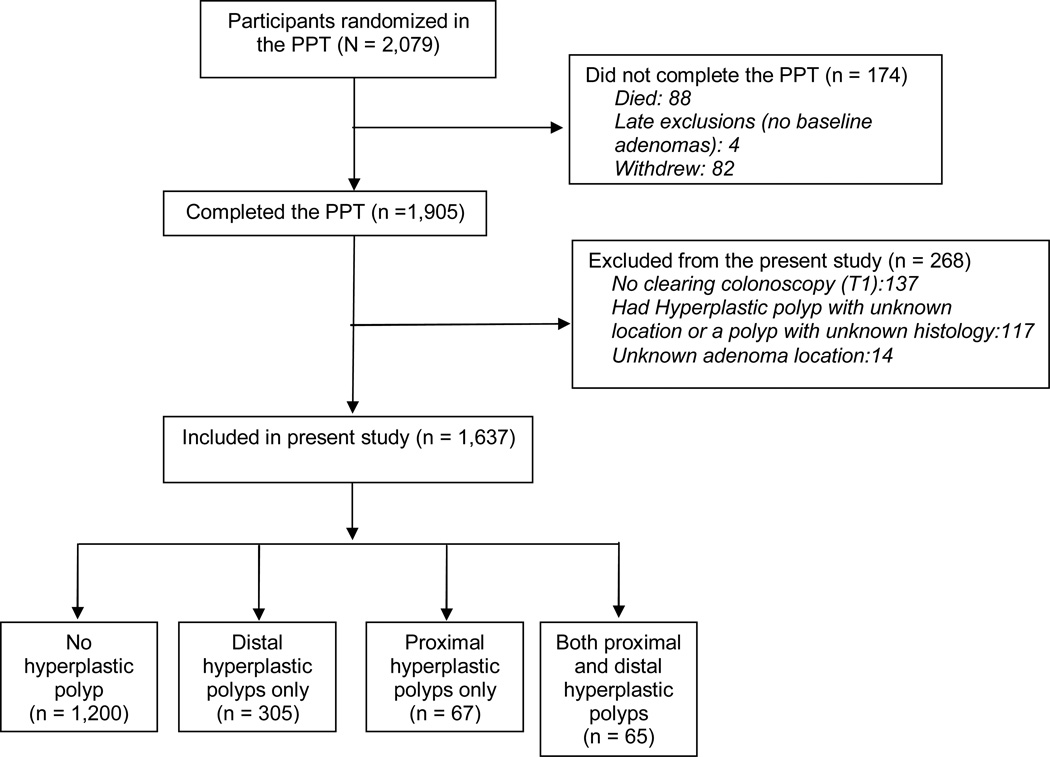
The present study involved 1,637 participants who completed the PPT, had all the scheduled colonoscopic examinations and had complete information on the location of all adenomatous and hyperplastic polyps (figure 1).
Figure 1.
Diagram of Flow of Participants through the Study
Exposure and outcome assessment
Information on the subject’s demographic characteristics and health-related lifestyle were obtained from every participant through direct interview. The participants underwent a clearing colonoscopy approximately one year after randomization (T1) to remove any lesion missed at qualifying colonoscopy (T0). The subjects were followed for approximately four years after randomization, and again underwent colonoscopy at the end of follow up (T4). For the present study, we combined results of the qualifying and clearing colonoscopy and considered them as baseline findings. Subjects were regarded as having hyperplastic polyps at baseline if present at either the qualifying or clearing colonoscopy. Serrated adenomas were not classified in this study. The occurrence of histologically confirmed adenomatous polyps after the clearing colonoscopy was defined as recurrence. The endoscopists’ colonoscopy reports provided information on size, multiplicity, and location of polyps. Histology and degree of atypia were confirmed by two central pathologists. The location of polyps removed from rectosigmoid to splenic flexure was defined as distal, while proximal location included transverse colon to cecum.
Statistical analyses
Statistical Analysis Systems (SAS) software (SAS Institute Inc, Cary, NC) was used for all analyses. We compared the baseline characteristics of subgroups of participants: participants without hyperplastic polyps versus all subjects with hyperplastic polyps, those with distal only, and those with any proximal hyperplastic polyps. We also compared subjects with any proximal hyperplastic polyps versus distal only. We calculated means, standard deviations and performed comparisons of continuous outcome variables using Student’s t- test. We calculated percentages and compared groups using Chi square statistics for categorical variables. We used unconditional logistic regression models to evaluate the association of coexisting hyperplastic polyps with adenomas and their location, with the risk of all adenoma and advanced adenoma recurrence versus no adenoma recurrence. We also evaluated the risk of adenoma recurrence associated with synchronous right sided hyperplastic and adenomatous polyps. We included age, sex, body mass index, smoking status, ≥3 non-advanced adenomas and advanced adenomas at baseline in the models.
Because we were interested in whether hyperplastic polyps increase the risk of adenoma recurrence specifically among individuals with low-risk adenomas, defined as 1 or 2 non-advanced adenomas, we restricted our study population to this subgroup of participants in an exploratory analysis. These individuals are recommended to have follow-up surveillance colonoscopy in 5 – 10 years in the current postpolypectomy guidelines.21 We evaluated the effect of the number of adenomatous polyps coexisting with hyperplastic polyps, size, and location of hyperplastic polyps on any adenoma and advanced adenoma recurrence within 3 years of follow-up. A total of 29 subjects did not have information on the size of their hyperplastic polyps and were excluded from analysis involving size. Only 13 subjects had large hyperplastic polyps (size ≥ 10mm) which precluded detailed analysis of this category. Therefore, we categorized subjects according to the largest hyperplastic polyps into < 6mm and ≥ 6mm. We calculated odds ratios with 95% confidence intervals. Further tests of whether the odds ratios were equal to 1 were performed using Wald tests conducted at the 0.05 significance level.
RESULTS
Baseline characteristics
A total of 305 (18.6%) subjects had only distal hyperplastic polyps, 67 (4.1%) had only proximal hyperplastic polyps, 65 (4.0%) had hyperplastic polyps in both the proximal and distal colon, and 1,200 (73.3%) did not have any hyperplastic polyps in the qualifying or clearing colonoscopy. Thus, 132 (8.1%) participants had any proximal hyperplastic polyp. When compared to subjects without hyperplastic polyps, participants who had hyperplastic polyps were more likely to be Caucasian, have a higher body mass index and be a current smoker (Table 1). There was no age or gender difference between subjects with and without hyperplastic polyps. Participants with only distal hyperplastic polyps were similar to those with proximal hyperplastic polyps except that smokers were more likely to have distal hyperplastic polyps (39.4% versus 24.9%, P = 0.009).
Table 1.
Selected Characteristics of the Subjects at Baseline
| Baseline characteristics | Overall (N = 1637) |
No hyperplastic polyp (n = 1200) |
Hyperplastic polyp (n = 437) |
P value* |
|---|---|---|---|---|
| Age in years, mean (SD) | 61.1 (9.9) | 61.0 (10.1) | 61.4 (9.2) | 0.422 |
| Male sex, n (%) | 1,055 (64.4) | 759 (63.3) | 296 (67.7) | 0.094 |
| Aspirin use, n (%) | 384 (23.5) | 281 (23.4) | 103 (23.6) | 0.948 |
| NSAIDs use, n (%)‡ | 557 (34.0) | 408 (34.0) | 149 (34.1) | 0.971 |
| Alcohol use, n (%) | 947 (57.8) | 683 (56.9) | 264 (60.4) | 0.205 |
| Physical activity, hours/week, mean (SD) | 12.0 (12.5) | 12.0 (12.6) | 11.9 (12.4) | 0.872 |
| Family history of colorectal cancer, n (%) | 445 (27.2) | 326 (27.2) | 119 (27.2) | 0.979 |
| Body mass index, kg/m2, n (%) | ||||
| <25 | 434 (26.5) | 337 (28.1) | 97 (22.2) | 0.041 |
| 25 – 29 | 779 (47.6) | 565 (47.1) | 214 (49.0) | |
| 30–38.8† | 424 (25.9) | 298 (24.8) | 126 (28.8) | |
| Smoking categories, n (%) | ||||
| Never | 657 (40.1) | 529 (44.1) | 128 (29.3) | <0.0001 |
| Former | 777 (47.5) | 554 (46.2) | 223 (51.0) | |
| Current | 203 (12.4) | 117 (9.8) | 86 (19.7) | |
| Race | ||||
| Caucasian, n (%) | 1472 (89.9) | 1061 (88.4) | 411 (94.1) | 0.004 |
| Blacks, n (%) | 129 (7.9) | 109 (9.1) | 20 (4.6) | |
| Other, n (%) § | 36 (2.2) | 30 (2.5) | 6 (1.4) | |
Comparison between subjects with hyperplastic versus no hyperplastic polyps at baseline.
Non steroidal anti-inflammatory drugs
High-risk adenoma refers to advanced adenoma /or ≥3 synchronous adenoma.
Advanced adenoma = any adenoma with size ≥ 10mm or high-grade dysplasia or villous histology
Exclusion criteria for the PPT included that participants must not weigh more than 150% of their ideal body weight
Others include Hispanic, Indian/Native American, Asian/Pacific Islanders
The highest number of hyperplastic polyps removed from any participant during colonoscopy was 16. Most hyperplastic polyps were diminutive in size. Only 19 subjects had ≥ 5 hyperplastic polyps during colonoscopy. Of these, only 2 individuals had ≥ 5 hyperplastic polyps in the proximal colon. Hyperplastic polyposis syndrome was recently defined for the World Health Organization International Classification of Tumors as: (1) at least five histologically diagnosed hyperplastic polyps proximal to the sigmoid colon, of which two are greater than 1 cm in diameter; or (2) any number of hyperplastic polyps occurring proximal to the sigmoid colon in an individual who has a first-degree relative with hyperplastic polyposis; or (3) greater than 30 hyperplastic polyps of any size distributed throughout the colon.29 Using this definition, nobody fulfilled the criteria in our study. One participant almost fulfilled the criteria, a 60-year-old Caucasian man without a family history of colorectal cancer. He had 3 non-advanced adenomas at qualifying and 2 non-advanced adenomas at clearing colonoscopy, respectively. He had 12 hyperplastic polyps at qualifying colonoscopy of which 7 were in the proximal colon. He had a total of 7 hyperplastic polyps ranging in size from 15 – 20mm but the largest proximal hyperplastic polyp was 8mm in size.
Adenoma recurrence
At follow-up (end point) colonoscopy, 1,007 (61.5%) participants had no adenoma recurrence, 104 (6.4%) subjects had advanced adenoma recurrence and 526 (32.1%) had non-advanced adenoma recurrence. When compared with participants without an adenoma recurrence at follow-up (n = 1,007), subjects with any adenoma recurrence (n = 630) were older (63.1 versus 59.8 years, P<0.001) and predominantly male (71.4% versus 60.8%, P<0.001).
Hyperplastic polyps as a predictor of adenoma recurrence
There was no statistically significant association between baseline coexistence of hyperplastic polyps with adenomas and recurrence of adenoma: OR 1.19 (95% CI: 0.94–1.51) or advanced adenoma: OR 1.25 (95% CI: 0.78–2.03) when compared with participants with only adenomas at baseline (Table 2). There was also no association between hyperplastic polyp location and adenoma recurrence: OR 1.01 (95% CI: 0.69–1.48) for any proximal hyperplastic polyp and OR 1.26 (95% CI: 0.96–1.65) for distal hyperplastic polyps (Table 2). Furthermore, there was no association between baseline synchronous right sided hyperplastic and adenomatous polyps and adenoma recurrence during follow-up OR 0.97 (95% CI: 0.58–1.63). The results were similar for males and females and there was no interaction between hyperplastic polyps and sex (P value = 0.129).
Table 2.
Association of Hyperplastic Polyps with Adenoma Recurrence
| Baseline | Follow-up colonoscopy findings | ||
|---|---|---|---|
| Characteristics | Any adenoma recurrence OR (95% CI) |
Advanced adenoma recurrence† OR (95% CI) |
|
| No hyperplastic polyp (N=1,200) |
Ref | Ref | Ref |
| All hyperplastic polyps (N=437) |
Unadjusted | 1.31 (1.05–1.64) | 1.13 (0.71–1.78) |
| Age adjusted | 1.30 (1.04–1.63) | 1.16 (0.73–1.84) | |
| Multivariable adjusted¶ |
1.19 (0.94–1.51) | 1.25 (0.78–2.03) | |
| Any proximal hyperplastic polyps (N=132) |
Unadjusted | 1.16 (0.80–1.67) | 1.26 (0.63–2.54) |
| Age adjusted | 1.15 (0.79–1.67) | 1.34 (0.65–2.70) | |
| Multivariable adjusted |
1.01 (0.69–1.48) | 1.33 (0.64–2.78) | |
| Distal hyperplastic polyps (N=305) |
Unadjusted | 1.38 (1.07–1.79) | 1.06 (0.62–1.83) |
| Age adjusted | 1.38 (1.06–1.78) | 1.08 (0.63–1.86) | |
| Multivariable adjusted |
1.26 (0.96–1.65) | 1.18 (0.67–2.08) | |
| Synchronous proximal hyperplastic and adenomatous polyps (N=68) |
Unadjusted | 1.28 (0.78–2.10) | 1.54 (0.63–3.75) |
| Age adjusted | 1.20 (0.73–1.98) | 1.51 (0.61–3.72) | |
| Multivariable adjusted |
0.97 (0.58–1.63) | 1.45 (0.57–3.70) | |
Advanced adenoma = any adenoma with size ≥ 10mm or high-grade dysplasia or villous histology
Multivariable adjustment for age, sex, ≥3 adenomas at baseline, baseline advanced adenoma, body mass index, and smoking
Exploratory analysis among subjects with low-risk adenomas
Among the 1,637 subjects in our study, 829 (50.6%) subjects had baseline low-risk adenomas (≤ 2 non-advanced adenomas). Of these, only 231 (27.9%) subjects had hyperplastic polyps. Overall, participants with 2 adenomas had increased risk of any adenoma when compared with subjects with one adenoma and no hyperplastic polyps irrespective of whether they had hyperplastic polyps or not (Table 3). The associations for advanced adenomas were of similar magnitude, but the numbers of cases were relatively small and the Odds ratios did not achieve statistical significance. There was no increased risk of adenoma recurrence among those with diminutive or moderate to large hyperplastic polyps when compared to those without hyperplastic polyps (Table 4). Also, we did not find statistically significant joint effects of location and size of hyperplastic polyps and adenoma recurrence (Table 5).
Table 3.
Effect of Presence of Hyperplastic Polyps on Adenoma Recurrence among Participants with 1 or 2 Low-Risk Adenomas at Baseline
| Baseline Characteristics | Follow-up colonoscopy findings | |||
|---|---|---|---|---|
| Number of adenomas |
Hyperplastic polyps present |
Number of subjects (%) N = 829 |
Any adenoma recurrence ¶ OR (95% CI) |
Advanced adenoma recurrence †¶ OR (95% CI) |
| 1 | No | 458 (55.2) | 1.0 (reference) | 1.0 (reference) |
| 1 | Yes | 158 (19.1) | 1.40 (0.95–2.08) | 1.39 (0.53–3.60) |
| 2 | No | 140 (16.9) | 2.27 (1.53–3.37) | 2.40 (0.96–5.98) |
| 2 | Yes | 73 (8.8) | 1.77 (1.06–2.97) | 2.31 (0.77–6.90) |
Advanced adenoma = any adenoma with size ≥ 10mm or high-grade dysplasia or villous histology
Multivariable adjustment for age, sex, body mass index and smoking
Table 4.
Effect of Size of Hyperplastic Polyps on Adenoma Recurrence among Participants with 1 or 2 Low-Risk Adenomas at Baseline*
| Baseline Characteristics | Follow-up colonoscopy findings | ||
|---|---|---|---|
| Largest size of hyperplastic polyps |
Number of subjects (%) |
Any adenoma recurrence ¶ OR (95% CI) |
Advanced adenoma recurrence †¶ OR (95% CI) |
| No hyperplastic polyp |
598 (74.8) | 1.0 (reference) | 1.0 (reference) |
| < 6 mm | 161 (20.1) | 1.06 (0.73–1.55) | 0.91 (0.35–2.36) |
| ≥ 6 mm | 41 (5.1) | 1.53 (0.79–2.96) | 2.96 (0.90–9.69) |
For 800 out of 829 participants; 29 participants with missing information on size of hyperplastic polyps were excluded
Advanced adenoma = any adenoma with size ≥ 10mm or high-grade dysplasia or villous histology
Multivariable adjustment for age, sex, body mass index and smoking
Table 5.
Effect of Location and Size of Hyperplastic Polyps on Adenoma Recurrence among Participants with 1 or 2 Low-Risk Adenomas at Baseline*
| Baseline Characteristics | Follow-up colonoscopy findings | ||||
|---|---|---|---|---|---|
| Largest size of hyperplastic polyps |
Location of hyperplastic polyps |
Number of subjects (%) |
Any adenoma recurrence ¶ OR (95% CI) |
Advanced adenoma recurrence †¶ OR (95% CI) |
|
| No hyperplastic polyp |
N/A | 598 (74.8) | 1.0 (reference) | 1.0 (reference) | |
| < 6 mm | Distal only | 124 (15.5) | 1.24 (0.83–1.87) | 1.04 (0.37–2.91) | |
| < 6 mm | Any proximal | 39 (4.9) | 0.63 (0.30–1.34) | 1.08 (0.28–4.88) | |
| ≥ 6 mm | Distal only | 19 (2.4) | 1.36 (0.53–3.51) | 1.30 (0.15–11.00) | |
| ≥ 6 mm | Any proximal | 20 (2.5) | 1.73 (0.68–4.34) | 3.74 (0.74–18.95) | |
For 800 out of 829 participants; 29 participants with missing information on size of hyperplastic polyps were excluded
Advanced adenoma = any adenoma with size ≥ 10mm or high-grade dysplasia or villous histology
Multivariable adjustment for age, sex, body mass index and smoking
DISCUSSION
Our study did not demonstrate a significantly increased risk of adenoma or advanced adenoma recurrence in subjects with hyperplastic polyps and adenomatous polyps compared to those with only adenomatous polyps. The location of hyperplastic polyps also did not affect the recurrence of any adenoma or advanced adenoma within 3 years of follow-up.
Although hyperplastic polyps are usually considered as benign findings, some retrospective studies have demonstrated a modest increase in the risk of metachronous adenomas among patients with hyperplastic polyps alone or with synchronous adenomas.15–17 Kellokumpu and colleagues15 investigated whether the presence of concomitant adenomas and hyperplastic polyps at the initial examination could predict a higher risk of new adenoma formation The authors reported a higher adenoma recurrence rate among patients with multiple adenomas with synchronous hyperplastic polyps at baseline compared to those with only adenomas (75% versus 21%, P<0.005), but the study only included 56 patients, of whom 16 (28.6%) had an incident adenoma over a median follow-up of 2.8 years. In a study of colonoscopic examinations performed by a single surgeon over a 20-year period, Huang and colleagues16 reported that among 42 patients with hyperplastic polyps, 18 (43%) patients developed incident adenomas. Among their polyp-free controls, only 77/362 (21%) developed subsequent adenomas over a mean follow-up of 4.3 years (Relative Risk 2.0; 95% CI: 1.2–3.4). Croizet and colleagues17 reported that subjects with hyperplastic polyps were 2.4 times more likely to develop metachronous adenomas within 5 years than polyp free controls, but had a lower, not statistically significant, rate of subsequent adenomas when compared to patients with adenomas at baseline. There were only 90 age and sex matched patients in each category. Although the data was not shown, the authors reported that hyperplastic polyp size, number, location, patient age, and symptoms were not associated with incident adenoma. None of these studies evaluated advanced adenoma recurrence.
To our knowledge, only one previous study has investigated the association between hyperplastic polyps and adenoma recurrence in a prospective manner.18 In that study, the authors combined data from two chemoprevention trials. In view of the fact that uniform data were not available to them about the presence or absence of hyperplastic polyps at the qualifying colonoscopy, they used only the results of the clearing colonoscopy performed approximately a year after randomization as the baseline findings, and categorized subjects into four groups: hyperplastic polyps only (n=168), adenomas only (n=360), both hyperplastic and adenomas (n=126) and no polyps (n=929) as the reference group. The study reported no association between hyperplastic polyps and adenoma recurrence within 3 years of follow-up (OR 1.06; 95% CI: 0.73–1.53). The effect of polyp location was not evaluated. However, there was an increased risk of adenoma recurrence among subjects with adenomas and an increased risk of hyperplastic polyp recurrence among subjects with hyperplastic polyps. A similar finding was reported by Lazarus and colleagues.30 In their retrospective analysis, the authors re-read the pathology slides of 239 consecutive patients who had a polypectomy and reclassified the polyp into hyperplastic polyps (n=56), serrated adenoma (n=38), admixed polyps (n=7) and conventional adenomas (n=138). The authors reported that subsequent polyp type was dependent on the index polyp with a higher recurrence of the index polyp type over a mean follow-up of 94 months.
Our study suggests that in the short term, neither the presence nor the location of hyperplastic polyps affect adenoma or advanced adenoma recurrence. It is noteworthy that no previous study, retrospective or prospective, has evaluated the risk of advanced adenoma recurrence associated with hyperplastic polyps. It may be that a follow-up duration of more than 3 years is necessary to observe an association between hyperplastic polyps and adenoma recurrence. In our exploratory analysis involving only participants with low-risk (1 or 2 non-advanced) adenomas, subjects with 2 adenomas had an increased risk of adenoma recurrence whether they had hyperplastic polyps or not. The size and the joint effects of location and size of hyperplastic polyp were not related to adenoma recurrence.
The PPT provides an opportunity to investigate baseline factors that are associated with adenoma recurrence in a large, geographically dispersed population. Other strengths of our study are that central pathologists were used providing improved standardization, information on candidate risk factors was gathered prospectively, and all patients had similar, timed, planned follow-up colonoscopic assessment for adenoma recurrence. Limitations of our study should also be acknowledged. Participants in the PPT were self selected and may be healthier than comparable members of the general population (the healthy volunteer effect), which may affect the generalizability of our findings. We could not assess the risk of hyperplastic polyps on incident adenoma development in subjects without adenomas at baseline in our study, since all participants in the PPT had a history of colorectal adenoma. It is unknown if the risk of incident adenomas will be different in this group of individuals, but the current recommendation is a 10-year surveillance interval similar to individuals without any polyps. Other limitations of our study include the relatively short 3-year duration of follow-up, most of the hyperplastic polyps in our study were small in size and we did not evaluate serrated adenomas specifically. When the pathology for the PPT was done, serrated adenomas were not widely discussed. Therefore, it is possible that some serrated lesions were classified as hyperplastic polyps. However, we do not feel that our results would change much because the proportion of lesions read as hyperplastic polyps that are truly serrated adenomas is probably small. Although the classification of serrated polyps is still evolving, some researchers have opined that serrated adenomas (formerly characterized as hyperplastic polyps) demonstrate crypt architectural alterations that reflect disordered growth. BRAF mutation and extensive DNA methylation have been described in these lesions and have been associated with CpG Island Methylation Phenotype (CIMP)-high and microsatellite instability (MSI) colon adenocarcinoma.2
In conclusion, we did not find any association between baseline hyperplastic polyps or their location and adenoma recurrence within 3 years of follow-up. Further studies that prospectively evaluate the risk of metachronous adenomatous polyps associated with baseline hyperplastic polyps and serrated adenomas, alone or coexisting with adenomas, in a screening population with long term follow-up may further enhance our understanding of the colorectal cancer risk associated with the serrated neoplasia pathway.
Acknowledgments
Grant support: The study was funded by Intramural Research Program of the Center for Cancer Research and Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health; and the Division of Cancer Prevention, National Cancer Institute, National Institutes of Health. The funding agency had a role in the design and reporting of the study and in the decision to submit the manuscript for publication and approved the final version of the manuscript.
Abbreviations used in this paper
- CI
Confidence interval
- OR
Odds ratio
- PPT
Polyp Prevention Trial
Footnotes
Financial Disclosures: None. No conflicts of interest exist.
REFERENCES
- 1.Jass JR. Hyperplastic polyps of the colorectum-innocent or guilty? Dis Colon Rectum. 2001;44(2):163–166. doi: 10.1007/BF02234287. [DOI] [PubMed] [Google Scholar]
- 2.O'Brien MJ. Hyperplastic and serrated polyps of the colorectum. Gastroenterol Clin North Am. 2007;36(4):947–968. doi: 10.1016/j.gtc.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Foutch PG, DiSario JA, Pardy K, et al. The sentinel hyperplastic polyp: a marker for synchronous neoplasia in the proximal colon. Am J Gastroenterol. 1991;86(10):1482–1485. [PubMed] [Google Scholar]
- 4.Ansher AF, Lewis JH, Fleischer DE, et al. Hyperplastic colonic polyps as a marker for adenomatous colonic polyps. Am J Gastroenterol. 1989;84(2):113–117. [PubMed] [Google Scholar]
- 5.Opelka FG, Timmcke AE, Gathright JB, Jr, et al. Diminutive colonic polyps: an indication for colonoscopy. Dis Colon Rectum. 1992;35(2):178–181. doi: 10.1007/BF02050675. [DOI] [PubMed] [Google Scholar]
- 6.Pinsky PF, Schoen RE, Weissfeld JL, et al. Predictors of advanced proximal neoplasia in persons with abnormal screening flexible sigmoidoscopy. Clin Gastroenterol Hepatol. 2003;1(2):103–110. doi: 10.1053/cgh.2003.50017. [DOI] [PubMed] [Google Scholar]
- 7.Blue MG, Sivak MV, Jr, Achkar E, et al. Hyperplastic polyps seen at sigmoidoscopy are markers for additional adenomas seen at colonoscopy. Gastroenterology. 1991;100(2):564–566. doi: 10.1016/0016-5085(91)90232-a. [DOI] [PubMed] [Google Scholar]
- 8.Pennazio M, Arrigoni A, Risio M, et al. Small rectosigmoid polyps as markers of proximal neoplasms. Dis Colon Rectum. 1993;36(12):1121–1125. doi: 10.1007/BF02052260. [DOI] [PubMed] [Google Scholar]
- 9.Lieberman DA, Weiss DG, Bond JH, et al. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343(3):162-8. Erratum in: N Engl J Med. 2000;343(16):1204. doi: 10.1056/NEJM200007203430301. [DOI] [PubMed] [Google Scholar]
- 10.Rex DK, Smith JJ, Ulbright TM, et al. Distal colonic hyperplastic polyps do not predict proximal adenomas in asymptomatic average-risk subjects. Gastroenterology. 1992;102(1):317–319. doi: 10.1016/0016-5085(92)91817-n. [DOI] [PubMed] [Google Scholar]
- 11.Brady PG, Straker RJ, McClave SA, et al. Are hyperplastic rectosigmoid polyps associated with an increased risk of proximal colonic neoplasms? Gastrointest Endosc. 1993;39(4):481–485. doi: 10.1016/s0016-5107(93)70155-8. [DOI] [PubMed] [Google Scholar]
- 12.Nusko G, Altendorf-Hofmann A, Hermanek P, et al. Correlation of polypoid lesions in the distal colorectum and proximal colon in asymptomatic screening subjects. Eur J Gastroenterol Hepatol. 1996;8(4):351–354. doi: 10.1097/00042737-199604000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Provenzale D, Garrett JW, Condon SE, et al. Risk for colon adenomas in patients with rectosigmoid hyperplastic polyps. Ann Intern Med. 1990;113(10):760–763. doi: 10.7326/0003-4819-113-10-760. [DOI] [PubMed] [Google Scholar]
- 14.Lin OS, Schembre DB, McCormick SE, et al. Risk of proximal colorectal neoplasia among asymptomatic patients with distal hyperplastic polyps. Am J Med. 2005;118(10):1113–1119. doi: 10.1016/j.amjmed.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Kellokumpu I, Kyllönen L. Multiple adenomas and synchronous hyperplastic polyps as predictors of metachronous colorectal adenomas. Ann Chir Gynaecol. 1991;80(1):30–35. [PubMed] [Google Scholar]
- 16.Huang EH, Whelan RL, Gleason NR, et al. Increased incidence of colorectal adenomas in follow-up evaluation of patients with newly diagnosed hyperplastic polyps. Surg Endosc. 2001;15(7):646–648. doi: 10.1007/s004640000389. [DOI] [PubMed] [Google Scholar]
- 17.Croizet O, Moreau J, Arany Y, et al. Follow-up of patients with hyperplastic polyps of the large bowel. Gastrointest Endosc. 1997;46(2):119–123. doi: 10.1016/s0016-5107(97)70058-0. [DOI] [PubMed] [Google Scholar]
- 18.Bensen SP, Cole BF, Mott LA, et al. Colorectal hyperplastic polyps and risk of recurrence of adenomas and hyperplastic polyps. Polyps Prevention Study. Lancet. 1999;354(9193):1873–1874. doi: 10.1016/s0140-6736(99)04469-4. [DOI] [PubMed] [Google Scholar]
- 19.Boolchand V, Olds G, Singh J, et al. Colorectal screening after polypectomy: a national survey study of primary care physicians. Ann Intern Med. 2006;145(9):654–659. doi: 10.7326/0003-4819-145-9-200611070-00007. [DOI] [PubMed] [Google Scholar]
- 20.Mysliwiec PA, Brown ML, Klabunde CN, et al. Are physicians doing too much colonoscopy? A national survey of colorectal surveillance after polypectomy. Ann Intern Med. 2004;141(4):264–271. doi: 10.7326/0003-4819-141-4-200408170-00006. [DOI] [PubMed] [Google Scholar]
- 21.Winawer SJ, Zauber AG, Fletcher RH, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. Gastroenterology. 2006;130(6):1872–1885. doi: 10.1053/j.gastro.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Rex DK, Petrini JL, Baron TH, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2006;101(4):873–885. doi: 10.1111/j.1572-0241.2006.00673.x. [DOI] [PubMed] [Google Scholar]
- 23.Jass JR, Baker K, Zlobec I, et al. Advanced colorectal polyps with the molecular and morphological features of serrated polyps and adenomas: concept of a 'fusion' pathway to colorectal cancer. Histopathology. 2006;49(2):121–131. doi: 10.1111/j.1365-2559.2006.02466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan TL, Zhao W, Leung SY, et al. BRAF and KRAS mutations in colorectal hyperplastic polyps and serrated adenomas. Cancer Res. 2003;63(16):4878–4881. [PubMed] [Google Scholar]
- 25.Spring KJ, Zhao ZZ, Karamatic R, et al. High prevalence of sessile serrated adenomas with BRAF mutations: a prospective study of patients undergoing colonoscopy. Gastroenterology. 2006;131(5):1400–1407. doi: 10.1053/j.gastro.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 26.Schatzkin A, Lanza E, Freedman LS, et al. The polyp prevention trial I: rationale, design, recruitment, and baseline participant characteristics. Cancer Epidemiol Biomarkers Prev. 1996;5(5):375–383. [PubMed] [Google Scholar]
- 27.Lanza E, Schatzkin A, Ballard-Barbash R, et al. The polyp prevention trial II: dietary intervention program and participant baseline dietary characteristics. Cancer Epidemiol Biomarkers Prev. 1996;5(5):385-92. Erratum in: Cancer Epidemiol Biomarkers Prev. 1996;5(7):584. [PubMed] [Google Scholar]
- 28.Schatzkin A, Lanza E, Corle D, et al. Lack of effect of a low-fat, high-fiber diet on the recurrence of colorectal adenomas. Polyp Prevention Trial Study Group. N Engl J Med. 2000;342(16):1149–1155. doi: 10.1056/NEJM200004203421601. [DOI] [PubMed] [Google Scholar]
- 29.Burt R, Jass JR. Hyperplastic polyposis. Lyon: IARC Press; 2000. [Google Scholar]
- 30.Lazarus R, Junttila OE, Karttunen TJ, et al. The risk of metachronous neoplasia in patients with serrated adenoma. Am J Clin Pathol. 2005;123:349–359. doi: 10.1309/VBAG-V3BR-96N2-EQTR. [DOI] [PubMed] [Google Scholar]