Abstract
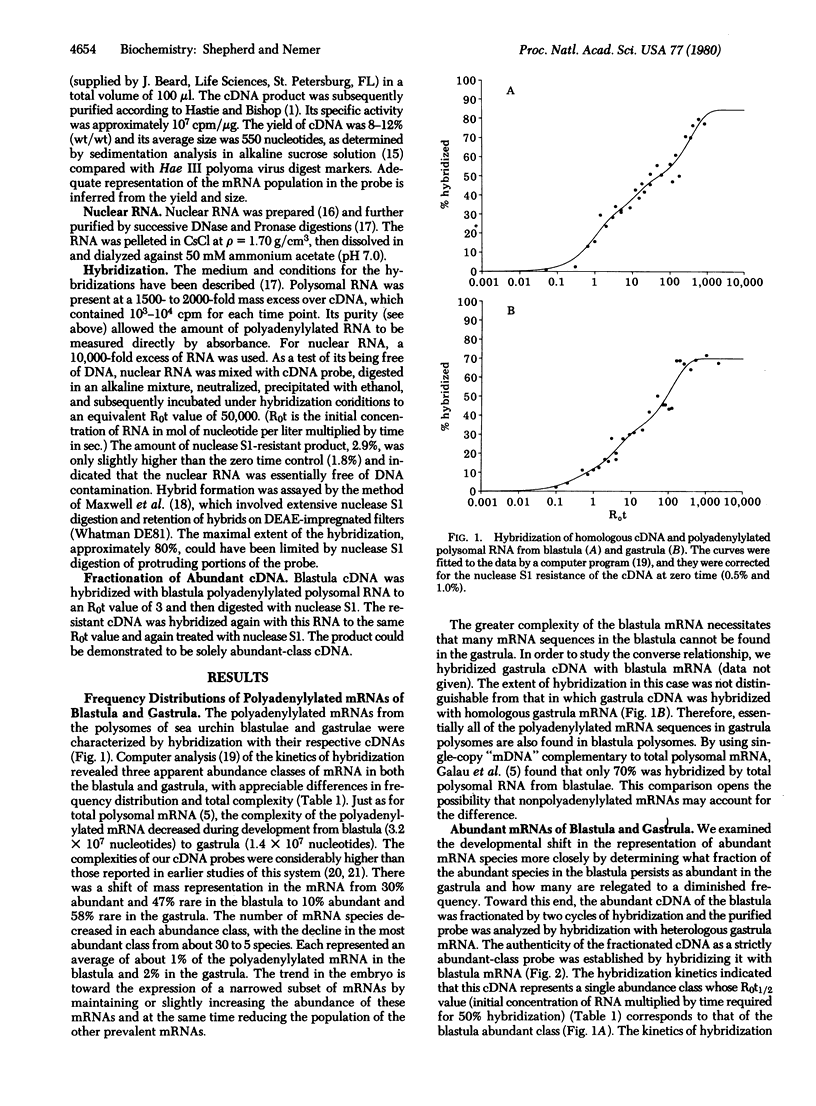
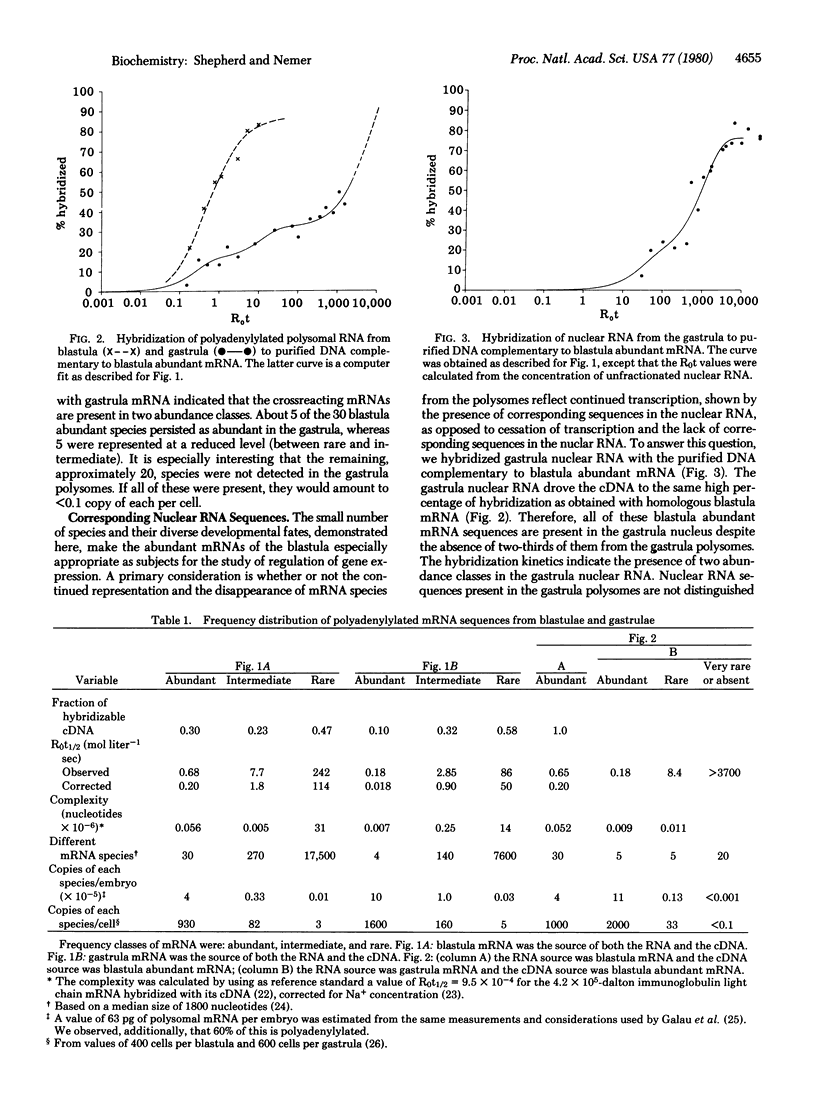
The frequency distributions of polyadenylylated RNAs from the polysomes of sea urchin blastulae and gastrulae were estimated from their kinetics of hybridization with complementary DNA. Developmental decreases in complexity were observed among abundant, intermediate, and rare frequency classes. The class of highest abundance in the blastula polysomes had a complexity of 5.6 X 10(4) nucleotides and contained about 30 mRNA species, which divided into subsets according to developmental fate. Studies with purified DNA complementary to this abundant class revealed that five of these mRNA species remained abundant in the gastrula, wherein each comprised 2% of the polyadenylylated RNA in the polysomes. Approximately 5 species decreased to a nearly rare frequency and 20 were absent or at the limits of detection in polyadenylylated RNA of gastrula polysomes. These distinctly different developmental fates suggest distinct modes of regulation of mRNA concentration for different subsets. Focusing on the small number of abundant blastula mRNAs, we ascertained that those which were absent from gastrula polysomes were nevertheless represented in the gastrula nuclear RNA. Therefore, the appearance of abundant mRNA species in polysomes can be regulated by posttranscriptional processes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Affara N., Daubas P. Regulation of a group of abundant mRNA sequences during Friend cell differentiation. Dev Biol. 1979 Sep;72(1):110–125. doi: 10.1016/0012-1606(79)90102-7. [DOI] [PubMed] [Google Scholar]
- Aviv H., Voloch Z., Bastos R., Levy S. Biosynthesis and stability of globin mRNA in cultured erythroleukemic Friend cells. Cell. 1976 Aug;8(4):495–503. doi: 10.1016/0092-8674(76)90217-8. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Graham D. E., Neufeld B. R. Analysis of repeating DNA sequences by reassociation. Methods Enzymol. 1974;29:363–418. doi: 10.1016/0076-6879(74)29033-5. [DOI] [PubMed] [Google Scholar]
- Dubroff L. M., Nemer M. Molecular classes of heterogeneous nuclear RNA in sea urchin embryos. J Mol Biol. 1975 Jul 5;95(3):455–476. doi: 10.1016/0022-2836(75)90203-x. [DOI] [PubMed] [Google Scholar]
- Galau G. A., Klein W. H., Davis M. M., Wold B. J., Britten R. J., Davidson E. H. Structural gene sets active in embryos and adult tissues of the sea urchin. Cell. 1976 Apr;7(4):487–505. doi: 10.1016/0092-8674(76)90200-2. [DOI] [PubMed] [Google Scholar]
- Galau G. A., Lipson E. D., Britten R. J., Davidson E. H. Synthesis and turnover of polysomal mRNAs in sea urchin embryos. Cell. 1977 Mar;10(3):415–432. doi: 10.1016/0092-8674(77)90029-0. [DOI] [PubMed] [Google Scholar]
- Groudine M., Weintraub H. Rous sarcoma virus activates embryonic globin genes in chicken fibroblasts. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4464–4468. doi: 10.1073/pnas.72.11.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpold M. M., Evans R. M., Salditt-Georgieff M., Darnell J. E. Production of mRNA in Chinese hamster cells: relationship of the rate of synthesis to the cytoplasmic concentration of nine specific mRNA sequences. Cell. 1979 Aug;17(4):1025–1035. doi: 10.1016/0092-8674(79)90341-6. [DOI] [PubMed] [Google Scholar]
- Hastie N. D., Bishop J. O. The expression of three abundance classes of messenger RNA in mouse tissues. Cell. 1976 Dec;9(4 Pt 2):761–774. doi: 10.1016/0092-8674(76)90139-2. [DOI] [PubMed] [Google Scholar]
- Herman R. C. Analysis of the message-sequence content of the pulse-labeled poly(A)+ heterogeneous nuclear RNA from HeLa cells by cDNA-excess hybridizations. Biochemistry. 1979 Mar 6;18(5):916–920. doi: 10.1021/bi00572a029. [DOI] [PubMed] [Google Scholar]
- Humphries S., Windass J., Williamson R. Mouse globin gene expression in erythroid and non-erythroid tissues. Cell. 1976 Feb;7(2):267–277. doi: 10.1016/0092-8674(76)90026-x. [DOI] [PubMed] [Google Scholar]
- Kleene K. C., Humphreys T. Similarity of hnRNA sequences in blastula and pluteus stage sea urchin embryos. Cell. 1977 Sep;12(1):143–155. doi: 10.1016/0092-8674(77)90192-1. [DOI] [PubMed] [Google Scholar]
- Leibovitch M. P., Leibovitch S. A., Harel J., Kruh J. Changes in the frequency and diversity of messenger RNA populations in the course of myogenic differentiation. Eur J Biochem. 1979 Jul;97(2):321–326. doi: 10.1111/j.1432-1033.1979.tb13117.x. [DOI] [PubMed] [Google Scholar]
- Marcu K. B., Valbuena O., Perry R. P. Isolation, purification, and properties of mouse heavy-chain immunoglobulin mRNAs. Biochemistry. 1978 May 2;17(9):1723–1733. doi: 10.1021/bi00602a022. [DOI] [PubMed] [Google Scholar]
- Maxwell I. H., Van Ness J., Hahn W. E. Assay of DNA-RNA hybrids by S1 nuclease digestion and adsorption to DEAE-cellulose filters. Nucleic Acids Res. 1978 Jun;5(6):2033–2038. doi: 10.1093/nar/5.6.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColl R. S., Aronson A. I. Changes in transcription patterns during early development of the sea urchin. Dev Biol. 1978 Jul;65(1):126–138. doi: 10.1016/0012-1606(78)90185-9. [DOI] [PubMed] [Google Scholar]
- Nemer M., Dubroff L. M., Graham M. Properties of sea urchin embryo messenger RNA containing and lacking poly(A). Cell. 1975 Oct;6(2):171–178. doi: 10.1016/0092-8674(75)90007-0. [DOI] [PubMed] [Google Scholar]
- Nemer M., Graham M., Dubroff L. M. Co-existence of non-histone messenger RNA species lacking and containing polyadenylic acid in sea urchin embryos. J Mol Biol. 1974 Nov 5;89(3):435–454. doi: 10.1016/0022-2836(74)90474-4. [DOI] [PubMed] [Google Scholar]
- Ono T., Getz M. J. Levels of ovalbumin messenger RNA sequences in nonoviduct tissues of the chicken. Dev Biol. 1980 Mar 15;75(2):481–484. doi: 10.1016/0012-1606(80)90180-3. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Davidson E. H., Britten R. J. A program for least squares analysis of reassociation and hybridization data. Nucleic Acids Res. 1977 Jun;4(6):1727–1737. doi: 10.1093/nar/4.6.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiners J. J., Jr, Busch H. Transcriptional and posttranscriptional modulation of cytoplasmic ribonucleic acids in regenerating liver and Novikoff hepatoma. Biochemistry. 1980 Mar 4;19(5):833–841. doi: 10.1021/bi00546a002. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Shepherd G. W., Flickinger R. Post-transcriptional control of messenger RNA diversity in frog embryos. Biochim Biophys Acta. 1979 Jul 26;563(2):413–421. doi: 10.1016/0005-2787(79)90060-1. [DOI] [PubMed] [Google Scholar]
- Sippel A. E., Hynes N., Groner B., Schütz G. Frequency distribution of messenger sequences within polysomal mRNA and nuclear RNA from rat liver. Eur J Biochem. 1977 Jul 1;77(1):141–151. doi: 10.1111/j.1432-1033.1977.tb11652.x. [DOI] [PubMed] [Google Scholar]
- Swaneck G. E., Nordstrom J. L., Kreuzaler F., Tsai M. J., O'Malley B. W. Effect of estrogen on gene expression in chicken oviduct: evidence for transcriptional control of ovalbumin gene. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1049–1053. doi: 10.1073/pnas.76.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold B. J., Klein W. H., Hough-Evans B. R., Britten R. J., Davidson E. H. Sea urchin embryo mRNA sequences expressed in the nuclear RNA of adult tissues. Cell. 1978 Aug;14(4):941–950. doi: 10.1016/0092-8674(78)90348-3. [DOI] [PubMed] [Google Scholar]
- Young B. D., Birnie G. D. Complexity and specificity of polysomal poly(A+) RNA in mouse tissues. Biochemistry. 1976 Jun 29;15(13):2823–2829. doi: 10.1021/bi00658a019. [DOI] [PubMed] [Google Scholar]



