Abstract
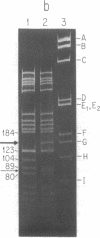
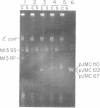
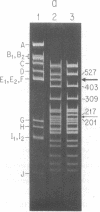
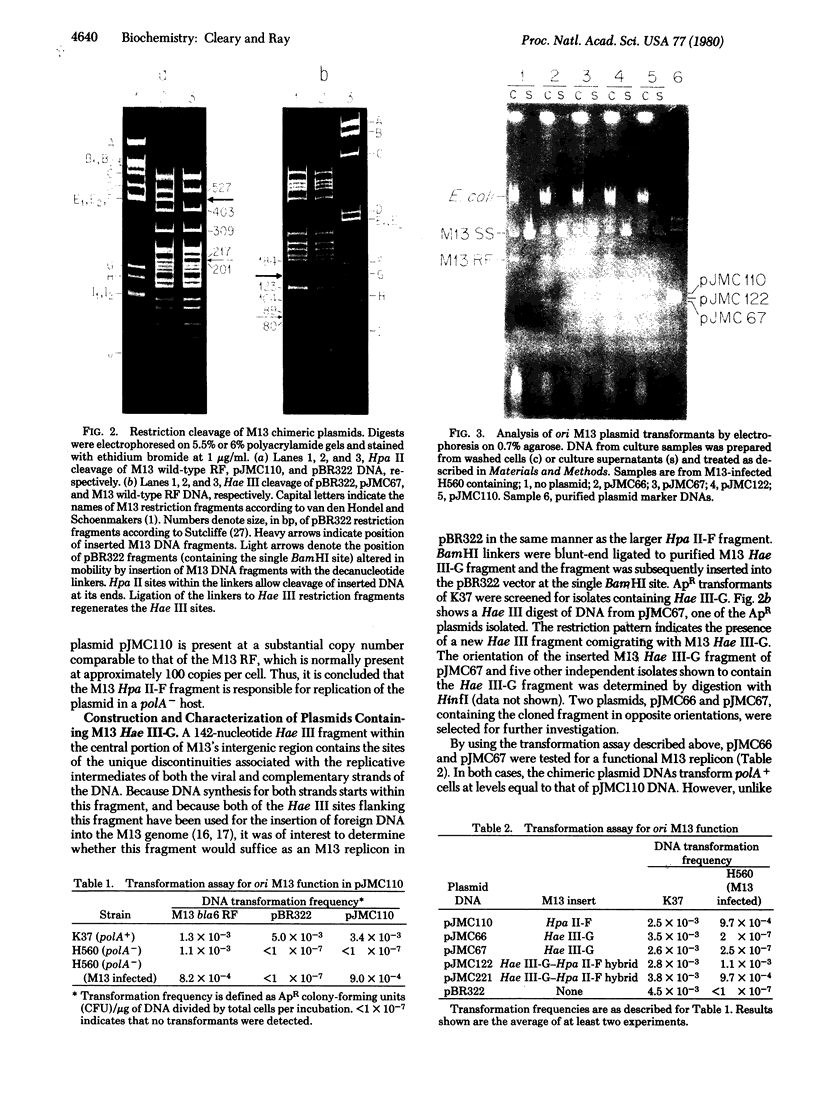
The replication origins of viral and complementary strands of bacteriophage M13 DNA are contained within a 507-nucleotide intergenic region of the viral genome. Chimeric plasmids have been constructed by inserting restriction endonuclease fragments of the M13 intergenic region into the plasmid pBR322. Replication of these hybrid plasmids, under conditions not permissive for the plasmid replicon, depends on specific segments of the M13 origin region and on the presence of M13 helper virus. Thus M13-infected polA- Escherichia coli can be transformed to ampicillin resistance by hybrid plasmids that have a functional M13 origin. Cells transformed to drug resistance by plasmids bearing M13 origin sequences contain the duplex chimeric DNA at high copy number but do not accumulate significant amounts of single-stranded plasmid DNA. Rare transducing phages carrying single-stranded chimeric DNA are produced and can be detected by their ability to transduce cells to ampicillin resistance. Plasmids containing a 270-nucleotide fragment from the gene II-proximal half of the intergenic region produce transformants at high frequency under nonpermissive conditions. A central Hae III fragment, Hae III-G, containing the nucleotide sequence coding for the RNA primer for the complementary strand and the nicking site for gene II protein, is sufficient for plasmid replication in M13-infected polA- cells but not for high frequency transformation. Additional sequence information on the gene II side of the Hae III-G fragment is necessary for efficient transformation by the plasmid DNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Backman K., Ptashne M., Gilbert W. Construction of plasmids carrying the cI gene of bacteriophage lambda. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4174–4178. doi: 10.1073/pnas.73.11.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahl C. P., Marians K. J., Wu R. A general method for inserting specific DNA sequences into cloning vehicles. Gene. 1976;1(1):81–92. doi: 10.1016/0378-1119(76)90008-1. [DOI] [PubMed] [Google Scholar]
- Barnes W. M. Construction of an M13 histidine-transducing phage: a single-stranded cloning vehicle with one EcoRI site. Gene. 1979 Feb;5(2):127–139. doi: 10.1016/0378-1119(79)90098-2. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Chen T. C., Ray D. S. Replication of bacteriophage M13. XIII. Structure and replication of cloned M13 miniphage. J Mol Biol. 1978 Oct 25;125(2):107–121. doi: 10.1016/0022-2836(78)90340-6. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enea V., Horiuchi K., Turgeon B. G., Zinder N. D. Physical map of defective interfering particles of bacteriophage f1. J Mol Biol. 1977 Apr 25;111(4):395–414. doi: 10.1016/s0022-2836(77)80061-2. [DOI] [PubMed] [Google Scholar]
- Fidanián H. M., Ray D. S. Replication of bacteriophage M13. VII. Requirement of the gene 2 protein for the accumulation of a specific RFII species. J Mol Biol. 1972 Dec 14;72(1):51–63. doi: 10.1016/0022-2836(72)90067-8. [DOI] [PubMed] [Google Scholar]
- Geider K., Beck E., Schaller H. An RNA transcribed from DNA at the origin of phage fd single strand to replicative form conversion. Proc Natl Acad Sci U S A. 1978 Feb;75(2):645–649. doi: 10.1073/pnas.75.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray C. P., Sommer R., Polke C., Beck E., Schaller H. Structure of the orgin of DNA replication of bacteriophage fd. Proc Natl Acad Sci U S A. 1978 Jan;75(1):50–53. doi: 10.1073/pnas.75.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi K., Zinder N. D. Origin and direction of synthesis of bacteriophage fl DNA. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2341–2345. doi: 10.1073/pnas.73.7.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaguni J., Ray D. S. Cloning of a functional replication origin of phage G4 into the genome of phage M13. J Mol Biol. 1979 Dec 25;135(4):863–878. doi: 10.1016/0022-2836(79)90516-3. [DOI] [PubMed] [Google Scholar]
- Lusky M., Hobom G. Inceptor and origin of DNA replication in lambdoid coliphages. I. The lambda DNA minimal replication system. Gene. 1979 Jun;6(2):137–172. doi: 10.1016/0378-1119(79)90068-4. [DOI] [PubMed] [Google Scholar]
- Messing J., Gronenborn B., Müller-Hill B., Hans Hopschneider P. Filamentous coliphage M13 as a cloning vehicle: insertion of a HindII fragment of the lac regulatory region in M13 replicative form in vitro. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3642–3646. doi: 10.1073/pnas.74.9.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray D. S., Kook K. Insertion of the Tn3 transposon into the genome of the single-stranded DNA phage M13. Gene. 1978 Oct;4(2):109–119. doi: 10.1016/0378-1119(78)90024-0. [DOI] [PubMed] [Google Scholar]
- Salstrom J. S., Pratt D. Role of coliphage M13 gene 5 in single-stranded DNA production. J Mol Biol. 1971 Nov 14;61(3):489–501. doi: 10.1016/0022-2836(71)90061-1. [DOI] [PubMed] [Google Scholar]
- Shen C. K., Hearst J. E. Psoralen-crosslinked secondary structure map of single-stranded virus DNA. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2649–2653. doi: 10.1073/pnas.73.8.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suggs S. V., Ray D. S. Nucleotide sequence of the origin for bacteriophage M13 DNA replication. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):379–388. doi: 10.1101/sqb.1979.043.01.044. [DOI] [PubMed] [Google Scholar]
- Suggs S. V., Ray D. S. Replication of bacteriophage M13. XI. Localization of the origin for M13 single-strand synthesis. J Mol Biol. 1977 Feb 15;110(1):147–163. doi: 10.1016/s0022-2836(77)80103-4. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. pBR322 restriction map derived from the DNA sequence: accurate DNA size markers up to 4361 nucleotide pairs long. Nucleic Acids Res. 1978 Aug;5(8):2721–2728. doi: 10.1093/nar/5.8.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak H. F., Griffith J., Geider K., Schaller H., Kornberg A. Initiation of deoxyribonucleic acid synthesis. VII. A unique location of the gap in the M13 replicative duplex synthesized in vitro. J Biol Chem. 1974 May 25;249(10):3049–3054. [PubMed] [Google Scholar]
- Van Den Hondel C. A., Pennings L., Schoenmakers J. G. Restriction-enzyme-cleavage maps of bacteriophage M13. Existence of an intergenic region on the M13 genome. Eur J Biochem. 1976 Sep;68(1):55–70. doi: 10.1111/j.1432-1033.1976.tb10764.x. [DOI] [PubMed] [Google Scholar]
- Vosberg H. P., Hoffmann-Berling H. DNA synthesis in nucleotide-permeable Escherichia coli cells. I. Preparation and properties of ether-treated cells. J Mol Biol. 1971 Jun 28;58(3):739–753. doi: 10.1016/0022-2836(71)90037-4. [DOI] [PubMed] [Google Scholar]
- Vovis G. F., Horiuchi K., Zinder N. D. Endonuclease R-EcoRII restriction of bacteriophage f1 DNA in vitro: ordering of genes V and VII, location of an RNA promotor for gene VIII. J Virol. 1975 Sep;16(3):674–684. doi: 10.1128/jvi.16.3.674-684.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W., Brutlag D., Schekman R., Kornberg A. RNA synthesis initiates in vitro conversion of M13 DNA to its replicative form. Proc Natl Acad Sci U S A. 1972 Apr;69(4):965–969. doi: 10.1073/pnas.69.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]