Abstract
Diabetic nephropathy is the major cause of end-stage renal disease worldwide. Although the renin-angiotensin system has been implicated in the pathogenesis of diabetic nephropathy, angiotensin I-converting enzyme (ACE) inhibitors have a beneficial effect on diabetic nephropathy independently of their effects on blood pressure and plasma angiotensin II levels. This suggests that the kallikrein-kinin system (KKS) is also involved in the disease. To study the role of the KKS in diabetic nephropathy, mice lacking either the bradykinin B1 receptor (B1R) or the bradykinin B2 receptor (B2R) have been commonly used. However, because absence of either receptor causes enhanced expression of the other, it is difficult to determine the precise functions of each receptor. This difficulty has recently been overcome by comparing mice lacking both receptors with mice lacking each receptor. Deletion of both B1R and B2R reduces nitric oxide (NO) production and aggravates renal diabetic phenotypes, relevant to either lack of B1R or B2R, demonstrating that both B1R and B2R exert protective effects on diabetic nephropathy presumably via NO. Here, we review previous epidemiological and experimental studies, and discuss novel insights regarding the therapeutic implications of the importance of the KKS in averting diabetic nephropathy.
Keywords: Bradykinin, Diabetic nephropathy, ACE inhibitors, Nitric oxide, Oxidative stress
Beneficial effects of genetically decreased ACE levels on diabetic nephropathy
Angiotensin I-converting enzyme (ACE), a zinc metallopeptidase widely distributed on the surface of endothelial and epithelial cells, plays a key role in the renin-angiotensin system (RAS) and in the kallikrein-kinin system (KKS). It catalyzes not only the conversion of angiotensin I to the vasoconstricting peptide angiotensin II, but also the conversion of active bradykinin, kallidin, and kallidin-like peptide to inactive bradykinin (1-7), kallidin (1-8), and kallidin-like peptide (1-8), respectively (1,2)(Figure 1). Since ACE has a 30 times lower Km and 10 times higher kcat / Km, it has higher affinity and efficiency for kinins than for angiotensin I (3), and it is responsible for ~47% of the total plasma kininase activity (4). Furthermore, computer simulations show that modest changes in ACE levels affect the levels of its substrates much more than the levels of its products, indicating that relatively small changes in ACE activity affect kinin levels more than angiotensin II levels (5,6). Indeed, an ACE inhibitor, perindopril, significantly increases plasma levels of bradykinin at doses much lower than those required to reduce angiotensin II levels (7).
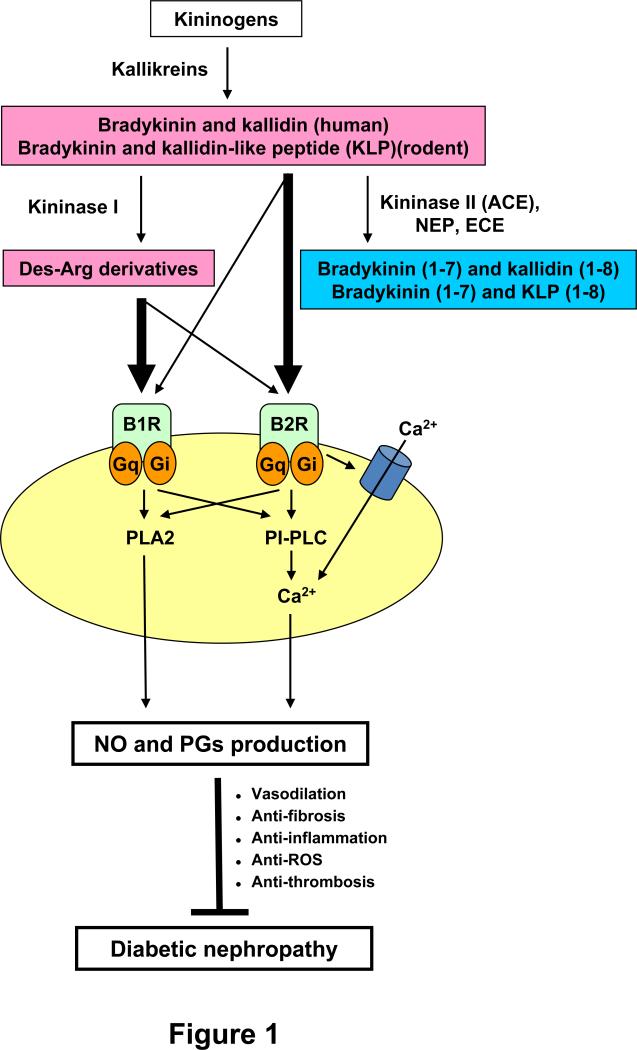
Figure 1.
The KKS components and protective effects of the KKS on diabetic nephropathy. The kinins, bradykinin and kallidin in humans or bradykinin and the kallidin-like peptide in rodents, are generated from kininogens by kallikreins. All the kinins are strong agonists of the bradykinin 2 receptor (B2R), and less so of the B1 receptor (B1R). Kininase I removes arginine from the carboxyl terminus of the kinins and generates their des-Arg derivatives, which are agonists mainly of B1R. Kininase II (ACE, angiotensin I-converting enzyme), neprilysin (NEP, endopeptidase 24.11), and endothelin-converting enzyme (ECE) all remove two amino acids (Phe and Arg) from the carboxyl terminus of the kinins, and inactivate them. Red and blue indicate active kinins and inactive kinins, respectively. The thickness of arrows arising from the kinins indicates the relative potency of each peptide to elevate intracellular calcium concentrations. Binding of the active kinins to both bradykinin receptors stimulates at least two intracellular signaling pathways, phospholipase A2 (PLA2)-dependent and phosphatidylinositol-specific phospholipase C (PI-PLC)-dependent pathways. The activated signaling pathways lead to the production of nitric oxide (NO) and prostaglandins (PGs), which exert protective effects on diabetic nephropathy. Gq indicates Gq protein; Gi, Gi protein. Adapted from Kakoki et al (2).
A common 287-bp insertion/deletion (I/D) polymorphism in intron 16 of the ACE gene occurs in humans. Importantly, the two alleles (I and D) are associated with different plasma ACE levels. The D/D and I/D genotypes show higher plasma levels of ACE than the I/I genotype by 65% and 30%, respectively (8). However, the ACE polymorphism does not significantly affect blood pressure, plasma angiotensin II or aldosterone levels (9). Nevertheless, the I and D human ACE alleles are associated with different risks for developing diabetic complications including nephropathy (10,11), neuropathy (12), retinopathy (13), myocardial infarction (14), and stroke (15). In all these diabetes-associated conditions, the D allele associated with higher levels of ACE confers the increased risk. The ACE I/D polymorphism also affects bradykinin metabolism in humans (16). Degradation of bradykinin through the ACE pathway in normotensive volunteers was greatest in D/D genotype, intermediate in I/D genotype, and least in I/I genotype, as measured by the ratio of bradykinin (1–5) (inactive stable metabolite of bradykinin) to bradykinin (16). Moreover, the ratio of bradykinin (1–5) to bradykinin positively correlated with plasma ACE activity. A recent report has further demonstrated that the D/D genotype in normotensive Brazilian male subjects has higher levels of plasma kallikrein activity than I/D and I/I genotypes by 30% and 60%, respectively, indicating possible compensation for the increased bradykinin degradation that occurs in the D/D genotype (17). Plasma ACE activity is also higher in the D/D genotype of this population. Together, these studies in humans demonstrate that the D allele is associated with not only enhanced plasma ACE activity, but also increased degradation of plasma bradykinin.
In genetically engineered mice having one, two, or three functional copies of the Ace gene at its normal chromosomal location, plasma ACE activities are 62% of normal in the one-copy animals, 100% in the two-copy animals (wild-type), and 144% in three-copy animals (18). Thus, these mouse models were originally expected to show different levels of blood pressures. However, the copy number of the Ace gene had no effect on blood pressure, an observation that supports the human polymorphism studies. Later work with the same series of mice suggests that quantitative changes in expression of the Ace gene may measurably affect blood pressures when accompanied by additional genetic or environmental factors that stress the homeostatic machinery. This was demonstrated by an experiment in which diabetes was induced with streptozotocin (STZ) treatment in mice having one, two, and three copies of the Ace gene (19). Twelve weeks later, the blood pressures of the one-copy mice and the wild-type (two-copy) mice were not affected by induction of diabetes. However, the blood pressure of the three-copy diabetic mice increased with time, and 12 weeks later were 10–20 mmHg higher than those of the one- and two-copy diabetic mice. Also the three-copy diabetic mice had overt proteinuria 12 weeks after induction of diabetes, whereas the one- and two-copy diabetic mice progressed much less rapidly. Importantly, proteinuria was significantly correlated with plasma ACE levels in the three-copy diabetic mice. Furthermore, urinary kallikrein significantly increased with increase in ACE copy number and tended to increase with diabetes, again implying the existence of possible compensation for increased bradykinin degradation.
Thus, a modest genetic increase in ACE levels is sufficient to aggravate nephropathy in diabetes, partly through increased bradykinin degradation.
Role of the KKS in the beneficial effect of ACE inhibitors on diabetic nephropathy
Several clinical studies have shown the beneficial effects of ACE inhibitors on diabetic nephropathy in patients with type 1 (20) or type 2 diabetes (21). The Collaborative Study demonstrated that an ACE inhibitor, captopril, reduces the risk of the combined end point of death, dialysis, and transplantation by 50% compared with placebo in patients with type 1 diabetes (20). Importantly, this beneficial effect of captopril is independent of blood pressure. The Bergamo Nephrologic Diabetes Complications Trial (BENEDICT) demonstrated that another ACE inhibitor, trandolapril, reduces the onset of microalbuminuria by 40-50% compared to placebo or the calcium-channel blocker verapamil in patients with hypertension, type 2 diabetes, and normal urinary albumin excretion (21). The reduced incidence of microalbuminuria is still significant even after adjustment for blood pressure. These clinical data provide convincing evidence that ACE inhibitors have blood pressure-independent beneficial effects on the diabetic nephropathy in both type 1 and type 2 diabetes (22).
Beneficial effects of angiotensin II type 1 receptor blockers (ARB) on diabetic nephropathy have also been reported. The RENAAL (Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan) study demonstrated that an ARB, losartan, reduced the risk of the primary end point, which was the composite of a doubling of the base-line serum creatinine concentration, end-stage renal disease, or death from any cause, by 16% compared with placebo in patients with type 2 diabetes (23). The Irbesartan Study also demonstrated that an another ARB, Irbesartan, reduced the risk of the primary end point, which was the same as the RENAAL Study, by 20% or 23% compared with placebo or with calcium-channel blocker amlodipine, respectively, in patients with type 2 diabetes (24). The beneficial effects of an ARB in both studies remain significant after adjustment for blood pressure.
In addition to the well-established protective role of angiotensin II blockade in systemic and intrarenal RAS (25-29), stimulation of the KKS partly explains the beneficial effects of ACE inhibitors and ARBs which occur independent of blood pressure in diabetic nephropathy. ACE inhibitors increase the plasma levels of bradykinin by inhibiting its degradation in rats and in humans (7,30,31). Also, ACE inhibition has been shown to be protective against diabetic nephropathy via the KKS in many animal models. The beneficial effects of ACE inhibitors are attenuated by a bradykinin B2 receptor (B2R) antagonist in STZ-treated rat (32,33), in obese Zucker diabetic fatty rats (34), and in C57BLKS db/db mice (35). These experiments strongly suggest that the protective effects of ACE inhibitors are partly mediated by B2R signaling.
ARBs increase plasma bradykinin levels by 2-fold in hypertensive humans, possibly due to reduction in pulmonary ACE and neutral endopeptidase activities (36). This implies that the beneficial effects of ARB are partly mediated by the KKS via the activation of angiotensin II type 2 receptors (AT2R)(37-41). Angiotensin II stimulates AT2R in vascular smooth muscle cells treated with an ARB. This stimulation leads to intracellular acidosis, mediated by inhibition of amiloride-sensitive Na+/H+ exchanger activity, resulting in enhancement of kininogenase activity, which increases bradykinin release (41).
These observations in humans and in animals emphasize the important role of the KKS in the beneficial effects on diabetic nephropathy of treatment with ACE inhibitors or ARB.
Are patients with the ACE D allele more responsive to ACE inhibitors?
Numerous studies indicate that the II genotype of the ACE polymorphism has a protective effect on the development of diabetic nephropathy, whereas the DD genotype confers an increased risk (10,11). Therefore, it might be expected that diabetic patients having the D allele would have a greater response to treatment with ACE inhibitors than those having the I allele manifested as a greater reduction in microalbuminuria or other renal end point. However, as reviewed by Ruggenenti (42), it is uncertain whether the ACE polymorphism affects the therapeutic benefits of ACE inhibitor treatment. Thus, the EUCLID trial shows that lisinopril, an ACE inhibitor, reduced albuminuria in type 1 diabetic patients compared with placebo by 51.5% in the II genotype, 14.8% in the ID genotype, and 7.7% in the DD genotype, indicating that patients with the I allele have more favorable response to ACE inhibitor than those with the D allele (43). Other studies in type 1 diabetic patients treated with ACE inhibitors also show that the I allele is associated with better renal outcomes compared with the D allele (44,45). In addition, a study of Chinese type 2 diabetic patients treated with an ACE inhibitor demonstrates that the II and ID genotypes are associated with reduced risk of mortality and occurrence of composite renal end point, but not the DD genotype (46). In contrast to treatment with ACE inhibitors, the RENAAL study demonstrated that losartan, an ARB, has a reduced risk of the primary endpoints compared with placebo by 5.8% in the II genotype, 17.6% in the ID genotype, and 27.9% in the DD genotype, indicating the greatest renoprotective response to ARB treatment in the DD genotype (47). Although no reasonable explanations regarding the conflicting results associated with ACE polymorphism have been published, selection bias of the patients and several confounding factors, such as changes in antihypertensive drugs, blood pressure, and blood glucose levels, all can influence the clinical outcome of the study. Further studies are warranted.
Kinin receptors in diabetic nephropathy
Kinins are potent renal vasodilators, and exhibit antithrombotic and antifibrotic activities. The kinins exert their biological effects through the activation of G protein-coupled receptors with seven transmembrane domains, designated B1 and B2 receptors (B1R and B2R) (48,49). B2R is constitutively expressed in most tissues, including all segments of the kidney, and it mediates the majority of the physiological effects of kinins in health. In contrast, B1R is expressed at only low levels under physiological conditions, but its expression is induced by various pathological stimuli such as ischemia-reperfusion injury (50), proinflammatory cytokines (51,52), diabetes (53), bacterial endotoxins (54), and angiotensin II (55), implying an important role of B1R activation under pathological situations. The cytokine-induced upregulation of B1R is mainly regulated by mitogen-activated protein kinase (MAPK) and nuclear factor-kappa B (NF-κB) (51,56,57). Indeed, an NF-κB binding site is detected in the promoter region of human B1R gene (51,58).
It is well documented that expression of the B1R and B2R compensate each other under a variety of conditions (Table). Expression of B1R in the kidney and heart is enhanced in B2R-null mice (59,60). Similarly, B2R gene expression is enhanced in the same tissues in B1R-null mice (61,62). Thus, absence of one bradykinin receptor may be compensated by over-expression of the remaining receptor. Such a compensative gene expression pattern of both receptors makes it difficult to elucidate pathological function and role of each receptor in diseases. In support of this mutual compensation, mice lacking both B1R and B2R show a reduced inflammatory response in a capsaicin-induced cutaneous neurogenic inflammation model, whereas the response is normal in mice lacking either B1R or B2R alone (63).
Table.
Compensative expression of the remaining bradykinin receptor and the outcome on diabetic complications in kinin-related mouse models.
| Genotype | Induction of diabetes | Organ | Expression | Outcome | Ref | |
|---|---|---|---|---|---|---|
| B1R | B2R | |||||
| B1R-null | STZ | Heart | - | ↑ | Reduced cardiac inflammation and fibrosis compared with STZ-treated wild-type mice. | 61 |
| B2R-null | Akita | Kidney | ↑ | - | Enhanced albuminuria and glomerulosclerosis compared with Akita diabetic mice having both B1R and B2R. | 59 |
| B2R-null | STZ | Kidney | ↑ | - | Reduced albuminuria and glomerulosclerosis compared with STZ-treated wild-type mice. | 72 |
| B1RB2R-null | Akita | Kidney | - | - | Enhanced albuminuria and glomerulosclerosis compared with B2R-null Akita diabetic mice. | 76 |
| B1RB2R-null | Akita | Heart | - | - | No adverse effects on cardiac function and no evidence of increased cardiac fibrosis compared with Akita diabetic mice having both B1R and B2R. | 109 |
| TK-null | STZ | Kidney | ↑ | ↑ | Enhanced albuminuria compared with STZ-treated wild-type mice. | 64 |
B1R indicates bradykinin B1 receptor; B2R, bradykinin B2 receptor; B1RB2R, both B1R and B2R; TK, tissue kallikrein; STZ, streptozotocin; Akita, Akita diabetic mice; Ref, reference.
The expression levels of both B1R and B2R in the diabetic kidney are enhanced in STZ-treated C57BL-6J mice (64) and rats (65,66), and in Akita diabetic mice (59), which are a type 1 diabetic animal model having a C96Y dominant negative mutation in the Insulin 2 (Ins2) gene (67). This upregulation of both receptors may be due to a direct effect of hyperglycemia, as suggested by in vitro experiments using rat endothelial cells and vascular smooth muscle cells (68,69). Bradykinin levels but not angiotensin II levels are also increased in both the plasma and kidney of STZ-treated rats, when compared with non-STZ-treated rats (27). In humans, although no differences are seen in circulating levels of bradykinin and kallidin peptides between type 2 diabetic patients and non-diabetic patients, plasma levels of tissue kallikrein, which is a serine protease that converts kininogen to the active bradykinin, are increased by 62% in diabetic patients (70). The plasma angiotensin II levels are not different between those patient groups. These findings suggest that the KKS plays a more significant role in the pathogenesis of diabetes and diabetic nephropathy than the RAS.
We have recently reported that loss of B2R in 6-month-old male Akita diabetic mice (B2R-null-Akita) exacerbates many of the renal diabetic phenotypes, including albuminuria (4-fold increase compared to Akita mice alone), interstitial fibrosis, and glomerular basement membrane (GBM) thickening (59) and induces premature aging (71). The mice also have a substantial increase in B1R gene expression in the kidney, but this does not protect them from developing diabetic nephropathy. In contrast, Tan et al have reported that deletion of B2R is protective against albuminuria and increased glomerular mesangial area which develop in diabetic nephropathy induced by STZ (72). These conflicting results may be due to differences in the strains and ages of the mice used, in the method of induction of diabetes, and in the severity of hyperglycemia, as we discussed previously (73). In particular, Tan et al have shown slightly lower hyperglycemia and the resulting lower urine volume in the diabetic B2R-null mice than in the diabetic wild-type mice during the first 11 weeks. The difference in the severity of diabetes between the two genotypes may have affected the development of diabetic nephropathy.
Whether the markedly increased expression in B1R gene that occurs in the kidney of mice lacking B2R with or without diabetes is beneficial or deleterious is debatable (59,72,74). To unravel the possible role of B1R in diabetes, mice lacking both B1R and B2R (B1RB2R-null mice) were required. However, it is impractical to make this animal model by simply crossbreeding B1R-null mice and B2R-null mice, because the genomic loci coding for B1R and B2R are only 11 kb apart in chromosome 12 and there is a very low probability of recombination at such a small genetic distance. Since no predicted coding genes are identified between the loci for B1R and B2R, we deleted the genomic region coding the two receptors and were successful in generating mice lacking both receptors (50). Another group has generated B1RB2R-null mice by disrupting B1R using embryonic stem cells obtained from B2R-null mice and has shown that the response to kinins, as evaluated by contractility studies in smooth muscle cells, is lacking, indicating that signaling via B1R and B2R mediates most of the effects of the kinins (75).
Using the B1RB2R-null animal model, we have recently demonstrated that the pathological changes characteristic of Akita diabetes, including nephropathy, bone mineral loss, and neuropathy, are all enhanced in 12-month-old male B1RB2R-null-Akita mice (76), despite similar levels of hyperglycemia and blood pressure. Urinary albumin excretion, already increased 10-fold in the Akita diabetic mice compared with wild-type mice, was increased to 17-fold in the B2R-null-Akita mice and to 29-fold in the B1RB2R-null-Akita mice, and there is a strong positive interaction between the two mutations. The enhancement of other diabetic renal phenotypes including glomerulosclerosis (Figure 2), interstitial fibrosis (Figure 3), and GBM thickening (Figure 4) in Akita mice caused by absence of both receptors is also greater than in Akita mice lacking only B2R. Thus, these results establish that the increased expression of B1R induced in the B2R-null-Akita mice has beneficial effects. In agreement with our observations, adeno-associated virus-mediated expression of the human tissue kallikrein has been shown to attenuate the renal diabetic phenotypes induced by STZ and high-fat diet as assessed by urinary albumin excretion, histological changes, creatinine clearance, and urinary osmolarity (77). More recently, Bodin et al have shown that mice lacking tissue kallikrein have a 2-fold increase in albuminuria compared to the wild-type mice when type I diabetes is induced by STZ, despite similar hyperglycemia and blood pressure (64). All these observations indicate that KKS signaling via both B1R and B2R plays a protective role in the development of diabetic nephropathy and suggest that its activation could be a beneficial target for drugs to prevent diabetic complications (Figure 1).
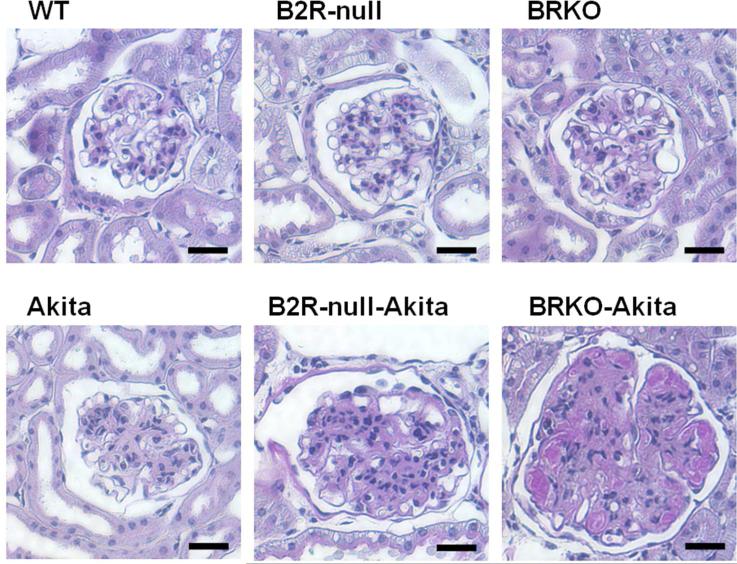
Figure 2.
Kidneys in 12-month-old male wild-type, B2R-null, B1RB2R-null, Akita, B2R-null-Akita, and B1RB2R-null-Akita mice stained with Periodic acid-Schiff (PAS) (76). Mesangial hypercellularity, accumulation of PAS-positive extracellular matrix, intra-arteriolar hyalinosis (insudation) in the renal glomerulus are observed in the Akita diabetic mice. The glomerular sclerosis caused by Akita diabetes are markedly enhanced by lack of B2R and more by lack of both receptors, even though histological changes in the non-diabetic B2R-null or B1RB2R-null mice are not remarkable at this age. Scale bar, 100 μm.
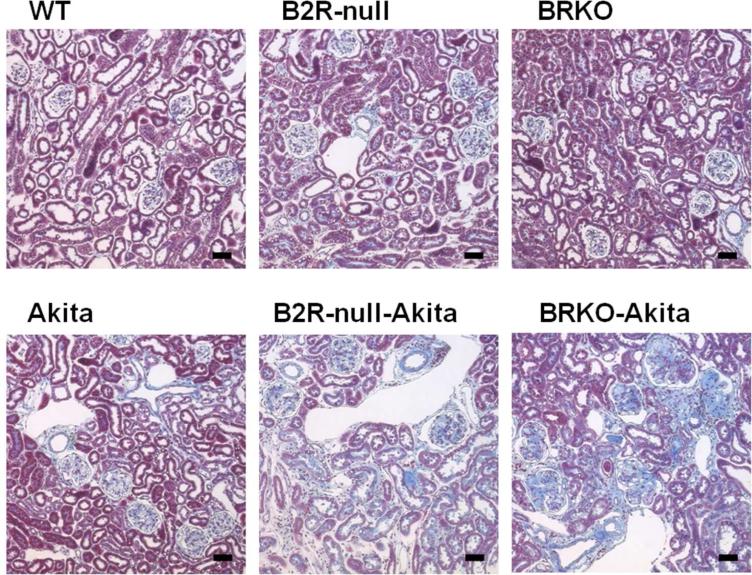
Figure 3.
Gomori's trichrome staining of renal cortex at the age of 12 months (76). The tubulointerstitial fibrosis observed in the Akita diabetic mice are markedly enhanced by lack of B2R and more by lack of both receptors. Scale bar, 100 μm.
Figure 4.
Electron micrographs of glomeruli at the age of 12 months (76). The thickening of the glomerular basement membrane (GBM) and foot process effacement of podocytes are observed in the Akita diabetes. The GBM thickening in the Akita diabetic mice is markedly enhanced by lack of B2R and more by lack of both receptors, although lack of the receptors in absence of diabetes has little or no effect. Scale bar, 1 μm.
The polymorphisms of bradykinin receptors
Four common polymorphisms in the gene for bradykinin receptors are described, one in the B1R promoter region (-699G/C), one in the B2R promoter region (-58C/T), one in the B2R exon 1 (+9/-9 bp insertion/deletion), and one in the B2R exon 2 (181C/T). In the +9/-9 genotype, the presence (+9) rather than absence (–9) of a 9 bp repeat sequence is associated with decreased B2R gene transcriptional activity and mRNA expression (78,79). In a mixed population of type 1 and type 2 diabetic patients, the +9 allele is associated with a lower urinary albumin-creatinine ratio (80). These findings suggest that reduced expression of the B2R gene has a protective effect against development of microalbuminuria. However, other association studies with this genotype show that the +9/+9 genotype is associated with the increased cardiovascular risk and systolic hypertension (81,82), implying a harmful effect of the +9/+9 genotype, which is the opposite direction against development of microalbuminuria. Further clinical studies associated with +9/-9 polymorphism on diabetic nephropathy are warranted in larger and separated populations of type 1 and type 2 diabetic patients.
Anti-fibrotic effect of the KKS
Glomerulosclerosis caused by accumulation of extracellular matrix proteins, including collagen types I, III, IV, and fibronectin in the mesangial interstitial space, plays a critical role in the development of diabetic nephropathy and in renal fibrosis (83,84). Mesangial cells are potential mediators of the glomerular damage and express both B1R and B2R, suggesting that the KKS is implicated in the glomerulosclerotic process (85-87).
A number of recent studies suggest a key role of B2R signaling in renal fibrosis in non-diabetic and diabetic settings. B2R activation exhibits an anti-proliferative effect in mesangial cells stimulated with several growth factors (87,88). In contrast, the B2R-null mice in the unilateral ureteral obstruction (UUO) model develop significantly more tubulointerstitial fibrosis than wild-type mice accompanied by decreased renal activity of plasminogen activator and matrix metalloproteinase 2 (89). Furthermore, benazepril, an ACE inhibitor, decreases plasminogen activator inhibitor-1 expression through B2R, thereby facilitating matrix degradation to reduce interstitial matrix deposition (90). Our observation that the glomerulosclerosis, tubulointerstitial fibrosis, and GBM thickening which develop in Akita diabetic mice are enhanced by lack of B2R supports a beneficial effect of B2R activation against diabetic renal fibrosis (59). The glomerulosclerosis developing in B2R-null-Akita mice is associated with increased megsin and nephrin expression. Megsin, a member of the serpin family of protease inhibitors, inhibits matrix protein degradation in the mesangium (91). Its expression is increased to approximately two times that of wild-type in the Akita diabetic mice and to approximately three times that of wild-type in the B2R-null Akita mice, which may be related to the extent of mesangial matrix expansion (59). Expression of nephrin, an essential component of the slit diaphragms of the glomerulus, is increased at early stages in STZ-induced diabetic rats (92), and nephrin expression is approximately 2 times wild-type in the Akita diabetic mice and approximately 4 times wild-type in B2R-null Akita mice (59). These studies indicate a protective effect of B2R activation in renal fibrosis.
Activation of B1R is associated with inflammatory response. Treatment with a B1R antagonist blocks macrophage infiltration and B1R-null mice have lower expression of pro-inflammatory molecules such as monocyte inflammatory protein 1 and interleukin-6, leading to a reduction of renal fibrosis in the UUO model (93,94). A B1R antagonist also reduces renal inflammation and focal and segmental glomerulosclerosis (FSGS) induced by an adriamycin (95). Furthermore, treatment with a B1R antagonist reduces renal chemokine expression and macrophage accumulation, and ameliorates the nephrotoxic serum-induced glomerulonephritis (96). These studies indicate a deleterious effect of B1R activation in renal fibrosis, despite the increase in B2R gene expression that occurs in either B1R blockade or deletion.
We have recently shown that the glomerulosclerosis, tubulointerstitial fibrosis, and GBM thickening which develop in Akita diabetic mice are more enhanced by lack of both B1R and B2R than by lack of B2R alone at the age of 12 months (Figures 2-4)(76). Expression of transforming growth factor β1 (TGFβ1), connective tissue growth factor (CTGF), and endothelin-1 (EDN1) is known to be increased in diabetic nephropathy (97-99). Furthermore, in vivo overexpression of TGFβ1, CTGF, or EDN1 is associated with the development of nephrosclerosis (100-102). The increased expression of these fibrogenic genes is likely to contribute to the enhanced glomerulosclerosis and fibrosis in the Akita mice lacking both B1R and B2R. These results show that the KKS signaling via both the B1R and B2R plays an important role in preventing renal fibrosis in diabetic nephropathy. In agreement with our study, bradykinin infusion prevents renal inflammation, apoptosis, and fibrosis via inhibition of oxidative stress and MAPK activity in high salt-induced renal lesion in rats (103), supportive of beneficial effect of the KKS.
In the heart, the protective effects of ACE inhibitors are partly abolished by B2R antagonists in ischemia-reperfusion injury (104,105) and by B2R gene deletion in chronic heart failure caused by coronary artery ligation (106). Lack of B2R has been reported by some investigators to cause cardiac hypertrophy and heart failure (107,108), although others have reported that lack of B2R does not affect cardiac function under normal physiological conditions (106). Lack of both B1R and B2R has no adverse effects on cardiac function evaluated by echocardiography and left-ventricular catheterization, and the mice lacking both receptors do not exhibit any evidence of increased fibrosis (109). On the other hand, deletion of B1R attenuates cardiac inflammation and fibrosis in STZ-induced diabetic mice, accompanied by an increase in B2R gene expression (61). This apparently beneficial effect of B1R deficiency disappears when accompanied by lack of B2R, since Akita diabetic mice lacking both receptors do not differ in histological changes and cardiac fibrosis compared with the Akita mice (109). These discordant findings in Akita diabetic mice lacking both B1R and B2R suggest the existence of distinct tissue-specific roles of kinin receptors in the pathogenesis of diabetic complications.
Role of nitric oxide in the protective effect of the KKS on diabetic nephropathy
Nitric oxide (NO) is synthesized enzymatically from L-arginine by NO synthase (NOS) in almost all mammalian cells (110,111). Three main NOS isoforms have been identified; neuronal NOS (nNOS or NOS1), inducible NOS (iNOS or NOS2), and endothelial NOS (eNOS or NOS3). All isoforms contain an N-terminal oxygenase domain and a C-terminal reductase domain, with a recognition sequence for the Ca2+ binding protein calmodulin located between the two domains (112). Since both nNOS and eNOS have a low affinity for Ca2+-calmodulin, they are activated by an elevation in intracellular Ca2+ levels (Ca2+-dependent isoforms). On the other hand, since iNOS binds calmodulin with a high affinity, its binding site is occupied at basal intracellular Ca2+ levels. Consequently, it is not further activated by an elevation in intracellular Ca2+ levels (Ca2+-independent isoform) (113-115).
Both the B1R and B2R are coupled with Gq- and Gi-proteins (49,116), and their stimulation activates phosphatidyl inositol-specific phospholipases, which elevates intracellular Ca2+ levels and activates the nNOS and eNOS (49,117,118). Expression of the Ca2+-independent isoform, iNOS, is also increased by bradykinin via both B1R (119) and B2R (120), which is dependent on intranuclear calcium and Akt signaling. The expression levels of iNOS gene are increased by lipopolysaccharide in wild-type mice, but this increase is diminished in mice lacking both B1R and B2R (75). Absence of B2R decreases the urinary excretion of stable metabolites of NO (NO2- and NO3-) (121), and lack of both B1R and B2R reduces fasting plasma NO2- / NO3- concentration more than that of B2R alone (50). These observations indicate that both the receptors play an important role in basal NO production.
Reduction of NO production is associated with the development of diabetic nephropathy. Consistent with this, a human polymorphism in intron 4 of the NOS3 gene has been associated with an increased risk for nephropathy in patients with either type 1 (122) or type 2 diabetes (123). L-arginine administration, the substrate of the NOS, decreases the proteinuria in STZ-induced diabetic rats (124). In contrast, L-NAME administration, a NOS inhibitor, aggravates the proteinuria and histological changes that occur in the diabetic nephropathy of Otsuka Long-Evans Tokushima Fatty rats (125). Lack of eNOS also accelerates the severity of diabetic nephropathy in C57BLKS/J db/db mice (126), lepr(db/db) mice (127), STZ-treated C57BL/6 mice (128), and Akita mice (129). In rats with STZ-induced diabetes, eNOS activity, assessed by endothelium-dependent vasodilation and NO release from the isolated kidney, are impaired, but administration of ACE inhibitors restores the eNOS function (130). Expression of iNOS is increased in the renal cortex of diabetic animals (131), but lack of iNOS increased mesangial hypercellularity and expansion as well as tubulointerstitial fibrosis in mice with STZ-induced diabetes (132). These findings suggest that the KKS exerts its beneficial effects on diabetic nephropathy at least in part via NO (Figure 1).
Role of oxidative stress in the effect of the KKS on diabetic nephropathy
The increased generation of reactive oxygen species (ROS) in the kidney is associated with the progression of diabetic nephropathy in humans and in animal models of diabetes (133-136). ROS, mostly generated through mitochondrial oxidative phosphorylation, stimulate several pathways involved in the pathogenesis of diabetic complications. These include the polyol pathway flux, formation of advanced glycation end products (AGEs), expression of the receptor for AGEs and its activating ligands, activation of protein kinase C isoforms, and overactivity of the hexosamine pathway. (137,138). Therefore, inhibition of ROS production has the potential of preventing ROS-induced kidney damage in diabetes. Consistent with this, overexpression of superoxide dismutase 1 attenuates glomerular injury and oxidative stress in STZ-induced diabetic mice (139) and in db/db mice (140). Oral administration of mitochondria-targeted antioxidants, MitoQ, for 12 weeks decreases urinary albumin levels and reduces renal interstitial fibrosis and glomerular damage in Akita diabetic mice (141).
Mitochondrial oxidative metabolism is reversibly inhibited by NO at the level of cytochrome c oxidase, a key enzyme in the electron transport chain (142-144). NO binds to this enzyme and inhibits its activity reversibly, thereby negatively regulating mitochondrial oxidative phosphorylation. Recent studies have shown that a cAMP-dependent pathway inhibits cytochrome c oxidase and activates the NADH-ubiquinone oxidoreductase activity of complex I, thereby decreasing mitochondrial respiration (145,146). Bradykinin stimulates eNOS activity in vascular endothelial cells and increases cAMP levels in kidney epithelial cells. Furthermore, the kinins facilitate the synthesis of prostanoids, including prostaglandin (PG) E2 and I2, which elevate intracellular cAMP levels (2). In contrast, ROS directly reduce both eNOS and prostacyclin synthase activity (137,138). Taken together, these findings suggest that the KKS may contribute to reducing oxidative stress via NO and PGs. In support of this possibility, we have shown that bradykinin reduces mitochondrial superoxide generation in human EA.hy926 vascular endothelial cells, an effect that is partly reversed by a NOS inhibitor (71). Protective effects of bradykinin against ROS-induced DNA damage and senescence in bovine aorta endothelial cells are also partly abolished by a NOS inhibitor (147). Furthermore, bradykinin infusion via implanted minipumps or tissue kallikrein gene delivery via tail-vein injection prevents high salt-induced renal injury in rats, being associated with increased renal NO levels and reduced ROS generation (103,148). Cicaprost, a PGI2 analog, also attenuates the progression of diabetic renal injury in STZ-treated rats (149). When bradykinin is administered to rats made hyperglycemic with STZ, it also reduces their oxidative stress phenotype as evaluated by hydrogen peroxide and malondialdehyde (MDA) levels (150). Moreover, the protective effects of ACE inhibitors against oxidative stress are attenuated by a B2R antagonist in STZ-treated rat assessed by serum MDA levels. Plasma levels of thiobarbituric acid-reactive substances (TBARS) are increased by 30% in B2R-null-Akita mice and further increased by 70% in B1RB2R-null-Akita mice compared with Akita mice alone (76). While further experimental data are needed, these findings support a protective role of bradykinin against ROS associated with diabetic nephropathy.
Conclusions
Many clinical studies and animal experiments have established the protective effects of ACE inhibitors on diabetic nephropathy. Accumulating evidence suggests that these beneficial effects, which are independent of changes in blood pressure and decreases in angiotensin II levels, are mediated in part by the KKS. Although whether non-selective stimulation of both B1R and B2R is more effective for the treatment of the diabetic nephropathy than B1R-specific or B2R-specific stimulation remains debatable; studies using mice lacking both B1R and B2R indicate that non-selective stimulation is likely to have the most therapeutic efficacy. The effects on diabetic complications of B1R and B2R stimulation may not be the same in all tissues. Clinical studies have given conflicting results on the differential efficacies of ACE inhibitors in diabetic patients with different ACE genotypes. Resolving these uncertainties requires further studies.
Acknowledgements
This work was supported by National Institutes of Health Grants DK76131 and HL49277, and by Career Development Award 2006-2-106 from Juvenile Diabetes Research Foundation.
Footnotes
Disclosure
None.
References
- 1.Skidgel RA, Engelbrecht S, Johnson AR, et al. Hydrolysis of substance p and neurotensin by converting enzyme and neutral endopeptidase. Peptides. 1984;5:769–776. doi: 10.1016/0196-9781(84)90020-2. [DOI] [PubMed] [Google Scholar]
- 2.Kakoki M, Smithies O. The kallikrein-kinin system in health and in diseases of the kidney. Kidney Int. 2009;75:1019–1030. doi: 10.1038/ki.2008.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaspard E, Wei L, Alhenc-Gelas F. Differences in the properties and enzymatic specificities of the two active sites of angiotensin I-converting enzyme (kininase II). Studies with bradykinin and other natural peptides. J Biol Chem. 1993;268:9496–9503. [PubMed] [Google Scholar]
- 4.Ishida H, Scicli AG, Carretero OA. Role of angiotensin converting enzyme and other peptidases in in vivo metabolism of kinins. Hypertension. 1989;14:322–327. doi: 10.1161/01.hyp.14.3.322. [DOI] [PubMed] [Google Scholar]
- 5.Smithies O, Kim HS, Takahashi N, et al. Importance of quantitative genetic variations in the etiology of hypertension. Kidney Int. 2000;58:2265–2280. doi: 10.1046/j.1523-1755.2000.00411.x. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi N, Hagaman JR, Kim HS, et al. Minireview: Computer simulations of blood pressure regulation by the renin-angiotensin system. Endocrinology. 2003;144:2184–2190. doi: 10.1210/en.2002-221045. [DOI] [PubMed] [Google Scholar]
- 7.Campbell DJ, Kladis A, Duncan AM. Effects of converting enzyme inhibitors on angiotensin and bradykinin peptides. Hypertension. 1994;23:439–449. doi: 10.1161/01.hyp.23.4.439. [DOI] [PubMed] [Google Scholar]
- 8.Rigat B, Hubert C, Alhenc-Gelas F, et al. An insertion-deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest. 1990;86:1343–1346. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lachurie ML, Azizi M, Guyene TT, et al. Angiotensin-converting enzyme gene polymorphism has no influence on the circulating renin-angiotensin-aldosterone system or blood pressure in normotensive subjects. Circulation. 1995;91:2933–2942. doi: 10.1161/01.cir.91.12.2933. [DOI] [PubMed] [Google Scholar]
- 10.Marre M, Bernadet P, Gallois Y, et al. Relationships between angiotensin I converting enzyme gene polymorphism, plasma levels, and diabetic retinal and renal complications. Diabetes. 1994;43:384–388. doi: 10.2337/diab.43.3.384. [DOI] [PubMed] [Google Scholar]
- 11.Tarnow L, Gluud C, Parving HH. Diabetic nephropathy and the insertion-deletion polymorphism of the angiotensin-converting enzyme gene. Nephrol Dial Transplant. 1998;13:1125–1130. doi: 10.1093/ndt/13.5.1125. [DOI] [PubMed] [Google Scholar]
- 12.Stephens JW, Dhamrait SS, Acharya J, et al. A common variant in the ACE gene is associated with peripheral neuropathy in women with type 2 diabetes mellitus. J Diabetes Complications. 2006;20:317–321. doi: 10.1016/j.jdiacomp.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Rabensteiner D, Abrahamian H, Irsigler K, et al. ACE gene polymorphism and proliferative retinopathy in type 1 diabetes: results of a case-control study. Diabetes Care. 1999;22:1530–1535. doi: 10.2337/diacare.22.9.1530. [DOI] [PubMed] [Google Scholar]
- 14.Cambien F, Poirier O, Lecerf L, et al. Deletion polymorphism in the gene for angiotensin-converting enzyme is a potent risk factor for myocardial infarction. Nature. 1992;359:641–644. doi: 10.1038/359641a0. [DOI] [PubMed] [Google Scholar]
- 15.Doi Y, Yoshinari M, Yoshizumi H, et al. Polymorphism of the angiotensin-converting enzyme (ACE) gene in patients with thrombotic brain infarction. Atherosclerosis. 1997;132:145–150. doi: 10.1016/s0021-9150(97)00080-4. [DOI] [PubMed] [Google Scholar]
- 16.Murphey LJ, Gainer JV, Vaughan DE, et al. Angiotensin-converting enzyme insertion-deletion polymorphism modulates the human in vivo metabolism of bradykinin. Circulation. 2000;102:829–832. doi: 10.1161/01.cir.102.8.829. [DOI] [PubMed] [Google Scholar]
- 17.Almeida SS, Barros CC, Moraes MR, et al. Plasma kallikrein and angiotensin I-converting enzyme N- and C-terminal domain activities are modulated by the insertion/deletion polymorphism. Neuropeptides. 2010;44:139–143. doi: 10.1016/j.npep.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Krege JH, Kim HS, Moyer JS, et al. Angiotensin-converting enzyme gene mutations, blood pressures, and cardiovascular homeostasis. Hypertension. 1997;29:150–157. doi: 10.1161/01.hyp.29.1.150. [DOI] [PubMed] [Google Scholar]
- 19.Huang W, Gallois Y, Bouby N, et al. Genetically increased angiotensin I-converting enzyme level and renal complications in the diabetic mouse. Proc Natl Acad Sci U S A. 2001;98:13330–13334. doi: 10.1073/pnas.231476798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis EJ, Hunsicker LG, Bain RP, et al. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 21.Ruggenenti P, Fassi A, Ilieva AP, et al. Preventing microalbuminuria in type 2 diabetes. N Engl J Med. 2004;351:1941–1951. doi: 10.1056/NEJMoa042167. [DOI] [PubMed] [Google Scholar]
- 22.Ruggenenti P, Cravedi P, Remuzzi G. The RAAS in the pathogenesis and treatment of diabetic nephropathy. Nat Rev Nephrol. 2010;6:319–330. doi: 10.1038/nrneph.2010.58. [DOI] [PubMed] [Google Scholar]
- 23.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 24.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 25.Carey RM, Siragy HM. The intrarenal renin-angiotensin system and diabetic nephropathy. Trends Endocrinol Metab. 2003;14:274–281. doi: 10.1016/s1043-2760(03)00111-5. [DOI] [PubMed] [Google Scholar]
- 26.Navar LG. The intrarenal renin-angiotensin system in hypertension. Kidney Int. 2004;65:1522–1532. doi: 10.1111/j.1523-1755.2004.00539.x. [DOI] [PubMed] [Google Scholar]
- 27.Campbell DJ, Kelly DJ, Wilkinson-Berka JL, et al. Increased bradykinin and “normal” angiotensin peptide levels in diabetic Sprague-Dawley and transgenic (mRen-2)27 rats. Kidney Int. 1999;56:211–221. doi: 10.1046/j.1523-1755.1999.00519.x. [DOI] [PubMed] [Google Scholar]
- 28.Hollenberg NK, Price DA, Fisher ND, et al. Glomerular hemodynamics and the renin-angiotensin system in patients with type 1 diabetes mellitus. Kidney Int. 2003;63:172–178. doi: 10.1046/j.1523-1755.2003.00701.x. [DOI] [PubMed] [Google Scholar]
- 29.Kobori H, Nangaku M, Navar LG, et al. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 30.Duncan AM, Kladis A, Jennings GL, et al. Kinins in humans. Am J Physiol Regul Integr Comp Physiol. 2000;278:R897–904. doi: 10.1152/ajpregu.2000.278.4.R897. [DOI] [PubMed] [Google Scholar]
- 31.Zeitz CJ, Campbell DJ, Horowitz JD. Myocardial uptake and biochemical and hemodynamic effects of ACE inhibitors in humans. Hypertension. 2003;41:482–487. doi: 10.1161/01.HYP.0000054976.67487.08. [DOI] [PubMed] [Google Scholar]
- 32.Tschope C, Seidl U, Reinecke A, et al. Kinins are involved in the antiproteinuric effect of angiotensin-converting enzyme inhibition in experimental diabetic nephropathy. Int Immunopharmacol. 2003;3:335–344. doi: 10.1016/S1567-5769(02)00273-4. [DOI] [PubMed] [Google Scholar]
- 33.Allard J, Buleon M, Cellier E, et al. ACE inhibitor reduces growth factor receptor expression and signaling but also albuminuria through B2-kinin glomerular receptor activation in diabetic rats. Am J Physiol Renal Physiol. 2007;293:F1083–1092. doi: 10.1152/ajprenal.00401.2006. [DOI] [PubMed] [Google Scholar]
- 34.Schafer S, Schmidts HL, Bleich M, et al. Nephroprotection in zucker diabetic fatty rats by vasopeptidase inhibition is partly bradykinin B2 receptor dependent. Br J Pharmacol. 2004;143:27–32. doi: 10.1038/sj.bjp.0705884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buleon M, Allard J, Jaafar A, et al. Pharmacological blockade of B2-kinin receptor reduces renal protective effect of angiotensin-converting enzyme inhibition in db/db mice model. Am J Physiol Renal Physiol. 2008;294:F1249–1256. doi: 10.1152/ajprenal.00501.2007. [DOI] [PubMed] [Google Scholar]
- 36.Campbell DJ, Krum H, Esler MD. Losartan increases bradykinin levels in hypertensive humans. Circulation. 2005;111:315–320. doi: 10.1161/01.CIR.0000153269.07762.3B. [DOI] [PubMed] [Google Scholar]
- 37.Liu YH, Yang XP, Sharov VG, et al. Effects of angiotensin-converting enzyme inhibitors and angiotensin II type 1 receptor antagonists in rats with heart failure. Role of kinins and angiotensin II type 2 receptors. J Clin Invest. 1997;99:1926–1935. doi: 10.1172/JCI119360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siragy HM, Carey RM. The subtype 2 (AT2) angiotensin receptor mediates renal production of nitric oxide in conscious rats. J Clin Invest. 1997;100:264–269. doi: 10.1172/JCI119531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Batenburg WW, Garrelds IM, Bernasconi CC, et al. Angiotensin II type 2 receptor-mediated vasodilation in human coronary microarteries. Circulation. 2004;109:2296–2301. doi: 10.1161/01.CIR.0000128696.12245.57. [DOI] [PubMed] [Google Scholar]
- 40.Hornig B, Kohler C, Schlink D, et al. AT1-receptor antagonism improves endothelial function in coronary artery disease by a bradykinin/B2-receptor-dependent mechanism. Hypertension. 2003;41:1092–1095. doi: 10.1161/01.HYP.0000064942.77814.26. [DOI] [PubMed] [Google Scholar]
- 41.Tsutsumi Y, Matsubara H, Masaki H, et al. Angiotensin II type 2 receptor overexpression activates the vascular kinin system and causes vasodilation. J Clin Invest. 1999;104:925–935. doi: 10.1172/JCI7886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruggenenti P, Bettinaglio P, Pinares F, et al. Angiotensin converting enzyme insertion/deletion polymorphism and renoprotection in diabetic and nondiabetic nephropathies. Clin J Am Soc Nephrol. 2008;3:1511–1525. doi: 10.2215/CJN.04140907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Penno G, Chaturvedi N, Talmud PJ, et al. Effect of angiotensin-converting enzyme (ACE) gene polymorphism on progression of renal disease and the influence of ACE inhibition in IDDM patients: Findings from the EUCLID Randomized Controlled Trial. EURODIAB Controlled Trial of Lisinopril in IDDM. Diabetes. 1998;47:1507–1511. doi: 10.2337/diabetes.47.9.1507. [DOI] [PubMed] [Google Scholar]
- 44.Jacobsen P, Rossing K, Rossing P, et al. Angiotensin converting enzyme gene polymorphism and ACE inhibition in diabetic nephropathy. Kidney Int. 1998;53:1002–1006. doi: 10.1111/j.1523-1755.1998.00847.x. [DOI] [PubMed] [Google Scholar]
- 45.Jacobsen P, Tarnow L, Carstensen B, et al. Genetic variation in the renin-angiotensin system and progression of diabetic nephropathy. J Am Soc Nephrol. 2003;14:2843–2850. doi: 10.1097/01.asn.0000092139.19587.51. [DOI] [PubMed] [Google Scholar]
- 46.So WY, Ma RC, Ozaki R, et al. Angiotensin-converting enzyme (ACE) inhibition in type 2, diabetic patients- interaction with ace insertion/deletion polymorphism. Kidney Int. 2006;69:1438–1443. doi: 10.1038/sj.ki.5000097. [DOI] [PubMed] [Google Scholar]
- 47.Parving HH, de Zeeuw D, Cooper ME, et al. ACE gene polymorphism and losartan treatment in type 2 diabetic patients with nephropathy. J Am Soc Nephrol. 2008;19:771–779. doi: 10.1681/ASN.2007050582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Regoli D, Rizzi A, Perron SI, et al. Classification of kinin receptors. Biol Chem. 2001;382:31–35. doi: 10.1515/BC.2001.005. [DOI] [PubMed] [Google Scholar]
- 49.Leeb-Lundberg LM, Marceau F, Muller-Esterl W, et al. International union of pharmacology. XLV. Classification of the kinin receptor family: From molecular mechanisms to pathophysiological consequences. Pharmacol Rev. 2005;57:27–77. doi: 10.1124/pr.57.1.2. [DOI] [PubMed] [Google Scholar]
- 50.Kakoki M, McGarrah RW, Kim HS, et al. Bradykinin B1 and B2 receptors both have protective roles in renal ischemia/reperfusion injury. Proc Natl Acad Sci U S A. 2007;104:7576–7581. doi: 10.1073/pnas.0701617104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ni A, Chao L, Chao J. Transcription factor nuclear factor kappaB regulates the inducible expression of the human B1 receptor gene in inflammation. J Biol Chem. 1998;273:2784–2791. doi: 10.1074/jbc.273.5.2784. [DOI] [PubMed] [Google Scholar]
- 52.Marceau F, Hess JF, Bachvarov DR. The B1 receptors for kinins. Pharmacol Rev. 1998;50:357–386. [PubMed] [Google Scholar]
- 53.Spillmann F, Altmann C, Scheeler M, et al. Regulation of cardiac bradykinin B1- and B2-receptor mRNA in experimental ischemic, diabetic, and pressure-overload-induced cardiomyopathy. Int Immunopharmacol. 2002;2:1823–1832. doi: 10.1016/s1567-5769(02)00174-1. [DOI] [PubMed] [Google Scholar]
- 54.Schremmer-Danninger E, Offner A, Siebeck M, et al. B1 bradykinin receptors and carboxypeptidase M are both upregulated in the aorta of pigs after LPS infusion. Biochem Biophys Res Commun. 1998;243:246–252. doi: 10.1006/bbrc.1997.7999. [DOI] [PubMed] [Google Scholar]
- 55.Morand-Contant M, Anand-Srivastava MB, Couture R. Kinin B1 receptor upregulation by angiotensin II and endothelin-1 in rat vascular smooth muscle cells: Receptors and mechanisms. Am J Physiol Heart Circ Physiol. 2010;299:H1625–1632. doi: 10.1152/ajpheart.00735.2009. [DOI] [PubMed] [Google Scholar]
- 56.Larrivee JF, Bachvarov DR, Houle F, et al. Role of the mitogen-activated protein kinases in the expression of the kinin B1 receptors induced by tissue injury. J Immunol. 1998;160:1419–1426. [PubMed] [Google Scholar]
- 57.Schanstra JP, Bataille E, Marin Castano ME, et al. The B1-agonist [des-Arg10]-kallidin activates transcription factor NF-kappaB and induces homologous upregulation of the bradykinin B1-receptor in cultured human lung fibroblasts. J Clin Invest. 1998;101:2080–2091. doi: 10.1172/JCI1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bachvarov DR, Hess JF, Menke JG, et al. Structure and genomic organization of the human B1 receptor gene for kinins (BDKRB1). Genomics. 1996;33:374–381. doi: 10.1006/geno.1996.0213. [DOI] [PubMed] [Google Scholar]
- 59.Kakoki M, Takahashi N, Jennette JC, et al. Diabetic nephropathy is markedly enhanced in mice lacking the bradykinin B2 receptor. Proc Natl Acad Sci U S A. 2004;101:13302–13305. doi: 10.1073/pnas.0405449101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Duka I, Kintsurashvili E, Gavras I, et al. Vasoactive potential of the B1 bradykinin receptor in normotension and hypertension. Circ Res. 2001;88:275–281. doi: 10.1161/01.res.88.3.275. [DOI] [PubMed] [Google Scholar]
- 61.Westermann D, Walther T, Savvatis K, et al. Gene deletion of the kinin receptor B1 attenuates cardiac inflammation and fibrosis during the development of experimental diabetic cardiomyopathy. Diabetes. 2009;58:1373–1381. doi: 10.2337/db08-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seguin T, Buleon M, Destrube M, et al. Hemodynamic and renal involvement of B1 and B2 kinin receptors during the acute phase of endotoxin shock in mice. Int Immunopharmacol. 2008;8:217–221. doi: 10.1016/j.intimp.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 63.Pietrovski EF, Otuki MF, Regoli D, et al. The non-peptide kinin receptor antagonists FR 173657 and SSR 240612: Preclinical evidence for the treatment of skin inflammation. Regul Pept. 2009;152:67–72. doi: 10.1016/j.regpep.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 64.Bodin S, Chollet C, Goncalves-Mendes N, et al. Kallikrein protects against microalbuminuria in experimental type 1 diabetes. Kidney Int. 2009;76:395–403. doi: 10.1038/ki.2009.208. [DOI] [PubMed] [Google Scholar]
- 65.Christopher J, Jaffa AA. Diabetes modulates the expression of glomerular kinin receptors. Int Immunopharmacol. 2002;2:1771–1779. doi: 10.1016/s1567-5769(02)00188-1. [DOI] [PubMed] [Google Scholar]
- 66.Mage M, Pecher C, Neau E, et al. Induction of B1 receptors in streptozotocin diabetic rats: possible involvement in the control of hyperglycemia-induced glomerular Erk 1 and 2 phosphorylation. Can J Physiol Pharmacol. 2002;80:328–333. doi: 10.1139/y02-024. [DOI] [PubMed] [Google Scholar]
- 67.Yoshioka M, Kayo T, Ikeda T, et al. A novel locus, Mody4, distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes. 1997;46:887–894. doi: 10.2337/diab.46.5.887. [DOI] [PubMed] [Google Scholar]
- 68.Rodriguez AI, Pereira-Flores K, Hernandez-Salinas R, et al. High glucose increases B1-kinin receptor expression and signaling in endothelial cells. Biochem Biophys Res Commun. 2006;345:652–659. doi: 10.1016/j.bbrc.2006.04.127. [DOI] [PubMed] [Google Scholar]
- 69.Christopher J, Velarde V, Zhang D, et al. Regulation of B(2)-kinin receptors by glucose in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2001;280:H1537–1546. doi: 10.1152/ajpheart.2001.280.4.H1537. [DOI] [PubMed] [Google Scholar]
- 70.Campbell DJ, Kladis A, Zhang Y, et al. Increased tissue kallikrein levels in type 2 diabetes. Diabetologia. 2010;53:779–785. doi: 10.1007/s00125-009-1645-8. [DOI] [PubMed] [Google Scholar]
- 71.Kakoki M, Kizer CM, Yi X, et al. Senescence-associated phenotypes in Akita diabetic mice are enhanced by absence of bradykinin B2 receptors. J Clin Invest. 2006;116:1302–1309. doi: 10.1172/JCI26958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tan Y, Keum JS, Wang B, et al. Targeted deletion of B2-kinin receptors protects against the development of diabetic nephropathy. Am J Physiol Renal Physiol. 2007;293:F1026–1035. doi: 10.1152/ajprenal.00203.2007. [DOI] [PubMed] [Google Scholar]
- 73.Brosius FC, III, Alpers CE, Bottinger EP, et al. Mouse models of diabetic nephropathy. J Am Soc Nephrol. 2009;20:2503–2512. doi: 10.1681/ASN.2009070721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lagneux C, Bader M, Pesquero JB, et al. Detrimental implication of B1 receptors in myocardial ischemia: evidence from pharmacological blockade and gene knockout mice. Int Immunopharmacol. 2002;2:815–822. doi: 10.1016/s1567-5769(02)00022-x. [DOI] [PubMed] [Google Scholar]
- 75.Cayla C, Todiras M, Iliescu R, et al. Mice deficient for both kinin receptors are normotensive and protected from endotoxin-induced hypotension. FASEB J. 2007;21:1689–1698. doi: 10.1096/fj.06-7175com. [DOI] [PubMed] [Google Scholar]
- 76.Kakoki M, Sullivan KA, Backus C, et al. Lack of both bradykinin B1 and B2 receptors enhances nephropathy, neuropathy, and bone mineral loss in Akita diabetic mice. Proc Natl Acad Sci U S A. 2010;107:10190–10195. doi: 10.1073/pnas.1005144107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yuan G, Deng J, Wang T, et al. Tissue kallikrein reverses insulin resistance and attenuates nephropathy in diabetic rats by activation of phosphatidylinositol 3-kinase-protein kinase B and adenosine 5'-monophosphate-activated protein kinase signaling pathways. Endocrinology. 2007;148:2016–2026. doi: 10.1210/en.2006-0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Braun A, Kammerer S, Maier E, et al. Polymorphisms in the gene for the human B2-bradykinin receptor. New tools in assessing a genetic risk for bradykinin-associated diseases. Immunopharmacology. 1996;33:32–35. doi: 10.1016/0162-3109(96)00079-3. [DOI] [PubMed] [Google Scholar]
- 79.Lung CC, Chan EK, Zuraw BL. Analysis of an exon 1 polymorphism of the B2 bradykinin receptor gene and its transcript in normal subjects and patients with C1 inhibitor deficiency. J Allergy Clin Immunol. 1997;99:134–146. doi: 10.1016/s0091-6749(97)70310-5. [DOI] [PubMed] [Google Scholar]
- 80.Maltais I, Bachvarova M, Maheux P, et al. Bradykinin B2 receptor gene polymorphism is associated with altered urinary albumin-creatinine values in diabetic patients. Can J Physiol Pharmacol. 2002;80:323–327. doi: 10.1139/y02-036. [DOI] [PubMed] [Google Scholar]
- 81.Dhamrait SS, Payne JR, Li P, et al. Variation in bradykinin receptor genes increases the cardiovascular risk associated with hypertension. Eur Heart J. 2003;24:1672–1680. doi: 10.1016/s0195-668x(03)00441-x. [DOI] [PubMed] [Google Scholar]
- 82.Van Guilder GP, Pretorius M, Luther JM, et al. Bradykinin type 2 receptor BE1 genotype influences bradykinin-dependent vasodilation during angiotensin-converting enzyme inhibition. Hypertension. 2008;51:454–459. doi: 10.1161/HYPERTENSIONAHA.107.102574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mason RM, Wahab NA. Extracellular matrix metabolism in diabetic nephropathy. J Am Soc Nephrol. 2003;14:1358–1373. doi: 10.1097/01.asn.0000065640.77499.d7. [DOI] [PubMed] [Google Scholar]
- 84.Qian Y, Feldman E, Pennathur S, et al. From fibrosis to sclerosis: mechanisms of glomerulosclerosis in diabetic nephropathy. Diabetes. 2008;57:1439–1445. doi: 10.2337/db08-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bascands JL, Pecher C, Rouaud S, et al. Evidence for existence of two distinct bradykinin receptors on rat mesangial cells. Am J Physiol. 1993;264:F548–556. doi: 10.1152/ajprenal.1993.264.3.F548. [DOI] [PubMed] [Google Scholar]
- 86.Bascands JL, Pecher C, Bompart G, et al. Bradykinin-induced in vitro contraction of rat mesangial cells via a B2 receptor type. Am J Physiol. 1994;267:F871–878. doi: 10.1152/ajprenal.1994.267.5.F871. [DOI] [PubMed] [Google Scholar]
- 87.Duchene J, Schanstra JP, Pecher C, et al. A novel protein-protein interaction between a G protein-coupled receptor and the phosphatase SHP-2 is involved in bradykinin-induced inhibition of cell proliferation. J Biol Chem. 2002;277:40375–40383. doi: 10.1074/jbc.M202744200. [DOI] [PubMed] [Google Scholar]
- 88.Alric C, Pecher C, Cellier E, et al. Inhibition of IGF-I-induced Erk 1 and 2 activation and mitogenesis in mesangial cells by bradykinin. Kidney Int. 2002;62:412–421. doi: 10.1046/j.1523-1755.2002.00475.x. [DOI] [PubMed] [Google Scholar]
- 89.Schanstra JP, Neau E, Drogoz P, et al. In vivo bradykinin B2 receptor activation reduces renal fibrosis. J Clin Invest. 2002;110:371–379. doi: 10.1172/JCI15493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Okada H, Watanabe Y, Kikuta T, et al. Bradykinin decreases plasminogen activator inhibitor-1 expression and facilitates matrix degradation in the renal tubulointerstitium under angiotensin-converting enzyme blockade. J Am Soc Nephrol. 2004;15:2404–2413. doi: 10.1097/01.ASN.0000136132.20189.95. [DOI] [PubMed] [Google Scholar]
- 91.Suzuki D, Miyata T, Nangaku M, et al. Expression of megsin mRNA, a novel mesangium-predominant gene, in the renal tissues of various glomerular diseases. J Am Soc Nephrol. 1999;10:2606–2613. doi: 10.1681/ASN.V10122606. [DOI] [PubMed] [Google Scholar]
- 92.Aaltonen P, Luimula P, Astrom E, et al. Changes in the expression of nephrin gene and protein in experimental diabetic nephropathy. Lab Invest. 2001;81:1185–1190. doi: 10.1038/labinvest.3780332. [DOI] [PubMed] [Google Scholar]
- 93.Klein J, Gonzalez J, Duchene J, et al. Delayed blockade of the kinin B1 receptor reduces renal inflammation and fibrosis in obstructive nephropathy. FASEB J. 2009;23:134–142. doi: 10.1096/fj.08-115600. [DOI] [PubMed] [Google Scholar]
- 94.Wang PH, Cenedeze MA, Campanholle G, et al. Deletion of bradykinin B1 receptor reduces renal fibrosis. Int Immunopharmacol. 2009;9:653–657. doi: 10.1016/j.intimp.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 95.Pereira RL, Buscariollo BN, Correa-Costa M, et al. Bradykinin receptor 1 activation exacerbates experimental focal and segmental glomerulosclerosis. Kidney Int. 2011;79:1217–1227. doi: 10.1038/ki.2011.14. [DOI] [PubMed] [Google Scholar]
- 96.Klein J, Gonzalez J, Decramer S, et al. Blockade of the kinin B1 receptor ameloriates glomerulonephritis. J Am Soc Nephrol. 2010;21:1157–1164. doi: 10.1681/ASN.2009090887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Riser BL, Denichilo M, Cortes P, et al. Regulation of connective tissue growth factor activity in cultured rat mesangial cells and its expression in experimental diabetic glomerulosclerosis. J Am Soc Nephrol. 2000;11:25–38. doi: 10.1681/ASN.V11125. [DOI] [PubMed] [Google Scholar]
- 98.Yamamoto T, Nakamura T, Noble NA, et al. Expression of transforming growth factor beta is elevated in human and experimental diabetic nephropathy. Proc Natl Acad Sci U S A. 1993;90:1814–1818. doi: 10.1073/pnas.90.5.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Minchenko AG, Stevens MJ, White L, et al. Diabetes-induced overexpression of endothelin-1 and endothelin receptors in the rat renal cortex is mediated via poly(ADP-ribose) polymerase activation. FASEB J. 2003;17:1514–1516. doi: 10.1096/fj.03-0013fje. [DOI] [PubMed] [Google Scholar]
- 100.Sanderson N, Factor V, Nagy P, et al. Hepatic expression of mature transforming growth factor beta 1 in transgenic mice results in multiple tissue lesions. Proc Natl Acad Sci U S A. 1995;92:2572–2576. doi: 10.1073/pnas.92.7.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yokoi H, Mukoyama M, Mori K, et al. Overexpression of connective tissue growth factor in podocytes worsens diabetic nephropathy in mice. Kidney Int. 2008;73:446–455. doi: 10.1038/sj.ki.5002722. [DOI] [PubMed] [Google Scholar]
- 102.Hocher B, Thone-Reineke C, Rohmeiss P, et al. Endothelin-1 transgenic mice develop glomerulosclerosis, interstitial fibrosis, and renal cysts but not hypertension. J Clin Invest. 1997;99:1380–1389. doi: 10.1172/JCI119297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chao J, Li HJ, Yao YY, et al. Kinin infusion prevents renal inflammation, apoptosis, and fibrosis via inhibition of oxidative stress and mitogen-activated protein kinase activity. Hypertension. 2007;49:490–497. doi: 10.1161/01.HYP.0000255925.01707.eb. [DOI] [PubMed] [Google Scholar]
- 104.Hartman JC, Wall TM, Hullinger TG, et al. Reduction of myocardial infarct size in rabbits by ramiprilat: reversal by the bradykinin antagonist HOE 140. J Cardiovasc Pharmacol. 1993;21:996–1003. doi: 10.1097/00005344-199306000-00022. [DOI] [PubMed] [Google Scholar]
- 105.Liu YH, Yang XP, Sharov VG, et al. Paracrine systems in the cardioprotective effect of angiotensin-converting enzyme inhibitors on myocardial ischemia/reperfusion injury in rats. Hypertension. 1996;27:7–13. doi: 10.1161/01.hyp.27.1.7. [DOI] [PubMed] [Google Scholar]
- 106.Yang XP, Liu YH, Mehta D, et al. Diminished cardioprotective response to inhibition of angiotensin-converting enzyme and angiotensin II type 1 receptor in B2 kinin receptor gene knockout mice. Circ Res. 2001;88:1072–1079. doi: 10.1161/hh1001.090759. [DOI] [PubMed] [Google Scholar]
- 107.Emanueli C, Maestri R, Corradi D, et al. Dilated and failing cardiomyopathy in bradykinin B2 receptor knockout mice. Circulation. 1999;100:2359–2365. doi: 10.1161/01.cir.100.23.2359. [DOI] [PubMed] [Google Scholar]
- 108.Maestri R, Milia AF, Salis MB, et al. Cardiac hypertrophy and microvascular deficit in kinin B2 receptor knockout mice. Hypertension. 2003;41:1151–1155. doi: 10.1161/01.HYP.0000064180.55222.DF. [DOI] [PubMed] [Google Scholar]
- 109.Wende AR, Soto J, Olsen CD, et al. Loss of bradykinin signaling does not accelerate the development of cardiac dysfunction in type 1 diabetic Akita mice. Endocrinology. 2010;151:3536–3542. doi: 10.1210/en.2010-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 111.Ignarro LJ, Cirino G, Casini A, et al. Nitric oxide as a signaling molecule in the vascular system: an overview. J Cardiovasc Pharmacol. 1999;34:879–886. doi: 10.1097/00005344-199912000-00016. [DOI] [PubMed] [Google Scholar]
- 112.Stuehr DJ. Mammalian nitric oxide synthases. Biochim Biophys Acta. 1999;1411:217–230. doi: 10.1016/s0005-2728(99)00016-x. [DOI] [PubMed] [Google Scholar]
- 113.Venema RC, Sayegh HS, Kent JD, et al. Identification, characterization, and comparison of the calmodulin-binding domains of the endothelial and inducible nitric oxide synthases. J Biol Chem. 1996;271:6435–6440. doi: 10.1074/jbc.271.11.6435. [DOI] [PubMed] [Google Scholar]
- 114.Andrew PJ, Mayer B. Enzymatic function of nitric oxide synthases. Cardiovasc Res. 1999;43:521–531. doi: 10.1016/s0008-6363(99)00115-7. [DOI] [PubMed] [Google Scholar]
- 115.Kuhr F, Lowry J, Zhang Y, et al. Differential regulation of inducible and endothelial nitric oxide synthase by kinin B1 and B2 receptors. Neuropeptides. 2010;44:145–154. doi: 10.1016/j.npep.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Couture R, Girolami JP. Putative roles of kinin receptors in the therapeutic effects of angiotensin 1-converting enzyme inhibitors in diabetes mellitus. Eur J Pharmacol. 2004;500:467–485. doi: 10.1016/j.ejphar.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 117.Drummond GR, Cocks TM. Endothelium-dependent relaxations mediated by inducible B1 and constitutive B2 kinin receptors in the bovine isolated coronary artery. Br J Pharmacol. 1995;116:2473–2481. doi: 10.1111/j.1476-5381.1995.tb15098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sangsree S, Brovkovych V, Minshall RD, et al. Kininase I-type carboxypeptidases enhance nitric oxide production in endothelial cells by generating bradykinin B1 receptor agonists. Am J Physiol Heart Circ Physiol. 2003;284:H1959–1968. doi: 10.1152/ajpheart.00036.2003. [DOI] [PubMed] [Google Scholar]
- 119.Ignjatovic T, Stanisavljevic S, Brovkovych V, et al. Kinin B1 receptors stimulate nitric oxide production in endothelial cells: signaling pathways activated by angiotensin I-converting enzyme inhibitors and peptide ligands. Mol Pharmacol. 2004;66:1310–1316. doi: 10.1124/mol.104.001990. [DOI] [PubMed] [Google Scholar]
- 120.Savard M, Barbaz D, Belanger S, et al. Expression of endogenous nuclear bradykinin B2 receptors mediating signaling in immediate early gene activation. J Cell Physiol. 2008;216:234–244. doi: 10.1002/jcp.21398. [DOI] [PubMed] [Google Scholar]
- 121.Schanstra JP, Duchene J, Praddaude F, et al. Decreased renal NO excretion and reduced glomerular tuft area in mice lacking the bradykinin B2 receptor. Am J Physiol Heart Circ Physiol. 2003;284:H1904–1908. doi: 10.1152/ajpheart.01150.2002. [DOI] [PubMed] [Google Scholar]
- 122.Zanchi A, Moczulski DK, Hanna LS, et al. Risk of advanced diabetic nephropathy in type 1 diabetes is associated with endothelial nitric oxide synthase gene polymorphism. Kidney Int. 2000;57:405–413. doi: 10.1046/j.1523-1755.2000.00860.x. [DOI] [PubMed] [Google Scholar]
- 123.Neugebauer S, Baba T, Watanabe T. Association of the nitric oxide synthase gene polymorphism with an increased risk for progression to diabetic nephropathy in type 2 diabetes. Diabetes. 2000;49:500–503. doi: 10.2337/diabetes.49.3.500. [DOI] [PubMed] [Google Scholar]
- 124.Reyes AA, Karl IE, Kissane J, et al. L-arginine administration prevents glomerular hyperfiltration and decreases proteinuria in diabetic rats. J Am Soc Nephrol. 1993;4:1039–1045. doi: 10.1681/ASN.V441039. [DOI] [PubMed] [Google Scholar]
- 125.Kamijo H, Higuchi M, Hora K. Chronic inhibition of nitric oxide production aggravates diabetic nephropathy in Otsuka Long-Evans Tokushima Fatty rats. Nephron Physiol. 2006;104:p12–22. doi: 10.1159/000093276. [DOI] [PubMed] [Google Scholar]
- 126.Zhao HJ, Wang S, Cheng H, et al. Endothelial nitric oxide synthase deficiency produces accelerated nephropathy in diabetic mice. J Am Soc Nephrol. 2006;17:2664–2669. doi: 10.1681/ASN.2006070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mohan S, Reddick RL, Musi N, et al. Diabetic eNOS knockout mice develop distinct macro- and microvascular complications. Lab Invest. 2008;88:515–528. doi: 10.1038/labinvest.2008.23. [DOI] [PubMed] [Google Scholar]
- 128.Nakagawa T, Sato W, Glushakova O, et al. Diabetic endothelial nitric oxide synthase knockout mice develop advanced diabetic nephropathy. J Am Soc Nephrol. 2007;18:539–550. doi: 10.1681/ASN.2006050459. [DOI] [PubMed] [Google Scholar]
- 129.Wang CH, Li F, Hiller S, et al. A modest decrease in endothelial NOS in mice comparable to that associated with human NOS3 variants exacerbates diabetic nephropathy. Proc Natl Acad Sci U S A. 2011;108:2070–2075. doi: 10.1073/pnas.1018766108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kakoki M, Hirata Y, Hayakawa H, et al. Effects of hypertension, diabetes mellitus, and hypercholesterolemia on endothelin type B receptor-mediated nitric oxide release from rat kidney. Circulation. 1999;99:1242–1248. doi: 10.1161/01.cir.99.9.1242. [DOI] [PubMed] [Google Scholar]
- 131.Choi KC, Lee SC, Kim SW, et al. Role of nitric oxide in the pathogenesis of diabetic nephropathy in streptozotocin-induced diabetic rats. Korean J Intern Med. 1999;14:32–41. doi: 10.3904/kjim.1999.14.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Trachtman H, Futterweit S, Pine E, et al. Chronic diabetic nephropathy: role of inducible nitric oxide synthase. Pediatr Nephrol. 2002;17:20–29. doi: 10.1007/s004670200004. [DOI] [PubMed] [Google Scholar]
- 133.Hakim FA, Pflueger A. Role of oxidative stress in diabetic kidney disease. Med Sci Monit. 2010;16:RA37–48. [PubMed] [Google Scholar]
- 134.Huang C, Kim Y, Caramori ML, et al. Diabetic nephropathy is associated with gene expression levels of oxidative phosphorylation and related pathways. Diabetes. 2006;55:1826–1831. doi: 10.2337/db05-1438. [DOI] [PubMed] [Google Scholar]
- 135.Kanwar YS, Wada J, Sun L, et al. Diabetic nephropathy: mechanisms of renal disease progression. Exp Biol Med (Maywood) 2008;233:4–11. doi: 10.3181/0705-MR-134. [DOI] [PubMed] [Google Scholar]
- 136.Ueno Y, Horio F, Uchida K, et al. Increase in oxidative stress in kidneys of diabetic Akita mice. Biosci Biotechnol Biochem. 2002;66:869–872. doi: 10.1271/bbb.66.869. [DOI] [PubMed] [Google Scholar]
- 137.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 138.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Craven PA, Melhem MF, Phillips SL, et al. Overexpression of Cu2+/Zn2+ superoxide dismutase protects against early diabetic glomerular injury in transgenic mice. Diabetes. 2001;50:2114–2125. doi: 10.2337/diabetes.50.9.2114. [DOI] [PubMed] [Google Scholar]
- 140.DeRubertis FR, Craven PA, Melhem MF, et al. Attenuation of renal injury in db/db mice overexpressing superoxide dismutase: evidence for reduced superoxide-nitric oxide interaction. Diabetes. 2004;53:762–768. doi: 10.2337/diabetes.53.3.762. [DOI] [PubMed] [Google Scholar]
- 141.Chacko BK, Reily C, Srivastava A, et al. Prevention of diabetic nephropathy in Ins2+/-AkitaJ mice by the mitochondria-targeted therapy mitoQ. Biochem J. 2010;432:9–19. doi: 10.1042/BJ20100308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cleeter MW, Cooper JM, Darley-Usmar VM, et al. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett. 1994;345:50–54. doi: 10.1016/0014-5793(94)00424-2. [DOI] [PubMed] [Google Scholar]
- 143.Brown GC, Cooper CE. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett. 1994;356:295–298. doi: 10.1016/0014-5793(94)01290-3. [DOI] [PubMed] [Google Scholar]
- 144.Schweizer M, Richter C. Nitric oxide potently and reversibly deenergizes mitochondria at low oxygen tension. Biochem Biophys Res Commun. 1994;204:169–175. doi: 10.1006/bbrc.1994.2441. [DOI] [PubMed] [Google Scholar]
- 145.Lee I, Salomon AR, Ficarro S, et al. cAMP-dependent tyrosine phosphorylation of subunit I inhibits cytochrome c oxidase activity. J Biol Chem. 2005;280:6094–6100. doi: 10.1074/jbc.M411335200. [DOI] [PubMed] [Google Scholar]
- 146.Piccoli C, Scacco S, Bellomo F, et al. cAMP controls oxygen metabolism in mammalian cells. FEBS Lett. 2006;580:4539–4543. doi: 10.1016/j.febslet.2006.06.085. [DOI] [PubMed] [Google Scholar]
- 147.Oeseburg H, Iusuf D, van der Harst P, et al. Bradykinin protects against oxidative stress-induced endothelial cell senescence. Hypertension. 2009;53:417–422. doi: 10.1161/HYPERTENSIONAHA.108.123729. [DOI] [PubMed] [Google Scholar]
- 148.Zhang JJ, Bledsoe G, Kato K, et al. Tissue kallikrein attenuates salt-induced renal fibrosis by inhibition of oxidative stress. Kidney Int. 2004;66:722–732. doi: 10.1111/j.1523-1755.2004.00794.x. [DOI] [PubMed] [Google Scholar]
- 149.Villa E, Rabano A, Ruilope LM, et al. Effects of cicaprost and fosinopril on the progression of rat diabetic nephropathy. Am J Hypertens. 1997;10:202–208. doi: 10.1016/s0895-7061(96)00319-6. [DOI] [PubMed] [Google Scholar]
- 150.Mikrut K, Paluszak J, Kozlik J, et al. The effect of bradykinin on the oxidative state of rats with acute hyperglycaemia. Diabetes Res Clin Pract. 2001;51:79–85. doi: 10.1016/s0168-8227(00)00222-9. [DOI] [PubMed] [Google Scholar]