Abstract
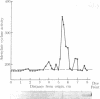
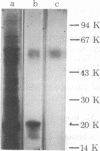
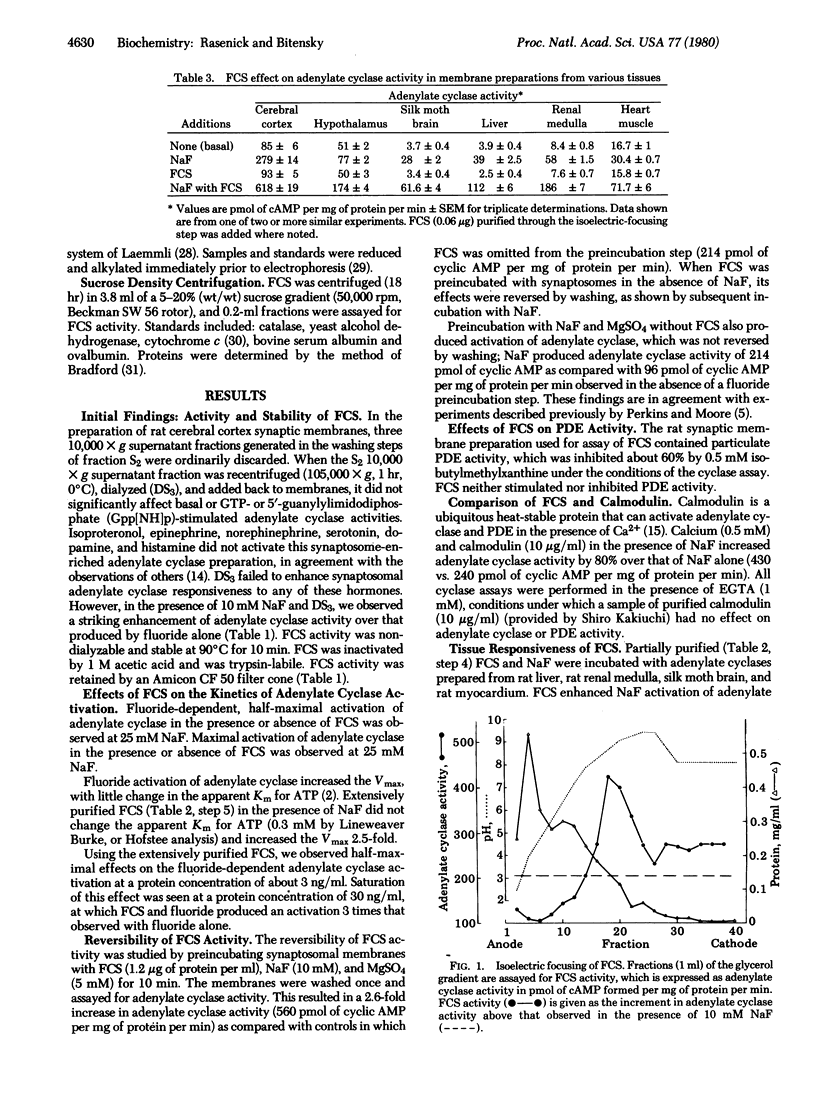
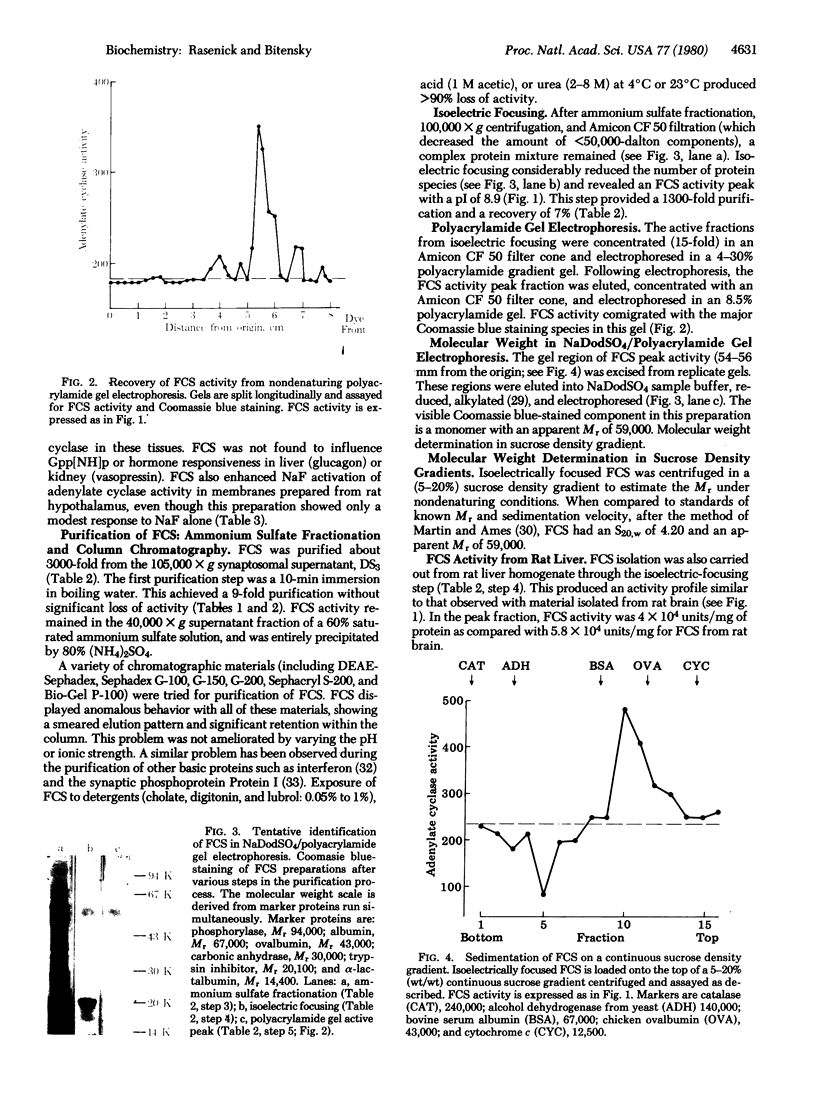
Fluoride activation of adenylate cyclase [ATP pyrophosphate-lyase (cyclizing), EC 4.6.1.1] is significantly enhanced (2 to 5 times) by a protein factor isolated from rat brain. The fluoride-dependent adenylate cyclase stimulator (FCS) is nondialyzable, trypsin-labile, and stable at 90 degrees C for 10 min. FCS stimulates adenylate cyclase activity only in the presence of NaF (2-25 mM) and this effect is independent of added GTP, 5'-guanylylimidodiphosphate, or calcium. FCS has been purified roughly 3000-fold from a 12,000 X g supernatant fraction of rat brain homogenate. Sodium dodecyl sulfate/polyacrylamide gel electrophoresis and sucrose density gradient sedimentation suggest that FCS is a monomer with an apparent Mr of 59,000. Isoelectric focusing indicates FCS has a pI of 8.9. FCS from rat brain stimulates fluoride-activated adenylate cyclase from a variety of cell types, and FCS can also be isolated from rat liver. The effects of FCS are not reversed by washing membranes when the membranes and FCS are preincubated with NaF. The Km of adenylate cyclase for ATP and the fluoride concentration causing half-maximal activation are unchanged by FCS; however, FCS increases the Vmax by 2.5-fold. FCS may act to increase the catalytic efficiency of fluoride-activated complexes of the GTP-binding unit with adenylate cyclase or to enhance the formation of additional active complexes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bitensky M. W., Russell V., Blanco M. Independent variation of glucagon and epinephrine responsive components of hepatic adenyl cyclase as a function of age, sex and steroid hormones. Endocrinology. 1970 Jan;86(1):154–159. doi: 10.1210/endo-86-1-154. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brostrom M. A., Brostrom C. O., Breckenridge B. M., Wolff D. J. Calcium-dependent regulation of brain adenylate cyclase. Adv Cyclic Nucleotide Res. 1978;9:85–99. [PubMed] [Google Scholar]
- Brown B. L., Ekins R. P., Albano J. D. Saturation assay for cyclic AMP using endogenous binding protein. Adv Cyclic Nucleotide Res. 1972;2:25–40. [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Mechanism of adenylate cyclase activation by cholera toxin: inhibition of GTP hydrolysis at the regulatory site. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3307–3311. doi: 10.1073/pnas.74.8.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dousa T. P. Effects of hormones on cyclic AMP formation in kidneys of nonmammalian vertebrates. Am J Physiol. 1974 May;226(5):1193–1197. doi: 10.1152/ajplegacy.1974.226.5.1193. [DOI] [PubMed] [Google Scholar]
- Downs R. W., Jr, Spiegel A. M., Singer M., Reen S., Aurbach G. D. Fluoride stimulation of adenylate cyclase is dependent on the guanine nucleotide regulatory protein. J Biol Chem. 1980 Feb 10;255(3):949–954. [PubMed] [Google Scholar]
- GRAY E. G., WHITTAKER V. P. The isolation of nerve endings from brain: an electron-microscopic study of cell fragments derived by homogenization and centrifugation. J Anat. 1962 Jan;96:79–88. [PMC free article] [PubMed] [Google Scholar]
- Harwood J. P., Rodbell M. Inhibition by fluoride ion of hormonal activation of fat cell adenylate cyclase. J Biol Chem. 1973 Jul 25;248(14):4901–4904. [PubMed] [Google Scholar]
- Hebdon M., Le Vine H., 3rd, Sahyoun N., Schmitges C. J., Cuatrecasas P. Properties of the interaction of fluoride- and guanylyl-5'-imidodiphosphate-regulatory proteins with adenylate cyclase. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3693–3697. doi: 10.1073/pnas.75.8.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen L. L. Catecholamine-sensitive adenylate cyclases in nervous tissues. J Neurochem. 1977 Jul;29(1):5–12. doi: 10.1111/j.1471-4159.1977.tb03918.x. [DOI] [PubMed] [Google Scholar]
- Izumi H., Oyama H., Ozawa H. The stimulatory effect of the boiled supernatant on cyclic AMP formation in synaptosomes from rat cerebral cortex. Jpn J Pharmacol. 1975 Aug;25(4):375–381. doi: 10.1254/jjp.25.375. [DOI] [PubMed] [Google Scholar]
- Katz M. S., Kelly T. M., Piñeyro M. A., Gregerman R. I. Activation of epinephrine and glucagon-sensitive adenylate cyclases of rat liver by cytosol protein factors. Role in loss of enzyme activities during preparation of particulate fractions, quantitation and partial characterization. J Cyclic Nucleotide Res. 1978 Oct;4(5):389–407. [PubMed] [Google Scholar]
- Kawakita M., Cabrer B., Taira H., Rebello M., Slattery E., Weideli H., Lengyel P. Purification of interferon from mouse Ehrlich ascites tumor cells. J Biol Chem. 1978 Jan 25;253(2):598–602. [PubMed] [Google Scholar]
- Kimmich G. A., Randles J., Brand J. S. Assay of picomole amounts of ATP, ADP, and AMP using the luciferase enzyme system. Anal Biochem. 1975 Nov;69(1):187–206. doi: 10.1016/0003-2697(75)90580-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane L. C. A simple method for stabilizing protein-sulfhydryl groups during SDS-gel electrophoresis. Anal Biochem. 1978 Jun 1;86(2):655–664. doi: 10.1016/0003-2697(78)90792-3. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Miki N., Baraban J. M., Keirns J. J., Boyce J. J., Bitensky M. W. Purification and properties of the light-activated cyclic nucleotide phosphodiesterase of rod outer segments. J Biol Chem. 1975 Aug 25;250(16):6320–6327. [PubMed] [Google Scholar]
- Neer E. J. Vasopressin-responsive, soluble adenylate cyclase from the rat renal medulla. J Biol Chem. 1973 May 25;248(10):3742–3744. [PubMed] [Google Scholar]
- Pecker F., Hanoune J. Activation of epinephrine-sensitive adenylate cyclase in rat liver by cytosolic protein-nucleotide complex. J Biol Chem. 1977 Apr 25;252(8):2784–2786. [PubMed] [Google Scholar]
- Perkins J. P. Adenyl cyclase. Adv Cyclic Nucleotide Res. 1973;3:1–64. [PubMed] [Google Scholar]
- Perkins J. P., Moore M. M. Adenyl cyclase of rat cerebral cortex. Activation of sodium fluoride and detergents. J Biol Chem. 1971 Jan 10;246(1):62–68. [PubMed] [Google Scholar]
- Queener S. F., Fleming J. W., Bell N. H. Analysis of sodium fluoride enhancement of calcitonin stimulation of renal cortical adenylate cyclase. J Biol Chem. 1978 Dec 25;253(24):9033–9040. [PubMed] [Google Scholar]
- RALL T. W., SUTHERLAND E. W. Formation of a cyclic adenine ribonucleotide by tissue particles. J Biol Chem. 1958 Jun;232(2):1065–1076. [PubMed] [Google Scholar]
- Ross E. M., Howlett A. C., Ferguson K. M., Gilman A. G. Reconstitution of hormone-sensitive adenylate cyclase activity with resolved components of the enzyme. J Biol Chem. 1978 Sep 25;253(18):6401–6412. [PubMed] [Google Scholar]
- Shahed A. R., Miller A., Chalker D., Allmann D. W. Effect of sodium fluoride on cyclic AMP production in rat hepatocytes. J Cyclic Nucleotide Res. 1979;5(1):43–53. [PubMed] [Google Scholar]
- Shinozawa T., Sen I., Wheeler G., Bitensky M. Predictive value of the analogy between hormone-sensitive adenylate cyclase and light-sensitive photoreceptor cyclic GMP phosphodiesterase: a specific role for a light-sensitive GTPase as a component in the activation sequence. J Supramol Struct. 1979;10(2):185–190. doi: 10.1002/jss.400100208. [DOI] [PubMed] [Google Scholar]
- Sitaramayya A., Virmaux N., Mandel P. On the mechanism of light activation of retinal rod outer segments cyclic GMP phosphodiesterase (light activation-influence of bleached rhodopsin and KF-deinhibition). Exp Eye Res. 1977 Aug;25(2):163–169. doi: 10.1016/0014-4835(77)90128-2. [DOI] [PubMed] [Google Scholar]
- Snyder F. F., Drummond G. I. Activation and stabilization of cardiac adenylate cyclase by GTP analog and fluoride. Arch Biochem Biophys. 1978 Jan 15;185(1):116–125. doi: 10.1016/0003-9861(78)90150-9. [DOI] [PubMed] [Google Scholar]
- Spiegel A. M., Downs R. W., Jr, Aurbach G. D. Separation of a guanine nucleotide regulatory unit from the adenylate cyclase complex with GTP affinity chromatography. J Cyclic Nucleotide Res. 1979;5(1):3–17. [PubMed] [Google Scholar]
- Ueda T., Greengard P. Adenosine 3':5'-monophosphate-regulated phosphoprotein system of neuronal membranes. I. Solubilization, purification, and some properties of an endogenous phosphoprotein. J Biol Chem. 1977 Jul 25;252(14):5155–5163. [PubMed] [Google Scholar]