Abstract
Microwave irradiation as the energy source for one–step direct transesterification of fatty acids in human serum lipids was examined in solvent system of methanol: hexane: acetyl chloride based on Lepage & Roy assay. Innovative and explosion proof single–mode or multimode microwave accelerate reaction system was employed. Recoveries were calculated as the percentage of fatty acid concentrations measured by microwave assay to those by reference method Lepage & Roy assay that utilized conductive heating at 100 °C for 60 min. At conditions of 100 °C for 1 min in Single–mode (S4–100×1), or 125 °C for 5 min in Multimode (M5–125×5), the recoveries were 100–103% for the total fatty acids and 96–106% for each categorized fatty acid, including saturates, monounsaturates, n-6 PUFA, and n-3 PUFA. For individual PUFA, the mean recoveries were 102–105% for 18:2n-6 and 18:3n-3; 99, 109, and 95% for 20:4n-6, 20:5n-3, and 22:6n-3, respectively. Thus, fatty acid concentrations determined by microwave fatty acid assay were accurate to those results by the reference method, when the microwave conditions were optimal. In summary, the microwave irradiation could replace conductive heating in one–step direct transesterification, and reduce duration from 60 min to 5 min or less. This methodology may be applied in both the absolute and relative quantification of serum total fatty acids.
Keywords: Lepage & Roy, Acetyl chloride, in situ, Transesterification, GC, Fatty acid profile
Introduction
Rapid and accurate quantification of the fatty acid composition of human and animal biological samples, such as serum, is fundamental to the evaluation of these fatty acids in diseases as diverse as cardiovascular disease [1], neurodegenerative diseases [2], stroke [3], inflammatory disorder [4], mental illness [5], and optimal neurodevelopment [6]. Measurements of fatty acids, either in isolated lipids or when derived from a biological matrix, can be carried out by gas chromatography coupled with flame ionization detector (GC) after the transesterification of fatty acid moieties into their corresponding methyl esters without prior extraction and/or saponification [7]. This reaction, referred as transmethylation, is usually completed through methanol with acidic [8-10] or alkaline catalysts [7] under conductive heating. The one–step direct transesterification of fatty acids in total lipids or glycerophospholipids in biology materials have greatly facilitated the throughput of fatty acid assay in a simple, rapid, and high throughput way [10-12]. However, the entire procedure for total lipids is time–consuming, requiring 60 min at 100 °C [10] or 2 hr at 80 °C [13] with conductive heating for a complete transesterification of fatty acids, and increases potential for oxidation.
Following the application of microwave irradiation in lipids extraction from foods [14], Lie Ken Jie and Yan–Kit initially applied microwave irradiation to the transformation of fatty acids in 1988 [15], including the esterification of free fatty acids within 5 min using a domestic microwave oven. Ever since, the application of the microwave as an energy source has been further developed in transesterification of phospholipid fatty acids from sheep brain serotonin receptor preparation [15, 16], human blood [17], vegetable and fish oil [18], and glycosphingolipids [19], with the reaction time as short as 20 sec [16]. The fatty acid structures of products are identical either from microwave irradiation or heatblock heating assay [18].
Nevertheless, compared to conductive heating, the transesterification of fatty acids by microwave irradiation using household microwave oven are incomplete with a recovery of 78% for total fatty acids in blood [17]. Clearly, the use of a domestic microwave oven in chemical synthesis presents significant safety issues. Thus, the application of microwave in fatty acid assay has not been further developed or optimally established. Tomas et al. first applied chemically safe, single–mode microwave oven to quantify meat acylglycerides with methanol and chlorotrimethylsilane [20]. In this study, we sought to optimize the conditions of transesterification in microwave reaction systems for the fatty acids in human serum total lipids using methanol:hexane with acetyl chloride as the catalyst. It proved that the microwave irradiation could significantly reduce the reaction time with complete transesterification of fatty acids at optimal conditions.
Materials and Methods
Chemicals
Methanol was purchased from Burdick & Jackson (Muskegon, MI); hexane from EMD Chemicals Inc (Gibbstown, NJ); acetyl chloride from Sigma–Aldrich (St. Louis, MO); 2, 6-Di-tert-butyl-4-methylphenol (BHT) from Acros (Geel, Belgium); sodium carbonate (anhydrous powder) from Mallinckrodt Baker, Inc. (Paris, KT). Standard docosatrienoic ethyl ester (22:3n-3) and GC reference standards GLC–462 were purchased from Nu–Chek Prep (Elysian, MN). The latter contains 28 fatty acid methyl esters (FAME). All chemicals were of analytical grade, commercially purchased, and used without further purification.
Human Serum
All serum samples analyzed in this report were from one research blood donor, with an omnivore diet, in the Clinical Center of the National Institutes of Health. A bulk of blood was collected by venipuncture, and sat at room temperature for one hour prior to being centrifuged at 1,700g for 15 min at 4 °C to collect the serum. The serum was aliquoted, frozen, and stored at −80 °C until analysis.
Instrumentation
Analog Drybath Incubator
An analog heatblock from VWR International, LLC (West Chester, PA) was the conductive heating source as utilized in the reference method for fatty acid determination.
Microwave Reaction Systems
Two microwave reaction systems from CEM Corporation (Matthews, NC) were employed in microwave irradiation fatty acid analysis (microwave assay). Both systems provided constant temperatures at pre–set degree during transesterification of human serum lipids.
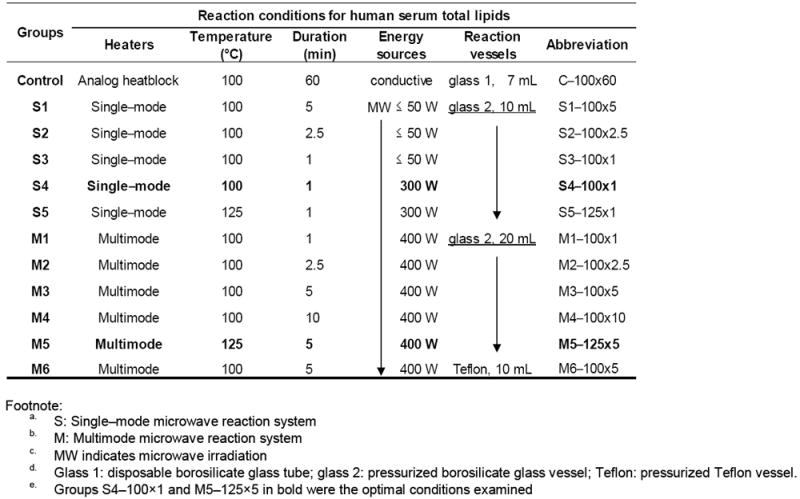
System S is a single–mode microwave reaction system (DISCOVER BenchMate), brief as Single–mode. Sample was processed one at a time in a pressurized 10 mL Pyrex glass reaction vessel with “snap–on” Teflon cap, which automatically vent when internal pressure reaches 300 psi (2068 pKa). Reaction temperature of 100 or 125 °C was pre–set and directly measured inside the glass vessel with one fiber optic temperature probe. Microwave power was initially set at no greater than 50 or 300 W, and automatically adjusted to maintain reaction temperature through temperature and pressure feedback in cavity. Reaction duration was examined at 1–5 min.
System M is a multimode microwave accelerate reaction system (MARS), brief as Multimode. It could process multiple reactions simultaneously, up to 24, 20 mL Pyrex glass vessels and up to 40, 10 mL Teflon reaction vessels. The accessory for glass vessels, GlassChem20, included one turntable with shield containing 24 receptacles. One set of reaction vessel was composed of glass vessel, vessel top, vent plug, and composite sleeve. Reaction temperature of 100 or 125 °C was pre–set, measured, and controlled through a single reference vessel using a fiber optic probe inserted into a thermowell that was in direct contact with the sample in reagent mixture. For the Teflon vessels, pressurized “snap–on” Teflon caps were employed, and an onboard infrared sensor was used to measure and control the temperature. After having reached the reaction temperature, 100 or 125 °C, the initial microwave power of 400 W (n ≤ 4) would automatically adjusted to hold the temperature until the end of reaction. Compressed air was applied to cool down sample. Reaction duration was examined at a range of 1–10 min.
Gas Chromatography
Agilent 6890 (Plus LAN) fast gas chromatography, coupled with a flame ionization detector and a 7683 series injector (Agilent Technologies, Inc., Santa Clara, CA), was employed to acquire the signal of FAME. A fused–silica, narrow–bore, high–efficiency DB–FFAP capillary column (15 m length 0.1 mm ID 0.1 μm film thickness) was used for chromatographic separation of FAME with hydrogen as the carrier gas at a constant pressure of 51.5 psi (355 kPa). Make–up nitrogen gas was set at a constant flow of 10 mL/min. The inlet and detector temperature were set–up at 250 °C. A split ratio of 50:1 was applied. Oven temperature program was initially set at 150 °C with a 0.25 min hold, ramped at 35 °C/min to 200 °C, further 8 °C/min ramp to 225 °C with a 3.2 min hold, and then 80 °C/min ramp to 245 °C with a 9 min hold to bake off column. A total of 28 FAME in GLC–462 were eluted in about 8 min with a total run of about 17 min [21]. GC ChemStation Rev. B.01.01 (164) SR1 was employed for data acquisition and peak integration.
Fatty Acid Direct Transesterification Method
The one–step direct transesterification in Lepage & Roy fatty acid assay [10, 13] was applied as the reference method. Compared to the conventional technique, the quantification of fatty acids in Lepage & Roy assay was carried out in methanol: hexane (4:1, by vol) with acetyl chloride without prior extraction of lipids. It is rapid and reliable; in particular, capable of maintaining short chain fatty acids, which are essential in dietary studies but easily underestimated in conventional method during lipid extraction and subsequent evaporation. Briefly, 100 μL of serum or 0.9% sodium chloride as solvent blank was added to a 16 × 100 mm disposable borosilicate glass test tube placed in ice containing 1.6 mL of methanol, 0.4 mL of hexane, and 200 μL of acetyl chloride. Standard 22:3n-3 ethyl ester (27.6 nmol per sample) was used as the internal standard (ISTD). The test tubes were then tightly closed under nitrogen with Teflon lined caps, and heated in an analog heating block at 100 °C for 60 min. Afterwards, the samples were chilled in ice and then neutralized by an addition of 5 mL of 6% Na2CO3 solution followed by centrifugation at 1,700g for 4 min. The hexane, served as the upper phase containing fatty acid methyl ester, was collected and the volume was reduced to ~30 μL prior to being placed in a GC autosampler tray. In general, one μL of aliquot was injected into GC inlet for data acquisition.
The microwave accelerated fatty acid assay was modified from the above Lepage & Roy procedures with only modification in the heating conditions, including energy source, temperature, duration, and reaction vessels. A set of experiments (n = 11) was designed to optimize the reaction duration at 100 or 125 °C with complete transesterification of fatty acids in the solvent system of methanol, hexane, and acetyl chloride. As presented in Table 1, all procedures across eleven microwave groups (series S and M) were the same except for the varied reaction temperatures (100 or 125 °C), duration (1, 2.5, 5, or 10 min), the initial power of microwave irradiation (50, 300, or 400 W), and materials of reaction vessels (disposal glass, pressurized glass or Teflon). The multispeed magnetic stirring and compressed air cooling were also employed. All of the microwave assays were carried out in triplicate except group M1 (duplicate).
Table 1.
Conditions of transesterification by either conductive heating or microwave irradiation
|
Calculation and Statistics
Data were expressed as mean SD in concentrations, μmol of fatty acid per L serum (μM), or the proportion of each fatty acid in total amount of the identified fatty acids in each sample (mol%). The concentrations were calculated by comparing the integrated areas of each fatty acid peak in the gas chromatograms with that of the known amount of ISTD added in the sample. Automated data processing through macro programming with Microsoft VBA 6.3 (Microsoft Corp; Seattle, WA) was performed during calculation of fatty acid values. Details are reported in the previous study [13]. Recovery of each fatty acid was defined as the percentage of fatty acid values determined by microwave assay to those by the reference method. The 20– and 22–carbon fatty acids with three or more carbon–carbon double bonds were categorized as highly unsaturated fatty acids (HUFA), which included 20:3n-6, 20:4n-6, 22:4n-6, and 22:5n-6 in n-6 PUFA; 20:5n-3, 22:5n-3, and 22:6n-3 in n-3 PUFA; and 20:3n-9. The proportion of n-6 or n-3 HUFA, that is n-6% or n-3% HUFA, was computed by dividing the sum of n-6 or n-3 HUFA by total HUFA in each sample and multiplying by 100.
Results of each fatty acid determined by multiple methods were compared by One-way ANOVA followed by Post Hoc Tamhane’s T2 test using SPSS 17.0 (SPSS Inc.; Chicago, IL). Statistic significance for a particular experimental condition was presented in comparison with the reference method at P <0.01.
Results and Discussion
This study focused on the microwave irradiation as an alternative energy source for one–step direct transesterification of fatty acid components of total lipids in human serum. The fatty acid concentrations (μmol/L) determined by microwave assays in methanol: hexane: acetyl chloride (4:1:0.2, by vol) were compared in Fig. 1, including total fatty acids (A), low abundance fatty acids (<50 μM; B), intermediate abundance (50–250 μM; C), and high abundance (>250 μM; D). Lepage & Roy assay was employed as the reference method, which applied the same solvent system but transesterified in heating block. The corresponding fatty acid profiles (mol%) were presented in Table 2.
Fig. 1.
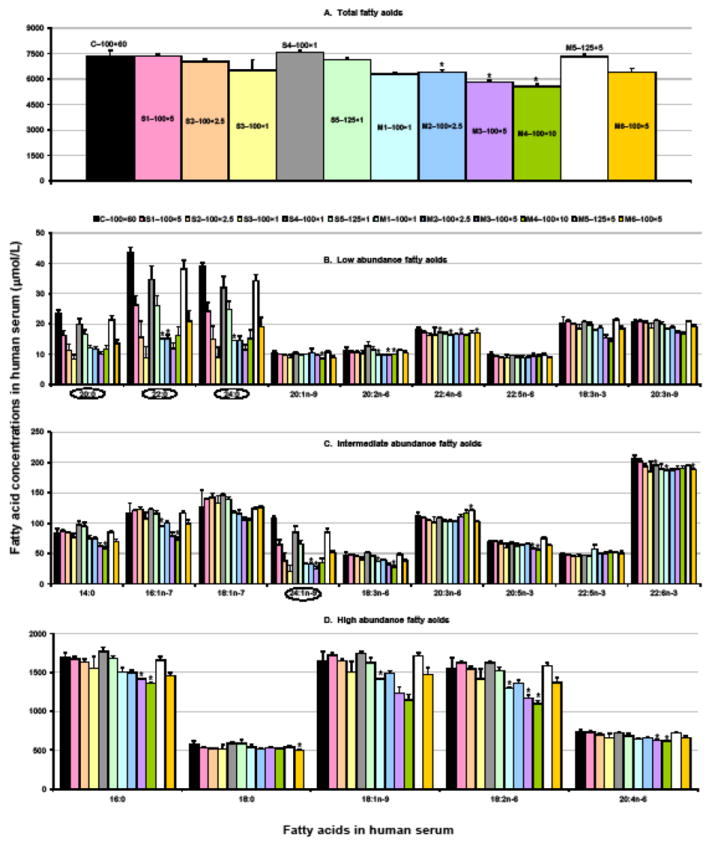
Comparison of the concentrations of fatty acids (μmol/L) in human serum determined by microwave accelerated assay for eleven conditions (S and M series) with those by Lepage & Roy assay, the reference method (control group C–100×60). Values are presented as mean ± SD, n = 3 except M1–100×5 (n = 2) and C–100×60 (n = 16). (A) Total fatty acids; (B) Low abundance fatty acids, <50 μM; (C) Intermediate abundance fatty acids, 50–250 μM; (D) High abundance fatty acids, >250 μM. Further details regarding the reaction conditions are presented in Table 1. One-way ANOVA followed by Tamhane’s T2 was applied to multiple comparison; asterisks indicate statistically different in comparison with control group at P <0.01. Unit conversion: 1 μmol/L of fatty acid ≈ 0.36 μg/mL
Table 2.
Comparison of fatty acid profiles in human serum total lipids determined by either Lepage & Roy assay or microwave irradiation assays
| Groups MW (Watt) Fatty acids | C-100×60 | S1-100×5 | S2-100×2.5 | S3-100×1 | S4-100×1 | S5-125×1 | M1-100×1 | M2-100×2.5 | M3-100×5 | M4-100×10 | M5-125×5 | M6-100×5 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n/a | 50 | 50 | 50 | 300 | 300 | 400 | 400 | 400 | 400 | 400 | 400 | |
| mol% of Total fatty acids | ||||||||||||
| 14:0 | 1.13 ± 0.1 | 1.18 ± 0.0 | 1.20 ± 0.0 | 1.18 ± 0.0 | 1.28 ± 0.1 | 1.33 ± 0.1 | 1.18 ± 0.1 | 1.17 ± 0.0 | 1.07 ± 0.1 | 1.02 ± 0.1 | 1.16 ± 0.0 | 1.09 ± 0.0 |
|
| ||||||||||||
| 16:0 | 23.2 ± 0.6 | 22.8 ± 0.0 | 23.2 ± 0.1 | 23.8 ± 0.6 | 23.4 ± 0.4 | 23.6 ± 0.6 | 23.9 ± 0.5 | 23.3 ± 0.2 | 24.4 ± 0.4 | 24.4 ± 0.4 | 22.6 ± 0.1 | 22.8 ± 0.4 |
|
| ||||||||||||
| 18:0 | 7.82 ± 0.8 | 7.27 ± 0.0 | 7.44 ± 0.1 | 7.94 ± 0.3 | 7.72 ± 0.1 | 8.26 ± 0.7 | 8.57 ± 0.3 | 8.09 ± 0.2 | 9.19 ± 0.4 | 9.36 ± 0.4 | 7.37 ± 0.0 | 7.80 ± 0.3 |
|
| ||||||||||||
| 20:0 | 0.32 ± 0.0 | 0.22 ± 0.0 | 0.16 ± 0.0 | 0.13 ± 0.0 | 0.26 ± 0.0 | 0.23 ± 0.0 | 0.19 ± 0.0 * | 0.18 ± 0.0 * | 0.17 ± 0.0 * | 0.21 ± 0.0 | 0.29 ± 0.0 | 0.21 ± 0.0* |
|
| ||||||||||||
| 22:0 | 0.60 ± 0.0 | 0.36 ± 0.0 | 0.22 ± 0.1 | 0.14 ± 0.1 | 0.46 ± 0.1 | 0.36 ± 0.0 | 0.24 ± 0.0 * | 0.24 ± 0.0 * | 0.20 ± 0.0 * | 0.29 ± 0.0 | 0.52 ± 0.0 | 0.33 ± 0.0 |
|
| ||||||||||||
| 24:0 | 0.54 ± 0.0 | 0.33 ± 0.0 | 0.21 ± 0.1 | 0.14 ± 0.1 | 0.42 ± 0.0 | 0.35 ± 0.0 | 0.23 ± 0.0 * | 0.22 ± 0.0 * | 0.20 ± 0.0 | 0.27 ± 0.0 | 0.47 ± 0.0 | 0.30 ± 0.0 |
|
| ||||||||||||
| 16:1n-7 | 1.59 ± 0.1 | 1.65 ± 0.0 | 1.74 ± 0.0 | 1.65 ± 0.1 | 1.63 ± 0.0 | 1.62 ± 0.1 | 1.51 ± 0.0 | 1.55 ± 0.0 | 1.36 ± 0.1 | 1.29 ± 0.1 | 1.60 ± 0.0 | 1.54 ± 0.0 |
|
| ||||||||||||
| 18:1n-9 | 22.5 ± 0.7 | 23.5 ± 0.1 * | 23.5 ± 0.2 * | 23.1 ± 0.2 | 23.0 ± 0.2 | 22.8 ± 0.5 | 22.5 ± 0.2 | 23.2 ± 0.2 | 21.2 ± 0.9 | 20.5 ± 0.9 | 23.4 ± 0.0 * | 23.1 ± 0.5 |
|
| ||||||||||||
| 18:1n-7 | 1.73 ± 0.3 | 1.91 ± 0.0 | 2.03 ± 0.1 | 2.03 ± 0.0 | 1.94 ± 0.1 | 1.96 ± 0.0 | 1.87 ± 0.1 | 1.80 ± 0.1 | 1.82 ± 0.1 | 1.89 ± 0.0 | 1.70 ± 0.1 | 1.97 ± 0.0 |
|
| ||||||||||||
| 20:1n-9 | 0.14 ± 0.0 | 0.14 ± 0.0 | 0.14 ± 0.0 | 0.13 ± 0.0 | 0.14 ± 0.0 | 0.14 ± 0.0 | 0.16 ± 0.0 * | 0.16 ± 0.0 | 0.16 ± 0.0 | 0.16 ± 0.0 | 0.15 ± 0.0 | 0.14 ± 0.0 |
|
| ||||||||||||
| 24:1n-9 | 1.48 ± 0.1 | 0.87 ± 0.1 | 0.54 ± 0.2 | 0.32 ± 0.1 | 1.13 ± 0.1 | 0.91 ± 0.1 | 0.51 ± 0.0 | 0.51 ± 0.0 * | 0.43 ± 0.1 * | 0.63 ± 0.1 | 1.16 ± 0.1 | 0.79 ± 0.0* |
|
| ||||||||||||
| 18:2n-6 | 21.2 ± 0.9 | 22.1 ± 0.0 | 22.0 ± 0.1 | 21.7 ± 0.4 | 21.5 ± 0.3 | 21.3 ± 0.5 | 20.6 ± 0.2 | 21.2 ± 0.3 | 20.1 ± 0.3 | 19.7 ± 0.3 | 21.7 ± 0.0 | 21.4 ± 0.4 |
|
| ||||||||||||
| 18:3n-6 | 0.6 ± 0.1 | 0.7 ± 0.0 | 0.6 ± 0.0 | 0.6 ± 0.0 | 0.7 ± 0.0 | 0.6 ± 0.0 | 0.6 ± 0.0 | 0.6 ± 0.0 | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.7 ± 0.0 | 0.6 ± 0.0 |
|
| ||||||||||||
| 20:2n-6 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 |
|
| ||||||||||||
| 20:3n-6 | 1.5 ± 0.1 | 1.5 ± 0.0 | 1.5 ± 0.0 | 1.6 ± 0.0 | 1.4 ± 0.0 | 1.4 ± 0.0 | 1.6 ± 0.0 | 1.6 ± 0.0 | 1.9 ± 0.1 | 2.1 ± 0.1 | 1.7 ± 0.0 | 1.6 ± 0.0 |
|
| ||||||||||||
| 20:4n-6 | 10.0 ± 0.2 | 10.0 ± 0.0 | 9.9 ± 0.1 | 10.0 ± 0.0 | 9.6 ± 0.1 | 9.6 ± 0.3 | 10.2 ± 0.1 | 10.2 ± 0.0 | 10.8 ± 0.3 | 11.1 ± 0.3 | 9.9 ± 0.1 | 10.3 ± 0.0* |
|
| ||||||||||||
| 22:4n-6 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 * | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.3 ± 0.0 |
|
| ||||||||||||
| 22:5n-6 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
|
| ||||||||||||
| 18:3n-3 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 |
|
| ||||||||||||
| 20:5n-3 | 0.9 ± 0.0 | 0.9 ± 0.0 | 0.9 ± 0.0 | 0.9 ± 0.0 | 0.9 ± 0.0 | 0.9 ± 0.1 | 1.0 ± 0.0 * | 1.0 ± 0.0 * | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 * | 1.0 ± 0.0* |
|
| ||||||||||||
| 22:5n-3 | 0.7 ± 0.0 | 0.7 ± 0.0 | 0.7 ± 0.0 | 0.7 ± 0.0 | 0.6 ± 0.0 | 0.6 ± 0.0 | 0.9 ± 0.1 | 0.8 ± 0.0 | 0.9 ± 0.0 | 0.9 ± 0.1 | 0.7 ± 0.0 | 0.8 ± 0.1 |
|
| ||||||||||||
| 22:6n-3 | 2.8 ± 0.1 | 2.7 ± 0.0 | 2.7 ± 0.0 | 2.8 ± 0.0 | 2.6 ± 0.0 | 2.7 ± 0.1 | 3.0 ± 0.0 | 2.9 ± 0.0 | 3.3 ± 0.1 | 3.4 ± 0.1 | 2.7 ± 0.0 | 3.0 ± 0.1 |
|
| ||||||||||||
| 20:3n-9 | 0.28 ± 0.0 | 0.28 ± 0.0 | 0.29 ± 0.0 | 0.29 ± 0.0 | 0.28 ± 0.0 | 0.28 ± 0.0 | 0.29 ± 0.0 | 0.29 ± 0.0 | 0.29 ± 0.0 | 0.30 ± 0.0 * | 0.29 ± 0.0 | 0.30 ± 0.0 |
| Summary | Fatty acid concentrations (μmol/L) | |||||||||||
|
| ||||||||||||
| Σ Fatty acids | 7304 ± 388 | 7340 ± 138 | 7024 ± 162 | 6526 ± 605 | 7562 ± 133 | 7131 ± 119 | 6297 ± 121 | 6419 ± 140 * | 5811 ± 111 * | 5577 ± 122 * | 7304 ± 181 | 6396 ± 223 |
|
| ||||||||||||
| Σ Saturates | 2450 ± 96 | 2361 ± 51 | 2280 ± 59 | 2172 ± 209 | 2534 ± 88 | 2432 ± 86 | 2291 ± 232 | 2133 ± 51 | 2048 ± 5 * | 1980 ± 13 * | 2370 ± 71 | 2077 ± 52 |
|
| ||||||||||||
| Σ Mono | 2009 ± 164 | 2067 ± 36 | 1967 ± 43 | 1779 ± 162 | 2126 ± 35 | 1971 ± 69 | 1842 ± 290 * | 1750 ± 43 * | 1456 ± 82 * | 1368 ± 88 | 2053 ± 48 | 1767 ± 93 |
|
| ||||||||||||
| Σ n-6 PUFA | 2480 ± 169 | 2552 ± 45 | 2431 ± 53 | 2248 ± 212 | 2550 ± 18 | 2392 ± 87 | 2287 ± 296 | 2197 ± 52 * | 1976 ± 38 * | 1900 ± 33 * | 2518 ± 55 | 2212 ± 85 |
|
| ||||||||||||
| Σ n-3 PUFA | 344 ± 9 | 340 ± 6 | 326 ± 7 | 308 ± 30 | 330 ± 3 | 316 ± 16 | 337 ± 19 | 320 ± 6 | 313 ± 5 | 313 ± 4 * | 342 ± 6 | 321 ± 2 * |
|
| ||||||||||||
| Σ n-6 HUFA | 873 ± 29 | 872 ± 16 | 829 ± 18 | 783 ± 75 | 862 ± 7 | 818 ± 31 | 813 ± 72 | 787 ± 14 | 767 ± 7 * | 762 ± 10* | 874 ± 17 | 794 ± 15 |
|
| ||||||||||||
| Σ n-3 HUFA | 323 ± 9 | 319 ± 6 | 306 ± 7 | 290 ± 28 | 309 ± 3 | 296 ± 15 | 318 ± 17 | 302 ± 6 | 298 ± 6 | 298 ± 5 | 321 ± 6 | 302 ± 2* |
|
| ||||||||||||
| Σ HUFA | 1217 ± 37 | 1211 ± 23 | 1155 ± 23 | 1092 ± 105 | 1192 ± 9 | 1134 ± 47 | 1150 ± 90 * | 1107 ± 19 | 1082 ± 12 * | 1077 ± 14* | 1215 ± 23 | 1116 ± 15* |
|
| ||||||||||||
| HUFA | Proportion | |||||||||||
|
| ||||||||||||
| n-6/n-3HUFA | 2.7 ± 0.1 | 2.7 ± 0.0 | 2.7 ± 0.0 | 2.7 ± 0.0 | 2.8 ± 0.0 * | 2.8 ± 0.0 | 2.6 ± 0.1 | 2.6 ± 0.0 | 2.6 ± 0.0 | 2.6 ± 0.0 * | 2.7 ± 0.0 | 2.6 ± 0.1 |
|
| ||||||||||||
| n-6% HUFA | 71.7 ± 0.4 | 72.0 ± 0.1 | 71.8 ± 0.2 | 71.7 ± 0.1 | 72.3 ± 0.0 * | 72.1 ± 0.3 | 70.7 ± 0.8 | 71.1 ± 0.2 | 70.9 ± 0.2 | 70.7 ± 0.0 * | 71.9 ± 0.1 | 71.2 ± 0.4 |
|
| ||||||||||||
| n-3% HUFA | 26.6 ± 0.4 | 26.3 ± 0.1 | 26.5 ± 0.2 | 26.5 ± 0.1 | 25.9 ± 0.1 * | 26.1 ± 0.3 | 27.7 ± 0.8 | 27.2 ± 0.2 | 27.5 ± 0.3 | 27.7 ± 0.1 * | 26.4 ± 0.1 | 27.1 ± 0.4 |
Values are presented as mean ± SD, n = 3 except M1-100×5 (2) and C-100×60 (16); value of “0.0” indicates < 0.05
One-way ANOVA followed by Tamhane’s T2 test was applied to multiple comparison; asterisks indicate statistically different in comparison with control group at P <0.01
Mono–monounsaturates; HUFA: highly unsaturated fatty acids; see footnote to Table 1 for further abbreviations
Unit conversion: 1 μmol/L of fatty acid 0.36 ≈ μg/mL
Complete Transesterification
It was apparent that the concentrations of various fatty acids in microwave groups S4–100×1 and M5–125×5 in Fig. 1 a–d were consistently close to those in the control group (C–100×60). As presented in Table 2, the total amount of fatty acids (μmol/L) was 7,562 (S4–100×1) and 7,304 (M5–125×5) in microwave groups compared to 7,304 in control group, which represented recoveries of 103 and 100%, respectively. Similarly, the recoveries of the categorized fatty acids, including saturates, monounsaturates, n-6 PUFA, and n-3 PUFA, were in ranges of 96–106% (S4–100×1) and 97–102% (M5–125×5). Compared to the control group, it was observed that the recoveries of individual fatty acids (n = 23) in both groups were 94% or greater for all except some minor fatty acids–20:0, 22:0, 24:0, and 24:1n-9, which were lower as 84% (range 79–91). These minor ones accounted less than 2.5% of moles of total fatty acids. In particular, the recoveries of individual PUFA in both groups were in range of 95–105% except for 18:3n-6 (109%) and 20:2n-6 (113%) in S4–100×1, and 20:3n-6 (109%) and 20:5n-3 (109%) in M5–125×5. Marginal greater recoveries for 20:5n-3 could be explained by the reduced potential oxidation in microwave assay, as observed by Khan and William [22]. It was likely similar to 20:3n-6. Furthermore, 100.0% of ISTD 22:3n-3 ethyl esters added in samples were transmethylated to 22:3n-3 methyl esters in M5–125×5, and 99.5% in S4–100×1. Thus, taken the above conditions as optimal, the fatty acids of interest in human serum were completely transesterified for all major ones and most minor ones with microwave irradiation heating at 100 °C for 1 min in Single-mode with initial power of 300 W (S4–100×1) or 125 °C for 5 min in Multimode with 400 W (M5–125×5).
Additionally, the recoveries for the categorized fatty acids in group S1–100×5, 100 °C for 5 min in Single–mode, were in range of 96–103% which indicated a complete transesterification as those in groups S4–100×1 and M5–125×5. However, upon inspection of panel B and C in Fig. 1, the minor fatty acids–20:0, 22:0, 24:0, and 24:1n-9, reached only 60–69%. Thus, this was not considered as optimal transesterification.
Given the varied features of two microwave reaction systems, the difference between two optimal conditions were expected and could be mainly derived from the different temperature control systems and the varied number of sample processed in each system. The actual temperature in Multimode was measured and controlled by internal fiber optic probe inserted the reference vessel, but in Single–mode by the cavity outside of the reaction vessel. In addition, it would be expected in Multimode if a much higher initial microwave power setting needed to maintain the optimal transesterification conditions when the number of samples in each batch increase.
The recent report by Tomas et al. [20] shows a similar result of range 103–117% of recoveries for 16:0, 18:0, 18:2n-6, and 20:4n-6 in meat acylglycerides using single mode microwave reaction system. However, the catalyst (chlorotrimethylsilane) applied in microwave irradiation accelerate transesterification is different from that in reference method (boron trifluoride). This might cause some variation.
Partial Transesterification
In the remaining nine microwave conditions tested, including groups S1–S3, S5, M1–M4, and M6, only 86–99% of 22:3n-3 ethyl esters were converted to methyl esters. Similarly, the recoveries (%) for total fatty acids, saturates, monounsaturates, and n-6 and n-3 PUFA, were in lower and wider ranges as 76–100, 81–96, 68–103, and 90–99, respectively. It was observable that fatty acids were partially transesterified under these conditions, which were thus considered as sub–optimal. However, after having been calibrated with ISTD, the concentrations of HUFA in these nine groups showed close results to those from the optimal conditions, groups S4–100×1 and M5–125×5, as summarized in Table 2. The average concentration of total HUFA in eleven microwave groups was 1,146 53 μmol/L serum with coefficient of variance as low as 4.6%. It reflected about 94% (range 89–100%) of that in Lepage & Roy control group (C–100×60).
In addition, n-6% HUFA exhibited very narrow range as 71–72%, while its counterpart, n-3% HUFA, as 26–28%, across the eleven microwave groups presented in Table 2. The ratio of n-6 to n-3 HUFA was 2.6–2.7. Compared to the reference method, their recoveries were 99–101, 98–104, and 95–103%, respectively. Apparently, these indices were very consistent with those in the control group. Similarity is observed in Armstrong’s study [17] that n-3% HUFA in human whole blood measured by microwave assay (53.6%) is close to that by conventional assay (54.4%) despite low recovery for the concentration of n-3 HUFA (74%). Thus, if only n-3% or n-6% HUFA was of interest in human serum total lipids, transesterification at both optimal and sub–optimal conditions in either single–mode or multimode microwave systems would be sufficient.
Low Efficiency Transesterification
The recoveries of fatty acids 20:0, 22:0, 24:0, and 24:1n-9 in microwave assay among sub–optimal reaction conditions were significantly lower than in optimal conditions; only 20–70%, as observable in the bar graph presented in Fig. 1 panel B and C. These low recoveries could not be calibrated by ISTD added prior to chemical analysis. The extension of the reaction time up to 10 min (M4–100×10) did not increase the efficiency of transesterification. This is in agreement with the reported study of microwave–assisted extraction of active ingredients of plants [23], in which longer time does not yield higher extraction recovery. It could possibly be explained as some lipids, such as sphingolipids, are more difficult to transesterify than other lipids regarding the microwave irradiation power, duration, and temperature, as suggested by Armstrong et al. [17].
Previously, the low recoveries of transesterification by microwave irradiation using a domestic microwave oven were extensively observed for all fatty acids, such as 78% for total fatty acids, 61–87% for PUFA, and 20–30% for 22:0, 24:0, and 24:1n-9 [17]. In addition to the non–professional microwave irradiation applied, this low efficiency derivatization is probably in part a contribution of the applied boron fluoride, which is a better catalyst for methanol methylation of non–esterified fatty acid [24] or transesterification of isolated lipids than the lipids complex in the biological samples.
Application and Limitation
Compared to the extreme explosive conditions explored in domestic microwave oven in previous report [16, 17], these innovative microwave reaction systems in this study were designed to be explosion proof for pressurized organic reactions. The transesterification of fatty acids in these systems was not only chemically safe, but also well controlled over the reaction temperature with automated, continuous adjustment of the microwave power. This made it possible to achieve complete transesterification of fatty acids in human serum total lipids at the conventional reaction temperatures as those in heatblock heating, but greatly reduced the reaction duration. Because of the disparity difference in the capacities, such as pressure control, temperature control, and reaction efficiency, no reaction conditions applied in domestic microwave oven would be examined in these microwave reaction systems.
However, this microwave fatty acid assay was validated only with transesterification of total lipids in one particular solvent system (methanol, hexane, and acetyl chloride) at small sampling size. Further studies are needed to define conditions for fatty acid determination in individual lipid class or for various tissues. Based on the present work it is expected that such applications could be validated with replacement of the heating block by microwave irradiation and with similar chemical procedures. Future work would also be needed to determine if even faster times were possible.
Summary
At optimal conditions (S4–100×1 and M5–125×5) utilizing either single–mode or multimode innovative microwave reaction system, microwave irradiation energy could replace the conductive heating to transesterify fatty acids in human serum lipids by methanol, hexane, and acetyl chloride. Microwave irradiation heating provided fatty acid quantifications which were comparable to the reference method but with greatly reduced reaction duration, from 60 min to 5 min or less. This microwave accelerated fatty acid assay could be useful in small– or large–scale clinical studies and laboratory research as a rapid, reliable, safe, and efficient method.
Acknowledgments
The authors wish to acknowledge Drs. Norman Salem Jr., Charlie Serhan, and William E.M. Lands for the valuable advice and encouragement on the method development. Thanks to Ms. Keller Barnhardt for set–up of the microwave reaction systems and to Ms. Cindy Clark from the NIH Library Writing Center for assistance with manuscript editing. This project was funded by the Intramural Research Program of the National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health.
Abbreviations
- BHT
2, 6-Di-tert-butyl-4-methylphenol
- FAME
Fatty acid methyl ester
- GC
Gas–liquid chromatography with flame ionization detector
- HUFA
Highly unsaturated fatty acid
- ISTD
Internal standard
- Multimode
Multimode microwave accelerate reaction system
- PUFA
Polyunsaturated fatty acid
- Single–mode
Single mode microwave reaction system
References
- 1.Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58:2047–2067. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- 2.Calon F, Lim GP, Morihara T, Yang F, Ubeda O, Salem N, Jr, Frautschy SA, Cole GM. Dietary n-3 polyunsaturated fatty acid depletion activates caspases and decreases nmda receptors in the brain of a transgenic mouse model of alzheimer’s disease. EurJNeurosci. 2005;22:617–626. doi: 10.1111/j.1460-9568.2005.04253.x. [DOI] [PubMed] [Google Scholar]
- 3.Mahe G, Ronziere T, Laviolle B, Golfier V, Cochery T, De Bray JM, Paillard F. An unfavorable dietary pattern is associated with symptomatic ischemic stroke and carotid atherosclerosis. J Vasc Surg. 2010;52:62–68. doi: 10.1016/j.jvs.2010.02.258. [DOI] [PubMed] [Google Scholar]
- 4.Cabre E, Manosa M, Gassull MA. Omega-3 fatty acids and inflammatory bowel diseases - a systematic review. Br J Nutr. 2012;107(Suppl 2):S240–252. doi: 10.1017/S0007114512001626. [DOI] [PubMed] [Google Scholar]
- 5.Hibbeln JR, Salem N., Jr Dietary polyunsaturated fatty acids and depression: When cholesterol does not satisfy. AmJClinNutr. 1995;62:1–9. doi: 10.1093/ajcn/62.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Hibbeln JR, Davis JM, Steer C, Emmett P, Rogers I, Williams C, Golding J. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (alspac study): An observational cohort study. Lancet. 2007;369:578–585. doi: 10.1016/S0140-6736(07)60277-3. [DOI] [PubMed] [Google Scholar]
- 7.Liu K-s. Preparation of fatty acid methyl esters for gas-chromatographic analysis of lipids in biological materials. JAOCS. 1994;71:1179–1187. [Google Scholar]
- 8.Stoffel W, Chu F, Ahrens EH. Analysis of long-chain fatty acids by gas-liquid chromatography. Anal Chem. 1959;31:307–308. [Google Scholar]
- 9.Morrison WR, Smith LM. Preparation of fatty acid methyl esters and dimethyl acetals from lipids with boron tri-fluoride-methanol. J Lipid Res. 1964;5:600–608. [PubMed] [Google Scholar]
- 10.Lepage G, Roy CC. Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res. 1986;27:114–120. [PubMed] [Google Scholar]
- 11.Glaser C, Demmelmair H, Koletzko B. High-throughput analysis of total plasma fatty acid composition with direct in situ transesterification. PLoS One. 2010;5:e12045. doi: 10.1371/journal.pone.0012045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glaser C, Demmelmair H, Koletzko B. High-throughput analysis of fatty acid composition of plasma glycerophospholipids. J Lipid Res. 2010;51:216–221. doi: 10.1194/jlr.D000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin YH, Salem N, Jr, Wells EM, Zhou W, Loewke JD, Brown JA, Lands WE, Goldman LR, Hibbeln JR. Automated high-throughput fatty acid analysis of umbilical cord serum and application to an epidemiological study. Lipids. 2012;47:527–539. doi: 10.1007/s11745-012-3661-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carrapiso AI, Garcia C. Development in lipid analysis: Some new extraction techniques and in situ transesterification. Lipids. 2000;35:1167–1177. doi: 10.1007/s11745-000-0633-8. [DOI] [PubMed] [Google Scholar]
- 15.Lie Ken Jie MSF, Cheung YK. The use of a microwave oven in the chemical transformation of long chain fatty acid esters. Lipids. 1988;23:367–369. doi: 10.1007/BF02537351. [DOI] [PubMed] [Google Scholar]
- 16.Banerjee P, Dawson G, Dasgupta A. Enrichment of saturated fatty acid containing phospholipids in sheep brain serotonin receptor preparations: Use of microwave irradiation for rapid transesterification of phospholipids. Biochim Biophys Acta. 1992;1110:65–74. doi: 10.1016/0005-2736(92)90295-w. [DOI] [PubMed] [Google Scholar]
- 17.Armstrong JM, Metherel AH, Stark KD. Direct microwave transesterification of fingertip prick blood samples for fatty acid determinations. Lipids. 2008;43:187–196. doi: 10.1007/s11745-007-3141-6. [DOI] [PubMed] [Google Scholar]
- 18.Dasgupta A, Banerjee P, Malik S. Use of microwave irradiation for rapid transesterification of lipids and accelearated synthesis of fatty acyl pyrrolidides for analysis by gas chromatography-mass spectrometry: Study of fatty acid profiles of olive oil, evening primrose oil, fish oils and phospholipids from mango pulp. Chem Phys Lipids. 1992;62:281–291. [Google Scholar]
- 19.Itonori S, Takahashi M, Kitamura T, Aoki K, Dulaney JT, Sugita M. Microwave-mediated analysis for sugar, fatty acid, and sphingoid compositions of glycosphingolipids. J Lipid Res. 2004;45:574–581. doi: 10.1194/jlr.D300030-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Tomas A, Tor M, Villorbina G, Canela R, Balcells M, Eras J. A rapid and reliable direct method for quantifying meat acylglycerides with monomode microwave irradiation. J Chromatogr A. 2009;1216:3290–3295. doi: 10.1016/j.chroma.2009.01.055. [DOI] [PubMed] [Google Scholar]
- 21.Masood A, Stark KD, Salem N., Jr A simplified and efficient method for the analysis of fatty acid methyl esters suitable for large clinical studies. J Lipid Res. 2005;46:2299–2305. doi: 10.1194/jlr.D500022-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.Khan MU, Williams JP. Microwave-mediated methanolysis of lipids and activation of thin-layer chromatographic plates. Lipids. 1993;28:953–955. [Google Scholar]
- 23.Deng C, Ji J, Li N, Yu Y, Duan G, Zhang X. Fast determination of curcumol, curdione and germacrone in three species of curcuma rhizomes by microwave-assisted extraction followed by headspace solid-phase microextraction and gas chromatography-mass spectrometry. J Chromatogr A. 2006;1117:115–120. doi: 10.1016/j.chroma.2006.03.066. [DOI] [PubMed] [Google Scholar]
- 24.Carvalho AP, Malcata FX. Preparation of fatty acid methyl esters for gas-chromatographic analysis of marine lipids: Insight studies. J Agric Food Chem. 2005;53:5049–5059. doi: 10.1021/jf048788i. [DOI] [PubMed] [Google Scholar]


