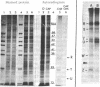
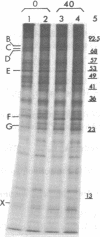
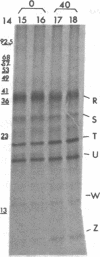
Abstract
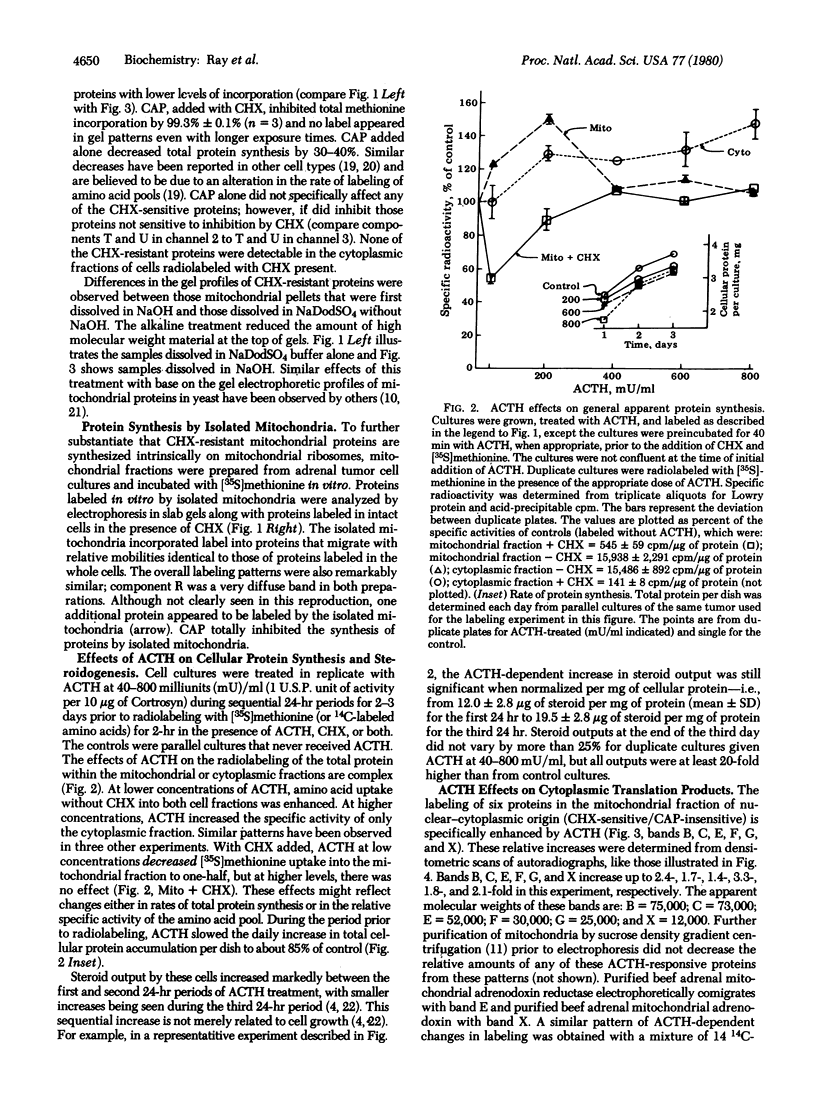
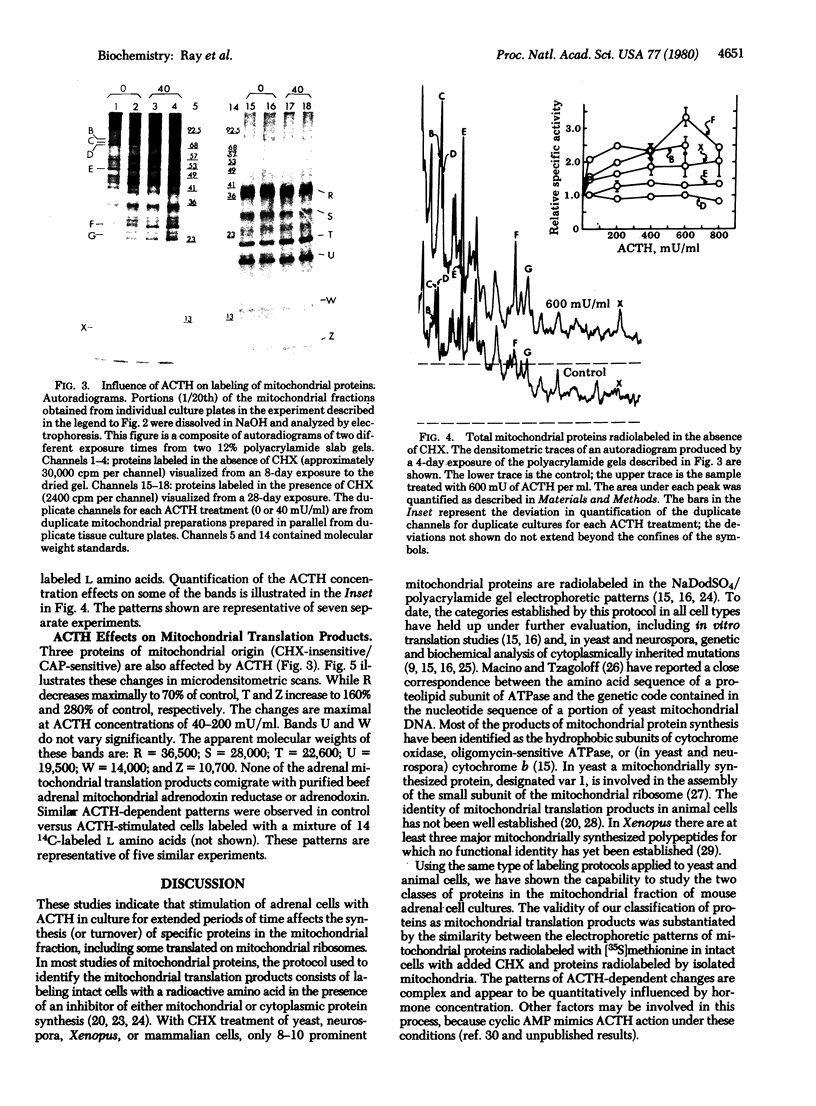
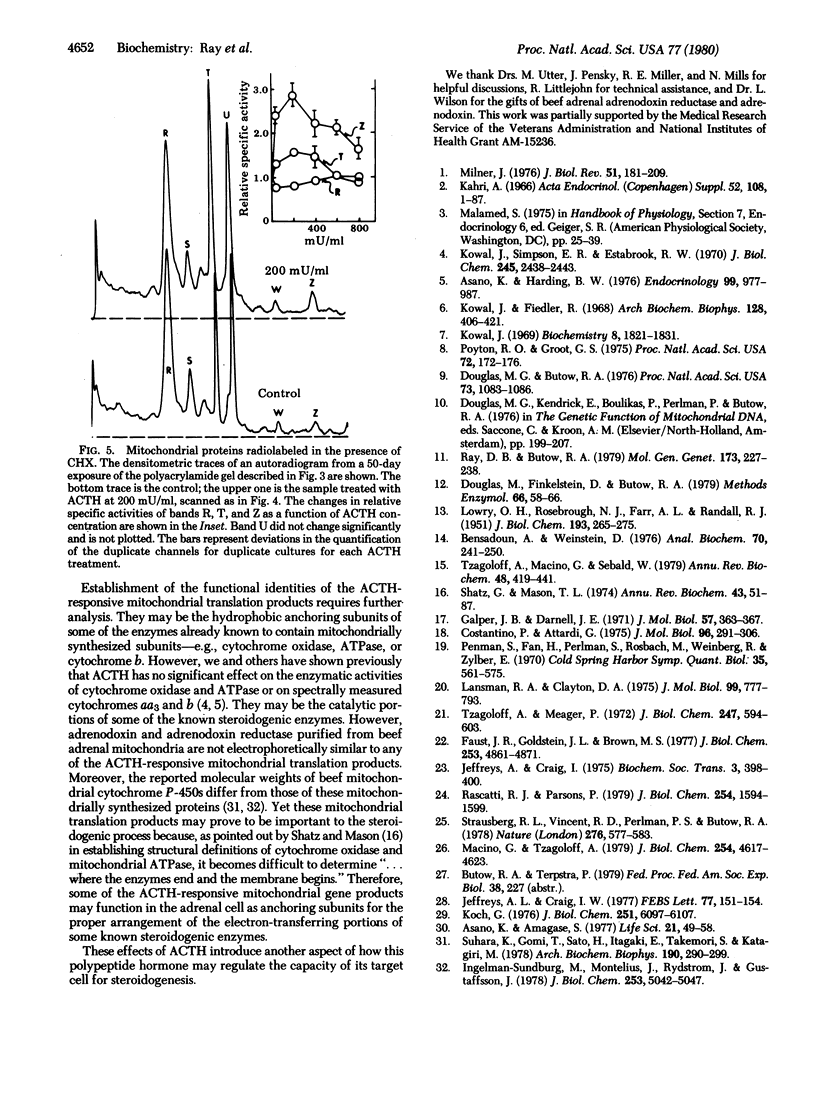
In addition to its stimulatory effects on steroidogenesis, adrenocorticotropic hormone (ACTH) also has a trophic action on the adrenal cell. This is manifested in part by increases in the levels of key mitochondrial steroidogenic enzymes. The mechanism by which this trophic action of ACTH occurs has been studied in monolayer cultures of mouse adrenal cortical tumor cells. ACTH treatment of these cells stimulates the relative incorporation of amino acids into at least eight specific proteins in mitochondrial preparations. Two of these ACTH-responsive proteins are among the nine major adrenal polypeptides that fulfill the criteria of mitochondrial translation products: (i) their synthesis in intact cells is specifically resistant to inhibition by cycloheximide yet uniquely sensitive to chloramphenicol and (ii) they are synthesized in vitro by isolated mitochondria. The other six ACTH-responsive proteins are within the much larger category of mitochondrial proteins that are synthesized on cytoplasmic ribosomes. One of the proteins synthesized in the cytoplasm electrophoretically comigrates with purified beef adrenodoxin reductase and another with beef adrenodoxin. These findings indicate that ACTH regulates the synthesis (and turnover, or both) of specific mitochondrial proteins that are synthesized inside as well as outside the mitochondria of these adrenal cells.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asano K., Amagase S. Adenosine-3',5'-cyclic monophosphate receptor protein in adrenal cortical mitochondria. Life Sci. 1977 Jul 1;21(1):49–58. doi: 10.1016/0024-3205(77)90423-4. [DOI] [PubMed] [Google Scholar]
- Asano K., Harding B. W. Biosynthesis of adrenodoxin in mouse adrenal tumor cells. Endocrinology. 1976 Oct;99(4):977–987. doi: 10.1210/endo-99-4-977. [DOI] [PubMed] [Google Scholar]
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Costantino P., Attardi G. Identification of discrete electrophoretic components among the products of mitochondrial protein synthesis in HeLa cells. J Mol Biol. 1975 Aug 5;96(2):291–306. doi: 10.1016/0022-2836(75)90349-6. [DOI] [PubMed] [Google Scholar]
- Douglas M. G., Butow R. A. Variant forms of mitochondrial translation products in yeast: evidence for location of determinants on mitochondrial DNA. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1083–1086. doi: 10.1073/pnas.73.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas M., Finkelstein D., Butow R. A. Analysis of products of mitochondrial protein synthesis in yeast: genetic and biochemical aspects. Methods Enzymol. 1979;56:58–66. doi: 10.1016/0076-6879(79)56009-1. [DOI] [PubMed] [Google Scholar]
- Faust J. R., Goldstein J. L., Brown M. S. Receptor-mediated uptake of low density lipoprotein and utilization of its cholesterol for steroid synthesis in cultured mouse adrenal cells. J Biol Chem. 1977 Jul 25;252(14):4861–4871. [PubMed] [Google Scholar]
- Galper J. B., Darnell J. E. Mitochondrial protein synthesis in HeLa cells. J Mol Biol. 1971 Apr 28;57(2):363–367. doi: 10.1016/0022-2836(71)90354-8. [DOI] [PubMed] [Google Scholar]
- Ingelman-Sundberg M., Montelius J., Rydström J., Gustafsson J. A. The active form of cytochrome P-45011beta from adrenal cortex mitochondria. J Biol Chem. 1978 Jul 25;253(14):5042–5047. [PubMed] [Google Scholar]
- Jeffreys A. J., Craig I. W. Differences in the mitochondrially synthesized subunits of human and mouse cytochrome c oxidase. FEBS Lett. 1977 May 15;77(2):151–154. doi: 10.1016/0014-5793(77)80223-8. [DOI] [PubMed] [Google Scholar]
- Jeffreys A., Craig I. Proteins made in mitochondria of cultured animal cells. Biochem Soc Trans. 1975;3(3):398–400. doi: 10.1042/bst0030398. [DOI] [PubMed] [Google Scholar]
- Koch G. Synthesis of the mitochondrial inner membrane in cultured Xenopus laevis oocytes. J Biol Chem. 1976 Oct 10;251(19):6097–6107. [PubMed] [Google Scholar]
- Kowal J. Adrenal cells in tissue culture. 3. Effect of adrenocorticotropin and 3',5'-cyclic adenosine monophosphate on 11 beta-hydroxylase and other steroidogenic enzymes. Biochemistry. 1969 May;8(5):1821–1831. doi: 10.1021/bi00833a007. [DOI] [PubMed] [Google Scholar]
- Kowal J., Fiedler R. Arenal cells in tissue culture. I. Assay of steroid products; steroidogenic responses to peptide hormones. Arch Biochem Biophys. 1968 Nov;128(2):406–421. doi: 10.1016/0003-9861(68)90047-7. [DOI] [PubMed] [Google Scholar]
- Kowal J., Simpson E. R., Estabrook R. W. Adrenal cells in tissue culture. V. On the specificity of the stimulation of 11beta-hydroxylation by adrenocorticotropin. J Biol Chem. 1970 May 10;245(9):2438–2443. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lansman R. A., Clayton D. A. Mitochondrial protein synthesis in mouse L-cells: effect of selective nicking of mitochondrial DNA. J Mol Biol. 1975 Dec 25;99(4):777–793. doi: 10.1016/s0022-2836(75)80184-7. [DOI] [PubMed] [Google Scholar]
- Macino G., Tzagoloff A. Assembly of the mitochondrial membrane system. The DNA sequence of a mitochondrial ATPase gene in Saccharomyces cerevisiae. J Biol Chem. 1979 Jun 10;254(11):4617–4623. [PubMed] [Google Scholar]
- Milner J. The functional development of mammalian mitochondria. Biol Rev Camb Philos Soc. 1976 May;51(2):181–209. doi: 10.1111/j.1469-185x.1976.tb01124.x. [DOI] [PubMed] [Google Scholar]
- Poyton R. O., Groot G. S. Biosynthesis of polypeptides of cytochrome c oxidase by isolated mitochondria. Proc Natl Acad Sci U S A. 1975 Jan;72(1):172–176. doi: 10.1073/pnas.72.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascati R. J., Parsons P. Biosynthesis of cytochrome c oxidase by isolated rat liver mitochondria. J Biol Chem. 1979 Mar 10;254(5):1594–1599. [PubMed] [Google Scholar]
- Ray D. B., Butow R. A. Regulation of mitochondrial ribosomal RNA synthesis in yeast. I. In search of a relaxation of stringency. Mol Gen Genet. 1979 Jun 20;173(3):227–238. doi: 10.1007/BF00268633. [DOI] [PubMed] [Google Scholar]
- Strausberg R. L., Vincent R. D., Perlman P. S., Butow R. A. Asymmetric gene conversion at inserted segments on yeast mitochondrial DNA. Nature. 1978 Dec 7;276(5688):577–583. doi: 10.1038/276577a0. [DOI] [PubMed] [Google Scholar]
- Suhara K., Gomi T., Sato H., Itagaki E., Takemori S., Katagiri M. Purification and immunochemical characterization of the two adrenal cortex mitochondrial cytochrome P-450-proteins. Arch Biochem Biophys. 1978 Sep;190(1):290–299. doi: 10.1016/0003-9861(78)90278-3. [DOI] [PubMed] [Google Scholar]
- Tzagoloff A., Macino G., Sebald W. Mitochondrial genes and translation products. Annu Rev Biochem. 1979;48:419–441. doi: 10.1146/annurev.bi.48.070179.002223. [DOI] [PubMed] [Google Scholar]
- Tzagoloff A., Meagher P. Assesmbly of the mitochondrial membrane system. VI. Mitochondrial synthesis of subunit proteins of the rutamycin-sensitive adenosine triphosphatase. J Biol Chem. 1972 Jan 25;247(2):594–603. [PubMed] [Google Scholar]