Abstract
Background
In addition to cognitive and emotional processing dysfunction, chronic cocaine users are also impaired at simple sensorimotor tasks. Many diseases characterized by compulsive movements, repetitive actions, impaired attention and planning are associated with dysfunction in frontal-striatal circuits. The aim of this study was to determine whether cocaine users had impaired frontal-striatal connectivity during a simple movement task and whether this was associated with sensorimotor impairment.
Methods
Functional MRI data was collected from 14 non-treatment seeking cocaine users and 15 healthy controls as they performed a finger-tapping task. Functional coupling was quantified by correlating the timecourses of each pair of anatomically connected regions of interest. Behavioral performance was correlated with all functional coupling coefficients.
Results
In controls there was a significant relationship between the primary motor cortex and the supplementary motor area (SMA), as well as the SMA and the dorsal striatum during ongoing movement. Cocaine users exhibited weaker fronto-striatal coupling than controls, while the cortical-cortical coupling was intact. Coupling strength between the SMA and the caudate was negatively correlated with reaction time in the users.
Conclusions
The observation that cocaine users have impaired cortical-striatal connectivity during simple motor performance, suggests that these individuals may have a fundamental deficit in information processing that influences more complex cognitive processes.
Keywords: addiction, connectivity, neuroimaging, neural networks, sensorimotor, striatum
1. Introduction
Chronic cocaine use is accompanied by disruptions in functional activity in multiple cortical and subcortical brain regions both at rest and during the performance of cognitive (Goldstein et al 2004; Hester and Garavan 2004), motivational (Risinger et al 2005; Sinha et al 2005), and sensorimotor tasks (Hanlon et al 2009; Hanlon et al 2010). Recent studies have demonstrated that, when challenged with a simple motor task, cocaine users have altered functional activity in both cortical and subcortical areas relative to controls. While the cocaine users made more errors and had slower reaction times than controls, behavioral impairment was not related to activity within the supplementary motor area of the frontal cortex, subdivisions of the striatum, or other portions of the motor circuit when these regions were investigated in isolation.
A recently published investigation of sensorimotor control in cocaine users demonstrated that long-term users have an elevated BOLD signal response in multiple cortical and subcortical regions during a simple finger-tapping task relative to non-drug using controls. The cocaine users also made more errors and had longer reaction times than controls. The behavioral impairment however, was not correlated with BOLD signal change in any single region considered in isolation. The aim of this study was to expand the analyses of that data to determine if impairment was related to the coupling among motor-related cortical areas and the dorsal striatum, rather than activity in those areas in isolation. This was motivated by prior studies in cocaine users which demonstrated a significant decrease in the white matter density in the area surrounding the prefrontal cortex and the dorsal striatum (Lim et al 2008). Furthermore, Li et al (2000) demonstrated that acute cocaine administration leads to a decrease in baseline functional correlation within the primary visual and motor cortices of chronic cocaine users, suggesting that chronic use may be associated with alterations between functionally relevant areas.
2. Methods and Materials
2.1 Participants
As documented in the previous investigation (Hanlon et al 2010) fifteen non-treatment seeking cocaine users and 14 control participants were recruited via local advertisements and word of mouth. All participants provided written informed consent to participate according to procedures approved by the Wake Forest University School of Medicine Institutional Review Board. All cocaine users met DSM-IV criteria for cocaine dependence and had used cocaine at least three times per week for a minimum of five years. All participants had positive urine drug screens for cocaine. Control participants did not have a history of substance dependence other than nicotine and were chosen to match the cocaine user population on the basis of gender, race, and education. As previously reported there was no significant difference in the average (± standard deviation) age (Controls: 31.1 ± 5.1, Users: 39.8 ± 3.7) or gender breakdown (Controls: 43% female, Users: 40% female) (Hanlon et al 2010).
2.2 Procedure and Task
On the scanning day, all participants arrived at the imaging center in the morning, approximately three hours prior to acquisition of their functional MRI scan. Urine samples were collected from all participants to test for pregnancy and drug use. No user displayed any overt behavioral signs of cocaine intoxication or craving. The details of the finger-sequencing task have previously been reported (Hanlon et al 2010). Briefly, participants were required to watch a movie of right hand finger tapping movements on a monitor and mimic the ongoing actions with their own fingers. In the MRI scanner the participants were able to see the movie of sequential finger-tapping movements via MR compatible goggles. Participants performed two, six minute runs of the task. Each functional run consisted of 30s movement blocks interspersed with 30s rest blocks. Following each rest block there was also a nine second preparation block in which the participant received a preparation cue which counted down the number of seconds remaining before the motor task began again. This preparation block was modeled separately in the statistical analysis and was used to eliminate the effects of attentional set-shifting to the visuo-motor performance data acquired in the task block. All participants performed a practice session before the functional MRI scan in order to limit the contribution of learning to the data acquired.
Functional data were acquired on a 1.5-T General Electric echo-speed Horizon LX scanner with a birdcage head coil (GE Medical Systems, Milwaukee, WI). For functional imaging, BOLD images were acquired using gradient echo echo-planar imaging (EPI) protocol (TR 3000 ms, TE 40 ms, flip angle 90, 3.75mm × 3.75mm in-plane resolution, 3.20mm slice thickness). The first six volumes of data were acquired before the task began to allow time for the signal to reach an equilibrium state before any stimulation onset. High-resolution T1-weighted anatomical images (3D SPGR, TI 600 ms; TR 9.56 ms; flip angle 20, TE 2.98 ms, 0.935mm × 0.935mm in-plane resolution, 1.50mm slice gap) were also acquired for each individual. Spatial preprocessing was performed with standard parametric mapping techniques (SPM5, London, UK). Briefly, the data were corrected for acquisition time (slice timing), realigned to the first volume (motion correction), normalized into a standardized neuroanatomical space (Montreal Neurological Institute (MNI) brain template), and smoothed using a Gaussian kernel of 8 mm for the group analysis to reduce the variance due to anatomical variability.
2.3 Functional Coupling Analysis
To test the hypothesis that functional coupling between brain regions involved in performance of ongoing movement tasks was significantly altered in cocaine users, the average BOLD timeseries was extracted from seven regions of interest that are involved in this finger tapping task (Hanlon et al 2010). Anatomically defined regions of interest (ROI) extracted from an MNI based atlas (WFU Pick Atlas, Wake Forest University) included the left primary motor cortex (Motor), supplementary motor area (SMA), anterior cingulate cortex (ACC), caudate (Cau), putamen (Put), thalamus (Thal) and the right cerebellum (Cereb). The timecourse for each ROI was extracted for each individual using MarsBaR 0.41. Within an individual, the timecourses of ROIs with established anatomical connectivity were then cross correlated with a sliding window (+/−1 volume) using MATLAB 7.0 (Mathworks, Natick, MA) (M1-SMA, SMA-Caud, SMA-Put, ACC-Caud, ACC-Put, Caud-Thal, Put-Thal, Thal-M1, Thal-Cereb, Cereb-SMA, Cereb-M1). The functional correlation at 0 lag was used for all participants in all interregion pairs. To assess the correlation of these regions independent of their correlation with the task or their association with the global signal, the data were orthogonalized to both the timecourse of the movement portions of the task, convolved with the hemodynamic response function, and the global signal. The global signal was calculated as the average of all voxels included in the mask over the timecourse of each run (Macey et al 2004). While our sampling frequency was lower than the cardiac rate, it is possible that respiratory rate and aliased cardiac rate may account for low frequency correlations among ROIs (Biswal et al 1995). To address this concern, as well as global signal drifts, a low-pass filter (0.1 Hz) was applied to the ROI timecourses.
Between and within group statistical comparisons of ROI coupling were performed using standard statistical analysis software (SPSS, Rel. 16. Chicago: SPSS Inc). The correlation coefficient for each pair of ROIs was used as a measure of functional coupling. The distribution of correlation coefficients in the did not violate the assumption of normality (Kolmogorov-Smirnov test) in either the control group (Z = 1.763, p > 0.10) or the user group (Z = 1.60, p > 0.10). Differences were evaluated by means of a mixed-model analysis of variance (group x ROI pair coupling) followed by appropriate post-hoc analyses. Within group differences amongst ROI coupling coefficients were assessed via Tukey’s honestly significant difference (HSD) test. Between group differences were assessed by means of Fishers least significant difference (LSD) test to determine significant differences between groups for a given ROI pairing. The relationship between motor performance (reaction time, error rate) and ROI pair coupling was assessed via Spearman’s rank correlation coefficient (to account for potential absence of normal distributions among variables).
3. Results
Analysis of variance revealed a significant interaction between group and ROI pairing (F = 2.70, df =10, p<0.02) (Table 1). Within controls there was a significant positive correlation between the M1-SMA, SMA-Caud, SMA-Put, ACC-Caud, Put-Thal, and the Thal-M1, but not the ACC-Put, Caud-Thal, Thal-Cereb, Cereb-SMA, nor Cereb-M1 (Figure 1a). Within the cocaine users there was a significant positive correlation between the M1-SMA, ACC-Caud, Put-Thal, and the Thal-M1, but not the SMA-Caud, SMA-Put, ACC-Put, Caud-Thal, Thal-Cereb, Cereb-SMA, or Cereb-M1 (Figure 1b). Between group comparisons revealed that the cocaine users had lower correlation coefficients in the SMA-Caud (F=3.9, p < 0.01) and the SMA-Put (F=2.6, p < 0.05).
Table 1.
Correlation of BOLD signal in pairs of anatomically connected regions of interest during ongoing movement
| Controls | Cocaine Users | |
|---|---|---|
| M1 - SMA | 0.65 (± 0.29)* | 0.63 ± 0.24* |
| SMA - Caud | 0.53 (± 0.33)* | 0.26# |
| SMA - Put | 0.59 ± 0.29* | 0.42# |
| ACC - Caud | 0.52 ± 0.32* | 0.54 ± 0.38* |
| ACC - Put | 0.43 | 0.40 |
| Caud - Thal | 0.50 | 0.42 |
| Put - Thal | 0.61 ± 0.21* | 0.59 ± 0.18* |
| Thal - M1 | 0.52 ± 0.41* | 0.61 ± 0.14* |
| Thal - Cerebl | 0.35 | 0.45 |
| Cerebl - SMA | 0.37 | 0.39 |
| Cerebl - M1 | 0.29 | 0.41 |
significant correlation within group (p<0.05)
significant difference between groups (p<0.05)
Figure 1.
Functional coupling in motor related regions during ongoing finger-tapping. Controls and non-treatment seeking cocaine users performed a sequential finger tapping task with their dominant right hand. The timecourse of the BOLD signal was extracted from seven regions of interest that are modulated by the task (M1-primary motor cortex, SMA-supplementary motor area, ACC- anterior cingulate cortex, C-caudate, P-putamen, T-thalamus, Cb-cerebellum). Cross correlation of the time courses in each of these anatomically connected regions revealed significant relationships between several of the pairs in the controls (A, green lines, p<0.05) and cocaine users (B, green lines, p<0.05). Between group comparisons revealed a significantly lower correlation between the frontal-striatal regions in the cocaine users (B, red lines, p<0.05).
Within cocaine users, there was a significant negative correlation between the strength of SMA-Caud coupling and reaction times (Figure 2). Within controls neither reaction time nor error rate was correlated with the strength of ROI coupling in the controls.
Figure 2.
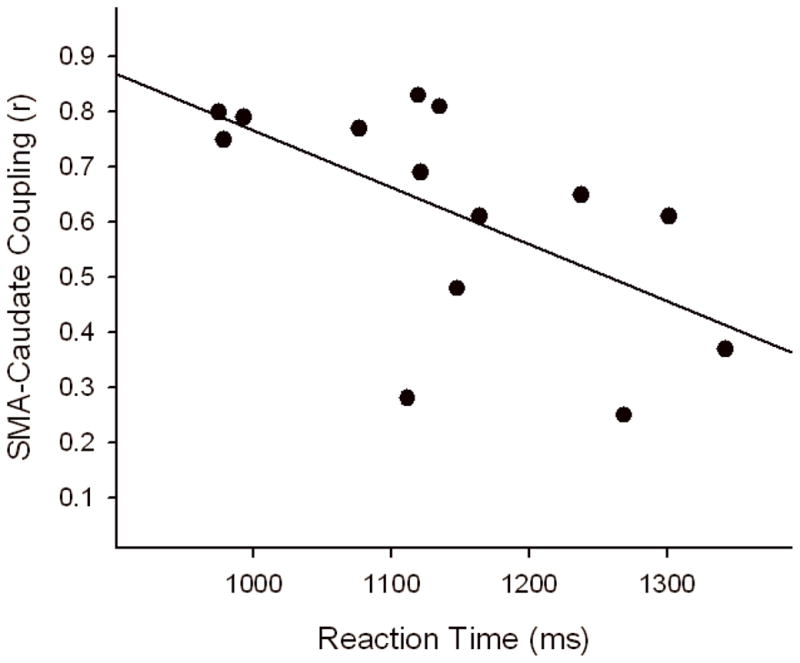
Relationship between SMA-caudate coupling and reaction time in cocaine users. The line of best fit is shown (r = −0.51, p<0.05).
4. Discussion
The present study demonstrates that cocaine users differ from controls in the pattern of functional connectivity observed during performance of a simple finger-tapping task. In cocaine users, activity in frontal cortical regions was not significantly correlated with activity in dorsal striatal areas involved in the task, despite intact cortical-cortical coupling. Successful execution of a simple finger-tapping task depends upon temporally coordinated activity in multiple cortical and subcortical brain regions. The presence of impaired functional coupling during this basic finger-tapping task suggests that chronic cocaine use may be associated with a fundamental deficit in neural network connectivity. Furthermore, the correlation between lower cortical-striatal correlation coefficients and longer reaction times in cocaine users demonstrates the behaviorally relevance of these relationships.
The specificity of this deficit to frontal-striatal connections suggests that cocaine addiction may share common neurobiological mechanisms with other clinical conditions such as obsessive compulsive disorder (Harrison et al 2009), Tourettes syndrome patients (Jeffries et al 2002), and attention deficit disorder (Cao et al 2009). Obsessive-compulsive disorder patients have significantly lower baseline connectivity between dorsal cortico-striatal regions than matched controls, and higher connectivity in ventral cortico-striatal connections. The strength of the cortico-striatal connectivity was proportional to symptom severity (Harrison et al 2009). As with these patients, the cocaine users in the current study also had a lower correlation between the functional timecourse in the frontal cortex and the dorsal striatum. Although the ventral striatum was not chosen as a region of interest in this study of simple finger-tapping performance, further investigations of connectivity between the frontal cortex and the ventral striatum in cocaine users may be insightful given that these regions are modulated in paradigms that elicit cocaine craving (Breiter et al 1997; Risinger et al 2005).
Although frontal-striatal connectivity has been investigated in several clinical diseases, it has not been widely addressed in addiction research (Ma et al 2010). A recent functional imaging study by Liu et al (2009) documented elevated connectivity of the SMA and the caudate in heroin users at rest. Given that these regions are associated with conflict monitoring (Garavan et al 2003) and inhibitory control (Dias et al 1997), the authors suggest that abnormal connectivity in these regions at rest may underscore behavioral deficits observed in these addicts. While lower functional connectivity between cortical and subcortical targets has been documented in cocaine users at rest (Gu et al 2007), the present investigation is the first to report lower frontal-striatal coupling during task performance in this population.
It should be noted that the participants in the current study were all non-treatment seeking active cocaine users. Although all had used cocaine within 72 hours of the time of study, none would be considered acutely intoxicated. It is possible that the lower frontal-striatal coupling observed here may be improved with abstinence from cocaine. It is further possible that the degree of connectivity, as a potential indicator of brain function, may serve as a marker for treatment outcome. A recent investigation in cigarette smokers beginning a treatment program, demonstrated that individuals with higher connectivity between the frontal cortex and the insula before the treatment program were less likely to relapse than those with lower connectivity (Janes et al. 2010). Future studies in treatment seeking individuals could determine whether frontal-striatal connectivity is influenced by abstinence and whether it may predict treatment outcome.
Further investigations are also necessary to determine whether the decrease in frontal-striatal cocaine users during a simple motor task generalizes to other deficits observed in this population during more complex cognitive tasks. Prior studies have demonstrated that, in addition to motor control, disruptions of frontal–striatal circuitry are also linked to impaired planning (Harrison et al 2009), error monitoring (Gehring and Knight 2000), and impulse control (Schmitz et al 2006) in other clinical populations. The results of the present investigation then, may suggest that poor frontal-striatal connectivity observed in cocaine users during a simple finger tapping task, may underlie deficits in these higher order cognitive functions often observed in this population.
References
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew RT, Rosen BR, Hyman SE. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19(3):591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Cao X, Cao Q, Long X, Sun L, Sui M, Zhu C, Zuo X, Zang Y, Wang Y. Abnormal resting-state functional connectivity patterns of the putamen in medication-naive children with attention deficit hyperactivity disorder. Brain Res. 2009;1303:195–206. doi: 10.1016/j.brainres.2009.08.029. [DOI] [PubMed] [Google Scholar]
- Desjardins AE, Kiehl KA, Liddle PF. Removal of confounding effects of global signal in functional MRI analyses. Neuroimage. 2001;13(4):751–8. doi: 10.1006/nimg.2000.0719. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociable forms of inhibitory control within prefrontal cortex with an analog of the Wisconsin Card Sort Test: restriction to novel situations and independence from “on-line” processing. J Neurosci. 1997;17(23):9285–97. doi: 10.1523/JNEUROSCI.17-23-09285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Kaufman J, Stein EA. A midline dissociation between error-processing and response-conflict monitoring. Neuroimage. 2003;20(2):1132–9. doi: 10.1016/S1053-8119(03)00334-3. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Knight RT. Prefrontal-cingulate interactions in action monitoring. Nat Neurosci. 2000;3(5):516–20. doi: 10.1038/74899. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Leskovjan AC, Hoff AL, Hitzemann R, Bashan F, Khalsa SS, Wang GJ, Fowler JS, Volkow ND. Severity of neuropsychological impairment in cocaine and alcohol addiction: association with metabolism in the prefrontal cortex. Neuropsychologia. 2004;42(11):1447–58. doi: 10.1016/j.neuropsychologia.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Gu H, Zhan W, Ross TJ, Salmeron BJ, Stein EA, Yang Y. Reduction of functional connectivity in cocaine users revealed by resting-state functional MRI. Proceedings of the International Society of Magnetic Resonance Medicine; Berlin, Germany. 2007. p. 548. [Google Scholar]
- Hanlon CA, Wesley MJ, Roth AJ, Miller MD, Porrino LJ. Loss of laterality in cocaine users: an fMRI investigation of sensorimotor control. Psychiatry Res. 2010;181(1):15–23. doi: 10.1016/j.pscychresns.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Wesley MJ, Porrino LJ. Loss of functional specificity in the dorsal striatum of chronic cocaine users. Drug Alcohol Depend. 2009;102(1–3):88–94. doi: 10.1016/j.drugalcdep.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BJ, Soriano-Mas C, Pujol J, Ortiz H, Lopez-Sola M, Hernandez-Ribas R, Deus J, Alonso P, Yucel M, Pantelis C, Menchon JM, Cardoner N. Altered corticostriatal functional connectivity in obsessive-compulsive disorder. Arch Gen Psychiatry. 2009;66(11):1189–200. doi: 10.1001/archgenpsychiatry.2009.152. [DOI] [PubMed] [Google Scholar]
- Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci. 2004;24(49):11017–22. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, deB Frederick B, Chuzi S, Pachas G, Culhane MA, Holmes AJ, Fava M, Evins AE, Kaufman MJ. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry. 2010;67(8):722–9. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffries KJ, Schooler C, Schoenbach C, Herscovitch P, Chase TN, Braun AR. The functional neuroanatomy of Tourette’s syndrome: an FDG PET study III: functional coupling of regional cerebral metabolic rates. Neuropsychopharmacology. 2002;27(1):92–104. doi: 10.1016/S0893-133X(01)00428-6. [DOI] [PubMed] [Google Scholar]
- Li SJ, Biswal B, Li Z, Risinger R, Rainey C, Cho JK, Salmeron BJ, Stein EA. Cocaine administration decreases functional connectivity in human primary visual and motor cortex as detected by functional MRI. Magn Reson Med. 2000;43(1):45–51. doi: 10.1002/(sici)1522-2594(200001)43:1<45::aid-mrm6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Lim KO, Wozniak JR, Mueller BA, Franc DT, Specker SM, Rodriguez CP, Silverman AB, Rotrosen JP. Brain macrostructural and microstructural abnormalities in cocaine dependence. Drug Alcohol Depend. 2008;92(1–3):164–72. doi: 10.1016/j.drugalcdep.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Liang J, Qin W, Tian J, Yuan K, Bai L, Zhang Y, Wang W, Wang Y, Li Q, Zhao L, Lu L, von Deneen KM, Liu Y, Gold MS. Dysfunctional connectivity patterns in chronic heroin users: an fMRI study. Neurosci Lett. 2009;460(1):72–7. doi: 10.1016/j.neulet.2009.05.038. [DOI] [PubMed] [Google Scholar]
- Ma N, Liu Y, Li N, Wang CX, Zhang H, Jiang XF, Xu HS, Fu XM, Hu X, Zhang DR. Addiction related alteration in resting-state brain connectivity. Neuroimage. 2010;49(1):738–44. doi: 10.1016/j.neuroimage.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey PM, Macey KE, Kumar R, Harper RM. A method for removal of global effects from fMRI time series. Neuroimage. 2004;22(1):360–6. doi: 10.1016/j.neuroimage.2003.12.042. [DOI] [PubMed] [Google Scholar]
- Risinger RC, Salmeron BJ, Ross TJ, Amen SL, Sanfilipo M, Hoffmann RG, Bloom AS, Garavan H, Stein EA. Neural correlates of high and craving during cocaine self-administration using BOLD fMRI. Neuroimage. 2005;26(4):1097–108. doi: 10.1016/j.neuroimage.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Schmitz N, Rubia K, Daly E, Smith A, Williams S, Murphy DG. Neural correlates of executive function in autistic spectrum disorders. Biol Psychiatry. 2006;59(1):7–16. doi: 10.1016/j.biopsych.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Sinha R, Lacadie C, Skudlarski P, Fulbright RK, Rounsaville BJ, Kosten TR, Wexler BE. Neural activity associated with stress-induced cocaine craving: a functional magnetic resonance imaging study. Psychopharmacology (Berl) 2005;183(2):171–80. doi: 10.1007/s00213-005-0147-8. [DOI] [PubMed] [Google Scholar]



