Abstract
The standard of care for primary central nervous system lymphoma (PCNSL) is systemic chemotherapy with or without whole brain radiotherapy or intrathecal chemotherapy. In contrast to treatment for other brain tumors, efforts at resection are discouraged. This is a secondary analysis of the German PCNSL Study Group–1 trial, a large randomized phase III study comprising 526 patients with PCNSL. Progression-free survival (hazard ratio [HR]: 1.39; 95% confidence interval [CI]: 1.10–1.74; P = .005) and overall survival (HR: 1.33; 95% CI: 1.04–1.70; P = .024) were significantly shorter in biopsied patients compared with patients with subtotal or gross total resections. This difference in outcome was not due to age or Karnofsky performance status (KPS). When controlled for the number of lesions, the HR of biopsy versus subtotal or gross total resection remained unchanged for progression-free survival (HR = 1.37; P = .009) but was smaller for overall survival (HR = 1.27; P = .085). This analysis of the largest PCNSL trial ever performed challenges the traditional view that the extent of resection has no prognostic impact on this disease. Therefore, we propose to reconsider the statement that efforts at resection should be discouraged, at least if resection seems safe, as is often the case in treatment of single PCNSL lesions.
Keywords: CNS lymphoma, resection, surgery
Primary central nervous system lymphoma (PCNSL) is a rare brain tumor with an annual incidence in the range of 0.5/100 000 (www.cbtrus.org). All treatment modalities except high-dose methotrexate (HD-MTX) have remained controversial, especially whole brain radiotherapy (WBRT) and intrathecal chemotherapy. Yet, a consistent recommendation of virtually all review articles and national or international guidelines, including those of the U.S. National Comprehensive Cancer Network, is that efforts at resection of PCNSL should not be undertaken. However, this recommendation is not based on contemporary reports. In 1974, Henry and colleagues1 reported survival of 3.3 months in 15 cases managed with supportive care alone and 4.6 months in 28 cases managed after surgery alone, but 15.2 months in 21 cases treated with radiotherapy, with or without surgery. De Angelis and colleagues2 observed no complications in 19 cases managed with stereotactic biopsies, whereas 4 of 10 patients who had had a complete resection suffered a severe postoperative deficit, indicating an increased surgical risk in this patient population. One of the most influential articles on the topic3 represents a retrospective analysis of 248 patients treated between 1980 and 1995: the survival rates at 1 year were 56.6% for completely resected patients, 31.8% for partially resected patients, and 48.6% for biopsied patients.3 Another, more recent retrospective study4 of 32 patients also questioned the value of surgical resection, although the authors essentially acknowledged that their study was inconclusive. The German PCNSL Study Group (G-PCNSL-SG)–1 trial, which examined the role of WBRT in the treatment of newly diagnosed PCNSL patients eligible for HD-MTX–based chemotherapy, provides a unique database to confirm or refute this statement on the lack of impact of surgery in PCNSL.5
Materials and Methods
This was an unplanned secondary analysis of the G-PCNSL-SG-1 trial for an association of the type of surgery and extent of resection as documented at study entry and the clinical outcome parameter response, complete remission (CR) rate at 6 months, progression-free survival (PFS), and overall survival (OS). Open and stereotactic biopsies were pooled. CR rate at 6 months, PFS, and OS were analyzed using the Kaplan–Meier method and the log-rank test. With 315 OS events and 414 PFS events in the Cox regression model, hazard ratios (HRs) >1.44 and >1.37, respectively, could be detected (type 1 error, .05; 2-sided type 2 error, .80) between patients with subtotal or gross total resection (pooled) and patients with biopsy. Populations are defined as follows5: 526 patients were eligible for study entry and entered the first study phase of HD-MTX–based chemotherapy (primary eligible population); 411 patients completed the first and entered the second phase of the trial, where randomization (WBRT vs no WBRT) should have become effective (the intent-to-treat [ITT] population); and 318 patients were treated as randomized (the per-protocol [PP] population).
Results
Of the 526 patients of the primary eligible population, 67 had a gross total resection, 70 had a subtotal resection, and 379 had a biopsy (48 open and 331 stereotactic). Gross total resection in cases with more than 1 lesion required gross total removal of all lesions. No data for type of surgery were provided for 10 patients. There was no difference in the 3 groups regarding age or KPS, which was determined at study entry, that is, after surgery (Table 1). There was also no such difference for age or KPS when gross total and subtotal resections were pooled and compared with the biopsied population (data not shown). Biopsied patients more often had multiple lesions than resected patients (P = .003).
Table 1.
Patient characteristics, response and outcome by type of surgical intervention
| Gross Total Resection | Subtotal Resection | Biopsy | |
|---|---|---|---|
| N patients (%) | 67 (13) | 70 (14) | 379 (73) |
| Median age, y (range) | 63 (19–80) | 62 (22–79) | 63 (19–84) |
| Number of lesions, n (%) | |||
| 1 | 43 (64.2) | 37 (52.8) | 176 (46.5) |
| >1 | 7 (10.4) | 24 (34.3) | 137 (36.1) |
| No data | 17 (25.4) | 9 (12.9) | 66 (17.4) |
| Median KPS (range)d | 80 (30–100) | 80 (30–100) | 70 (20–100) |
| Complete remission rate at 6 months (%) | 38/67 (56.7) | 29/70 (41.4) | 130/379 (34.3) |
| OR = 0.54, 95% CI: 0.27–1.06, P = .074a | OR = 0.40, 95% CI: 0.24–0.68, P < .001a | ||
| OR = 0.74, 95% CI: 0.44–1.24, P = .252b | |||
| OR = 0.55, 95% CI: 0.37–0.81, P = .003c | |||
| Median PFS (95% CI) | 11 months (5–18) | 15 months (0–31) | 6 months (4–8) |
| PFS events (%) | 48/67 (72) | 49/70 (70) | 317/379 (84) |
| HR = 0.97, 95% CI: 0.65–1.44, P = .87a | HR = 1.35, 95% CI: 0.99–1.83, P = .053a | ||
| HR = 1.42, 95% CI: 1.05–1.91, P = .023b | |||
| HR = 1.39, 95% CI: 1.10–1.74, P = .005c | |||
| Median OS (95% CI) | 32 months (18–46) | 31 months (21–40) | 18 months (14–23) |
| OS events (%) | 39/67 (58) | 44/70 (63) | 268/379 (71) |
| HR = 1.26, 95% CI: 0.81–1.96, P = .297a | HR = 1.44, 95% CI: 1.03–2.02, P = .032a | ||
| HR = 1.22, 95% CI: 0.89–1.68, P = .218b | |||
| HR = 1.33, 95% CI: 1.04–1.70, P = .024c | |||
Abbreviation: OR, odds ratio; PFS, progression-free survival; OS, overall survival; KPS, Karnofsky performance status; HR, hazards ratio; CI, confidence interval.
aVs gross total resection.
bVs subtotal resection.
cVs subtotal + gross total resections pooled.
dAt study entry, after surgery.
The CR rate at 6 months was 56.7% for gross totally resected patients, 41.4% for subtotally resected patients, and 34.3% for biopsied patients (P = .001). Of note, we do not attribute the increase in the CR rate at 6 months to surgery alone, because PCNSL is a very aggressive lymphoma expected to recur within 6 months, even after gross total resection, without adequate chemotherapy or chemoradiotherapy.
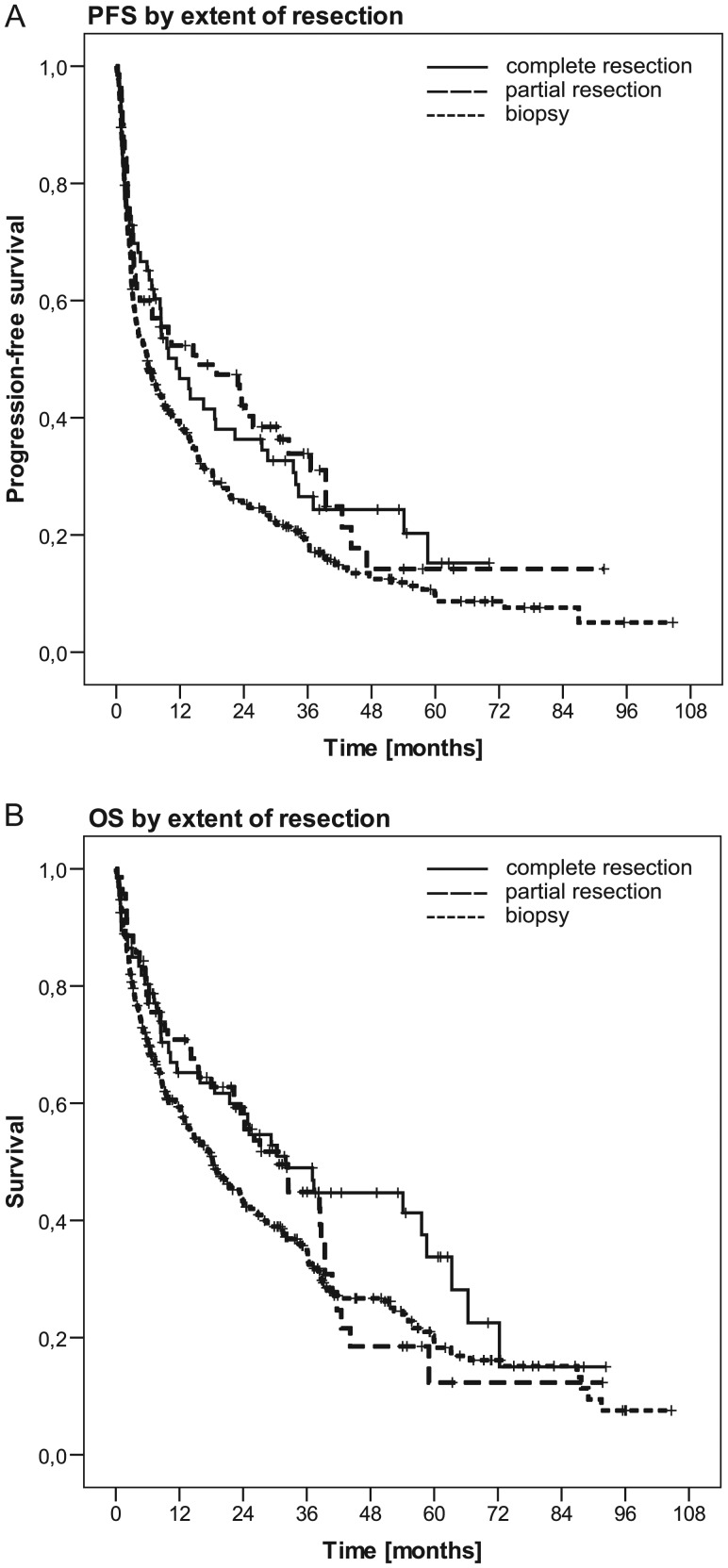
There was no difference in PFS or OS between patients with a gross total and a subtotal resection. However, biopsied patients had inferior PFS and OS compared with gross totally resected patients or gross totally + subtotally resected patients pooled (Table 1, Fig. 1). When biopsied patients were compared with subtotally or gross totally resected patients, HRs for PFS were 1.39 (95% confidence interval [CI]: 1.10–1.74, P = .005) in the primary eligible population (all 526 patients); 1.34 (95% CI: 1.00–1.79, P = .047) for the PP population; 1.57 (95% CI: 0.80–3.08, P = .186) for the ITT population; and 1.09 (95% CI: 0.68–1.74, P = .73) for patients not entered into the ITT population. The corresponding HRs for OS were 1.33 (95% CI: 1.04–1.70, P = .024), 1.30 (95% CI: 0.94–1.79, P = .116), 1.33 (95% CI: 0.62–2.85, P = .46), and 1.04 (95% CI: 0.65–1.65, P = .88).
Fig. 1.
PFS (A) and OS (B) by extent of resection: gross total resection vs subtotal resection vs biopsy in the primary eligibility population of 526 patients (PFS: P = .005 for biopsy vs gross or subtotal resection, P = .023 for gross total vs subtotal resection; OS: P = .024 for biopsy vs gross or subtotal resection, P = .218 for gross total vs subtotal resection; see also Table 1).
In a sensitivity analysis of the primary eligibility population (n = 526), we investigated whether the number of lesions was a confounder, ie, whether patients with a larger number of lesions with presumed worse prognosis underwent surgery less frequently. To this aim, we subdivided our sample into patients with 1 lesion (60.8%, n = 262) and those with 2 or more lesions (39.2%, n = 169). For 95 patients, the number of lesions was not documented. We found indeed that 19% of the patients with more than 1 lesion underwent gross total or subtotal resection, in contrast to 31% of the patients with only 1 lesion (P = .005). Moreover, the number of lesions was indeed a prognostic factor (PFS HR = 1.40, 95% CI: 1.13–1.73, P = .002; OS HR = 1.40, 95% CI: 1.11–1.77, P = .005). However, after adjustment for the number of lesions, HR of biopsy vs subtotal or gross total resection remained unchanged for PFS (1.39; 95% CI: 1.08–1.79, P = .012) and was only slightly smaller for OS (1.27; 95% CI: 0.97–1.67, P = .085). Comparable results were obtained when the number of lesions was used as a continuous covariate or when patients with 1 or 2 lesions were compared with patients with 3 or more lesions. The location of lesions (supratentorial, cortical, subcortical, spinal, or cerebellar/brainstem) was not associated with OS or PFS (data not shown).
Discussion
This analysis of the largest PCNSL trial ever performed challenges the traditional view that the extent of resection has no prognostic impact in this disease and that efforts at resection should therefore be avoided. We observed that gross totally or subtotally resected patients appeared to derive a benefit from surgery (Fig. 1). Differences in neither age nor postoperative KPS accounted for these differences in outcome (Table 1). The impact of extent of resection was similar for PFS but less prominent for OS when adjusted for the number of lesions. However, the benefit from surgery did not become apparent in the negatively selected population of 115 patients from the primary eligibility population who started HD-MTX–based chemotherapy but did not enter into the second phase of the study. This raises the possibility that there is a subpopulation of patients with aggressive, treatment-resistant tumors for whom cytoreductive surgery does not result in improved outcome.
Limitations of this analysis, which was not planned in the study protocol, include its retrospective nature and the lack of a central review of neuroimaging for extent of resection. Yet, the determination of the extent of resection was among the prospectively collected, prespecified parameters of study documentation. Moreover, pooling of gross total and subtotal resections avoids the problem of not having performed a central review of early postoperative scans to assess extent of resection.
The determination of KPS after surgery might also be a limitation because patients may have an improved KPS after gross total or subtotal resection, supported by the use of steroids. It is possible that low preoperative KPS values dissuaded surgeons from performing resections and that this bias enriched the group of biopsied patients somewhat for poor KPS. Yet, postoperative KPS was similar and apparently independent of type of surgery (Table 1), indicating that such biases were not introduced to a relevant extent.
The largest previous analysis of biopsy vs subtotal vs gross total resection, which indicated an inferior outcome at least with subtotal resection, may no longer be appropriate to estimate safety and efficacy because neurosurgery has developed, standards of adjuvant therapy have dramatically changed, and patients were treated.3 Accordingly, given that no prospective study to look at the role of surgery in isolation will ever be performed, we propose to reconsider the statement that efforts at resection should be discouraged, at least if resection seems safe, eg, in cases of single lesions, and we suggest that extent of resection should be considered for stratification or at least be assessed in future PCNSL trials.
Funding
The G-PCNSL-SG-1 trial was supported by the German Cancer Aid grant 70–2838-Th 2 to E.T. and M.W.
Acknowledgments
We thank the German Cancer Society for awarding its Certificate of Quality (Gütesiegel A) to the G-PCNSL-SG-1 trial. The authors thank all institutions, investigators, patients, and families who supported this trial.
Conflict of interest statement. M.W. received research support from Merck Serono and Roche and honoraria for advisory board and lecture activities from Merck Serono, MSD, Roche, and Magforce. P.M. received honoraria from Parexel for serving on a safety board. P.R. reports no disclosures. E.T. received honoraria, grants, and congress support from Pfizer, Amgen, and Celgene. A.K. reports no disclosures.
References
- 1.Henry JM, Heffner RR, Dillard SH, et al. Primary malignant lymphomas of the central nervous system. Cancer. 1974;34:1293–1302. doi: 10.1002/1097-0142(197410)34:4<1293::aid-cncr2820340441>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 2.De Angelis LM, Yahalom J, Heinemann MH, et al. Primary CNS lymphoma: combined treatment with chemotherapy and radiotherapy. Neurology. 1990;40:80–86. doi: 10.1212/wnl.40.1.80. [DOI] [PubMed] [Google Scholar]
- 3.Bataille B, Delwail V, Menet E, et al. Primary intracerebral malignant lymphoma: report of 248 cases. J Neurosurg. 2000;92:261–266. doi: 10.3171/jns.2000.92.2.0261. [DOI] [PubMed] [Google Scholar]
- 4.Bellinzona M, Roser F, Ostertag H, et al. Surgical removal of primary central nervous system lymphomas (PCNSL) presenting as space occupying lesions: a series of 33 cases. Eur J Surg Oncol. 2005;31:100–105. doi: 10.1016/j.ejso.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Thiel E, Korfel A, Martus P, et al. High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): a phase 3, randomised, non-inferiority trial. Lancet Oncol. 2010;11:1036–1047. doi: 10.1016/S1470-2045(10)70229-1. [DOI] [PubMed] [Google Scholar]