Abstract
In alphabetic language systems, converging evidence indicates that developmental dyslexia represents a disorder of phonological processing both behaviorally and neurobiologically. However, it is still unknown whether, impaired phonological processing remains the core deficit of impaired English reading in individuals with English as their second language and how it is represented in the neural cortex. Using functional magnetic resonance imaging, the present study investigated the neural responses to letter rhyming judgment (phonological task) and letter same/different judgment (orthographic task) in Chinese school children with English and Chinese reading impairment compared to typically developing children. Whole brain analyses with multiple comparison correction revealed reduced activation within the left lingual/calcarine gyrus during orthographic processing in children with reading impairment compared to typical readers. An independent region of interest analysis showed reduced activation in occipitotemporal regions during orthographic processing, and reduced activation in parietotemporal regions during phonological processing, consistent with previous studies in English native speakers. These results suggest that similar neural deficits are involved for impaired phonological processing in English as both the first and the second language acquired. These findings pose implications for reading remediation, educational curriculum design, and educational policy for second language learners.
Keywords: Children, impaired reading, Phonological processing, Orthographic processing, Second language learning, fMRI developmental dyslexia
Introduction
Phonological skills have been shown to be important during reading development (Wagner and Torgesen, 1987; Bradley and Bryant, 1983; Lukatela and Turvey, 1994). Longitudinal and intervention studies demonstrated that phonological skills could predict and might play a causal role in literacy development (Bradley and Bryant, 1978, 1983; Lundberg et al., 1988). Phonological abilities are therefore suggested to be essential for success in learning to read. Deficits of phonological skills are also found to be a most prominent characteristic of developmental dyslexia (Ramus et al., 2003; Shaywitz and Shaywitz, 2005; Shaywitz et al., 1998).
Little is known about the development of phonological or orthographic skills in children with English as their second language (ESL), and especially in ESL children who show reading deficits in English with varying abilities in their mother tongue. It is therefore important to understand whether children’s different linguistic backgrounds influence the process of learning to read in English and whether the underlying neural mechanisms for reading sub skills, such as phonological or orthographic processing, are similar in children with English as their first language compared to ESL children. Previous research has suggested that educational variables such as program type, method of instruction, socioeconomic status or characteristics of the native language of the child may impact literacy proficiency in ESL children (August and Hakuta, 1997; Fitzgerald, 1995; Hakuta, 1999; Tabors and Snow, 2001). However, no study to date has compared the underlying neural mechanisms of phonological and orthographic processing in ESL children with and without reading impairment in English.
The neural correlates of phonological processing have been identified in typically developing English (as the first language) speaking readers (L1), which is a dorsal pathway in the left-hemisphere including the inferior parietal lobule and the posterior aspect of the superior temporal gyrus (see review, Pugh et al., 2000; Temple, 2002). Converging evidence also showed reduced activation in individuals with impaired reading compared to typical readers in the left posterior parietotemporal region during letter rhyming (Temple et al., 2001; Hoeft et al., 2006), nonword rhyming (Shaywitz et al., 2002) and semantic category judgment (Shaywitz et al., 2002). The parietotemporal regions have also been suggested to be involved in the mapping of phonology onto orthography (Hoeft et al., 2007). Moreover, there is evidence that the reduced activation in this area in individuals with developmental dyslexia is consistent across different language systems (Seki et al., 2001; Paulesu et al., 2001).
On the other hand, orthographic processing skills have also been shown to be fundamental in the visual recognition of words and reading (LaBerge and Samuels, 1974; McClelland and Rumelhart, 1981; Coltheart et al., 1993). Behaviorally, orthographic skills have been shown to serve as a potential source for variance in reading acquisition (Stanovich and West, 1989; Cunningham and Stanovich, 1990). Furthermore, impaired visual processing in individuals with reading impairments has also been reported, suggesting that orthographic processing still plays an independent role in reading even if controlled for phonological processing (Stanovich and West, 1989; Cunningham et al., 2001; Cunningham and Stanovich, 1990; Berninger, 1994).
Moreover, activation in the left occipitotemporal area has been found during visual language processing across different language systems (Bolger et al., 2005; Price, 2000; Xue et al., 2005), indicating its role in processing the orthographic structure of well-learned visual word forms (Cohen et al., 2000; Kuo et al., 2004; Binder et al., 2006; Kronbichler et al., 2004; Puce et al., 1996). In addition, evidence from developmental studies (Booth et al., 2001; Turkeltaub et al., 2003) revealed engagement of this region during printed-word recognition with increased reading skills. In addition, individuals with developmental dyslexia in both alphabetic (Brunswick et al., 1999; Shaywitz et al., 2003; Cao et al., 2006; Maurer et al., 2007) and non-alphabetic (Siok et al., 2004) language systems exhibited hypoactivation in the occipitotemporal region during visual word processing when compared to typical individuals, suggesting a cross-language deficit for orthographic processing. However, the precise role of the left occipitotemporal area during orthographic processing has been debated. Some suggest that this area is responsible for the extraction of abstract visual word form, i.e., feature-invariant, pre-lexical, visual word recognition (Binder et al., 2006; Dehaene et al., 2005); some argue that it is more likely to be involved in lexical processing (Kronbichler et al., 2006, 2007).
Furthermore, a number of other studies suggest that the occipitotemporal region is not sensitive to familiar orthography (Price et al., 1996; Binder et al., 2003; Tagamets et al., 2000), and that its activation may be modulated by various factors beyond orthographic processing, such as phonology (Xue et al., 2006, 2008; Xue and Poldrack, 2007; Brem et al., 2010). Based on these findings, one can argue that the hypoactivation in the occipototemporal brain region may not reflect a specific orthographic deficit, but a deficit in the interaction or connectivity between visual and phonological processing components necessary for reading (e.g.; McCrory et al., 2005).
In short, despite the literature focusing on the role for phonological and orthographic processing at either the behavior level or the neural correlates for these two processes in first language (L1), be it an alphabetical or non-alphabetical language system, the neural correlates of phonological and orthographic processing in the second language (L2) remain unknown.
Most research studies exploring difficulties with English reading in Chinese children have utilized behavioral measures of phonological processing but the results are somewhat controversial (Wang et al., 2002; Yu and Wang, 2001; Ho and Fong, 2005). Specifically, Wang et al. (2002) and Yu and Wang (2001) found no significant group differences in phonological awareness between impaired English readers and typical readers. Furthermore, contrary to the findings in alphabetic language system, regression analyses revealed a negative relationship between phonological awareness and English reading comprehension among adolescence (Wang et al., 2002; Yu and Wang, 2001), which may result from the traditional Chinese reading pedagogy, addressing word forms instead of phonology. However, Ho and Fong (2005) revealed that Chinese children with developmental dyslexia demonstrated a weakness in English reading and phonological processing when compared to typical readers. Additionally, the Chinese children’s phonological performance in English exhibited a significantly positive correlation with their English word reading, suggesting that phonological skills are also fundamental in learning English as a second language. Nevertheless, differences in age and type of subjects (Chinese children with poor English skills vs. Children with Chinese reading impairment) in different studies should be noted. Moreover, these studies addressed phonological processing in English in Chinese native speakers, but they neglected measuring orthographic skills, which might serve as an important factor in a logographic language system like Chinese (Chen and Juola, 1982; Leck et al., 1995).
The neural substrates for phonological and orthographic processes in L2 remain unclear. Only a few studies have investigated the neural correlates for English learning in native Chinese, and most of them focused more on the differential neural activation between L1 and L2, without further discussion of the actual activation in L2 (Ding et al., 2003; Tan et al., 2003). Furthermore, only a few studies have directly investigated both of these two processes within the same group of subjects, which makes the comparison and contrast between neural correlates for these two processes less comparable due to variations in subjects and experimental paradigms across different studies.
Here, using functional magnetic resonance imaging (fMRI), we investigated both English orthographic and phonological processing in Chinese school children with English reading impairment and typically developing children. Brain activation during a letter matching task (e.g. do D and D match?) and rhyme judgment task (e.g. do D and T rhyme?; Temple et al., 2001, 2003) was compared between 12 children with impaired English reading and 16 age- and IQ-matched typical developing peers respectively. The aim of this study was to identify the neural substrates and deficits of English orthographic and phonological processing in Chinese school children with reading impairments in English. If the neurocognitive deficits for impaired English reading are universal regardless of which first language is learned first, we expect the atypical activation pattern for impaired English readers among Chinese-speaking children to be similar to the activation pattern of children with English as their first language. On the other hand, if the neural impairments of impaired English reading among Chinese children are different from native English speakers in orthographic and/or phonological processing, it may suggest that reading in the second language English in Chinese children has specific neural correlates. These findings will provide theoretical and practical implications for English as a second language (ESL) teaching pedagogy. This is the first study to investigate the underlying neural mechanisms of reading deficits in second language learners and will therefore contribute to the growing body of behavioral studies investigating challenges of acquisition as well as instruction of English as a second language (ESL).
Methods
Participants
Thirty-six children in grades 4, 5, and 6 participated in the present study (note: fMRI and behavioral analyses were based on twenty-eight children, see the fMRI data analysis for more details). They were screened in several primary schools in Beijing. None of the participants had a history of neurological diseases, head injury, or psychiatric disorders. The DSM-IV Attention-Deficit/Hyperactivity Disorder (ADHD) Scale (American Psychiatric Association, 1994) was also used to exclude children with ADHD. All the participants were right handed according to self report (Edinburgh Handedness Inventory (Oldfield, 1971)), and had normal or corrected-to-normal vision. All of these children were native speakers of Chinese, the official dialect of Mainland China and the language taught in school, and started learning English formally as their second language from schooling age (about age 6). Informed consent was obtained from each subject and their parents before participation. This study was approved by the ethical review board at the State Key Laboratory of Cognitive Neuroscience and Learning of Beijing Normal University.
The 36 participants were selected among 857 children in grades 4, 5 and 6, and divided into two groups: 19 impaired and 17 typically developing readers, according to a number of standardized English tests (see below and Table 1).
Table 1.
Participants’ characteristics and mean scores for reading measures, with minimum and maximum (in parenthesis).
| Variable | Impaired readers | Typical readers | p |
|---|---|---|---|
| Sample size | 12 | 16 | |
| Age (years) | 9.9(8.3–11.0) | 10.0(8.9–11.1) | ns |
| Gender (male/female) | 7/5 | 3/13 | |
| Raven’sa | 74% (50%–95%) | 77% (50%–95%) | ns |
| Spellingb | 79.71(68.04–87.65) | 122.37(110.77–137.83) | <0.001 |
| WRAT-spellingc | 0.8(0–3.0) | 5.1(1.0–15.0) | <0.001 |
| Word readingc | 7.5(1.0–17.0) | 39.1(33.0–44.0) | <0.001 |
| Woodcock–Johnson Reading Masteryc | |||
| Word identification (word reading) | 15.3(5.0–19.0) | 27.0(21.0–33.0) | <0.001 |
| Word-attack (non-word decoding) | 2.8(0–7.0) | 14.4(7.0–22.0) | <0.001 |
| Phonological Awareness Testc | |||
| Rhyme detection | 5.1(2.0–9.0) | 8.5(5.0–10.0) | <0.001 |
| Oral cloze | 0.6(0–4.0) | 8.4(6.0–10.0) | <0.001 |
| Syllable identification | 6.2(6.0–8.0) | 6.8(6.0–8.0) | <0.05 |
| Initial phoneme deletion | 3.9(0–8.0) | 7.4(4.0–8.0) | <0.005 |
| Chinese Reading Testb | |||
| Reading Fluency Test | 96.74(78.18–109.64) | 108.37(82.60–132.73) | <0.05 |
| Chinese Vocabulary Test | 90.37(59.17–114.19) | 114.05(103.21–126.04) | <0.001 |
Items for each test listed in raw scores: WRAT-spelling=40, Word reading=45, Word identification=58, Word-attack=30, Rhyme detection=10, Oral cloze=10, Syllable identification=8, Initial phoneme deletion=8.
Percentiles.
Standard scores.
Raw scores.
The WRAT-spelling test (Wide Range Achievement Test-Revision 3, Wilkinson, 1993) was first used as the main screening test for impaired English readers. However, we found that this test was too difficult for Chinese children in grades 4–6, with a mean score of 1.72 out of 40 (n=857). Such a floor effect meant a narrow range and further led to a seriously skewed distribution of scores, which resulted in a poor differentiation of subjects. Therefore, raw scores of this test are listed for each group for reference.
Instead, a spelling test was developed as the main screening test for impaired English readers by us because there was no standardized English test for Chinese children. 279 words were chosen from primary school English textbooks for Chinese-speaking children; then 158 children from grades 1, 3 and 5 were asked to rate the familiarity of each word on a 5-point scale. The average familiarity score for each word was taken as its indication for word frequency. In addition, grapheme-to-phoneme regularity was taken into account when a word was chosen. Forty words were used in the Spelling test, half identified as high-frequency words, and the other half low-frequency ones. In each word group with high/low word frequency, half followed the grapheme-to-phoneme conversion rules, and the other half did not. During test administration, each word was read aloud two times and the participants were required to write down the word on the answer sheet. The test–retest reliability of this test and its correlation with WRAT-spelling were 0.96 and 0.78 respectively. As for the Spelling test, in order to compare scores from different grades, scores for all tested children were converted to standard scores using the following procedure. Firstly, means and standard deviations (SD) for each grade were calculated. Secondly, the raw score of each individual was transformed into Z-scores based on the mean and SD of his/her grade (see Liu et al., 2009). Finally, Z-scores were converted to standard scores with a Mean of 100 and a SD of 15.
An additional Word Reading test, a subtest of the English Phonological Awareness test, was also used as a complementary screening test for impaired readers. 45 English words, chosen mainly from primary school English textbooks were included. The children were asked to read aloud as many words as possible until 4 consecutive word errors, and the number of correct responses was recorded as raw scores.
Furthermore, the Raven Standard Progressive Matrices were used to measure children’s nonverbal IQ. Scoring procedures were based on the Chinese norm (Zhang and Wang, 1985).
Three criteria needed to be met for selecting English reading impaired children (IR), as follows: first, the percentile in the Raven test needed to be above the 50th percentile to ensure average IQ; second, the standard score for the Spelling test needed to be at most 88 (below standard score 90); third, the raw scores for the Word Reading test needed to be below the grade average. The age- and grade-matched typical developing readers were selected among the reading impaired children’s peers. For children defined as typical English readers (TR), despite normal IQ as measured by the Raven test, the standard score for the Spelling test needed to be above the 70th percentile and that for the Word Reading test needed to be above the grade average. Overall, 19 impaired English readers and 17 typical English readers match the criteria for analysis. Similar standards for recruiting children with dyslexia or with reading impairment were implemented by Siok et al. (2004, 2008).
A battery of assessments was administered to measure reading, decoding, and phonological abilities: the subtests word identification and word attack from the Woodcock Diagnostic Reading Battery (Woodcock, 1987) and Phonological Awareness Tests. The English Phonological Awareness Test we used here was designed to assess the English phonological awareness in Chinese-speaking children. Four subtests were administered: rhyme detection, oral cloze, syllable identification and initial phoneme deletion. The overall test–retest reliability of this test is 0.92, which was calculated using a sample of 171 subjects who had done the test twice within an interval of 3 weeks.
Table 1 shows the average percentile for the Raven’s test and the average standard score for Spelling in the two groups, with minimum and maximum for each test in parenthesis. Means and ranges for raw scores of WRAT- spelling, word identification, word attack and Phonological Awareness Tests are shown in the table. Scores could not be converted into standard score because the tests either had skewed distribution resulting from the floor effect, or contained too few items which led to less differentiation. Seven reading impaired children and one typical control were excluded in the final fMRI analysis due to image quality or technical problems.
Chinese reading ability was also tested through a Reading Fluency Test and a Chinese written vocabulary test. The Reading Fluency Test measuring reading comprehension had 95 sentences. Each sentence was paired with 5 multiple choice pictures. Participants were asked to read each sentence and select from the five pictures the one that best illustrated the meaning of the sentence. Children were encouraged to complete as many paragraphs as possible within a 10-minute time period. The performance score was determined by the total number of sentences the participants could understand. Rapid retrieval and retention of lexical information and construction of sentential representation were needed to complete the task.
The standardized written vocabulary test (Wang and Tao, 1996) involved 210 characters divided into 10 groups based on their difficulty level in reading. Participants were asked to write down a compound word based on a constituent morpheme provided on the sheet. Performance was measured by the total number of correct characters (morphemes) the participants could make use of in word-compositions. Participants had to know morpheme combination rules to form a compound word.
Standard scores for these two tests were calculated following similar steps than performed for the English spelling test. The impaired English readers showed reduced performance compared to the typical readers on both Chinese written vocabulary test and Reading Fluency Test (see Table 1). Specifically, for the impaired English reading group, 4 children scored less than 90 in the Reading Fluency Test, the other 4 scored less than 90 in the Chinese written vocabulary test. Additionally, 3 children scored less than 100 in the Reading Fluency Test and 5 scored less than 100 in the vocabulary test. However, for the typical English readers, only 2 out 16 showed scores less than 100 in the Reading Fluency Test and no child performed below 100 for the written vocabulary test. Such results indicated that children in the impaired English reading group also, to some extent, showed impaired reading in Chinese when compared to the control group.
Design and materials
Task design
A phonological and an orthographical processing task were used in the fMRI scanner (Fig. 1). Each consisted of an active condition and a rest condition with fixation.
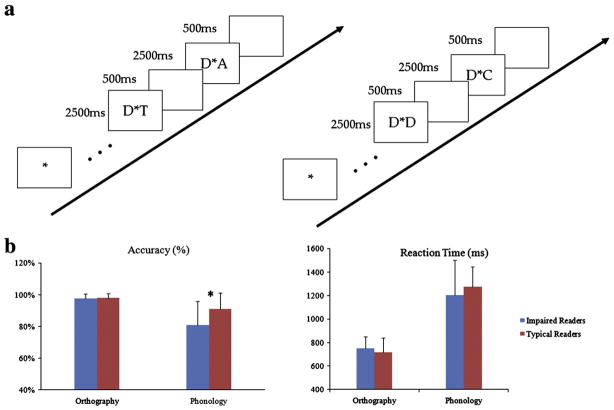
Fig. 1.
(a) Graphical description of task design for both tasks: phonology (left) and orthography (right). In each trial, a pair of two letters was synchronously shown for 2500 ms, one left and one right to a fixation asterisk, followed by a 500 ms blank interval. The participants were asked to indicate via button press whether these two letters rhyme or are the same within the 2500 ms from the onset of the stimulus. (b) Behavioral results. Accuracy (left) and reaction time (right) for typical English readers (in red or gray) and impaired English readers (in blue or black) for two tasks (see results). Impaired readers were less accurate than typical ones for the phonological task, while the two groups showed no significant difference in accuracy of the orthographical task. For reaction time, there is no group difference for both tasks.
During the phonological processing task, participants judged whether two letters, visually presented at the same time on the screen, rhymed (e.g., D and T) or not (e.g., D and A), using a button response. During the orthographic task, children were asked to indicate whether two visually presented letters were the same (e.g., D and D) or not (e.g., D and A). During the rest condition, children were required to fixate an asterisk presented in the middle of the screen and no response was required.
The two tasks were presented separately in a block design, where six blocks of letter-rhyme/letter-form judgments were alternated with seven blocks of rest conditions. We used varying durations for activation blocks in both the phonological and orthographic tasks to reduce the potential confound resulting from periodic noise either from physiological rhythms or scanners or participants’ expectation. The average time for each block was 33 s for each activation block and 24 s for each rest block in both tasks. Each activation block included 8–12 trials. In each trial, a pair of two letters was synchronously exposed for 2500 ms, one on the left and one on the right side of a fixation asterisk, followed by a 500 ms blank interval. The participants were asked to response within the 2500 ms after the onset of the stimulus. Measures of task accuracy and reaction time (RT) were obtained.
Image acquisition
The MRI imaging and imaging related procedures were performed at the Brain Imaging Center (State Key Laboratory of Cognitive Neuroscience and Learning, Beijing Normal University). A 3.0 T Siemens Trio scanner was used. A T2*-weighted gradient-echo planar Imaging (EPI) sequence sensitive to blood oxygen level-dependent contrast was used for fMRI scans with the following acquisition parameters: repetition time, 3000 ms; echo-time, 30 ms; flip-angle, 90°; field-of-view, 20×20 cm, matrix size, 64×64, 30 slices (4 mm).
fMRI data analysis
Eight participants, seven reading impaired and one typical control, were excluded in the analysis due to observable poor image quality screened by an experienced MRI technician, or technical problems of stimuli display during the scanning. The data of twenty-eight participants was analyzed with statistical parametric mapping software (SPM5; Wellcome Department of Cognitive Neurology, London, UK). After discarding the first 4 volumes of each subject in order to obtain T1 equilibration, the functional images were realigned to the first volume in the scanning session using affine transformations. No participant had more than 3.0 mm of movement in any plane. Then, the images were co-registered to their corresponding anatomical volumes, and normalized to Montreal Neurological Institute (MNI) stereotaxic space using parameters obtained from anatomical segmentation, and resampled to voxel size of 2×2×2 mm. Spatial smoothing was performed with a Gaussian filter (8 mm full width at half maximum). Although conditions were blocked, to exclude the possibility that incorrect responses might confound the results, we adopted an event related analysis to dissociate correct from incorrect responses (Hu et al., 2010) for the first level analysis, in which a canonical hemodynamic response function was convolved with event-related delta functions, resulting in separate models of correct responses for both orthographic and phonological tasks. The condition effects for individual participants were estimated using the general linear model (GLM). Group analysis was carried out with a random effects model (Friston et al., 1999). For orthographic processing, regions of activation were identified through letter-form judgment (Temple et al., 2001) vs. rest condition (fixation) contrast images using one-sample t-tests for each group separately. The statistical threshold was set at p<0.001(FDR corrected) with extend threshold of 10 voxels. Next, a two-sample t test was performed (p=0.005 uncorrected; extent threshold=10) to access significant difference in brain activation between the two groups. For phonological processing, regions of activation were defined through letter-rhyme judgment vs. letter-form judgment contrast images using one-sample t-test for each group separately. The statistical threshold for phonological processing was set at p<0.025 uncorrected (ET=80) for increased sensitivity. As for the direct comparison between two groups, a two-sample t test was implemented with p<0.005 uncorrected and ET=10.
In order to deal with the multiple comparisons issue, alphasim correction was conducted across the whole brain (Yan et al., 2009). The between-group statistical threshold was set at p<0.005 and cluster size>153 mm3, which corresponded to a corrected p<0.05.
Also, we conducted independent regions of interests (ROIs) analysis with Marsbar (http://marsbar.sourceforge.net) for left inferior occipital and left angular brain regions, where deficits in individuals with developmental dyslexia have been found in previous studies (Cao et al., 2006; Brunswick et al., 1999; Meyler et al., 2008; Shaywitz et al., 1998; Temple et al., 2001). The co-ordinates of the two spherical ROIs (8 mm radius) were defined from Brunswick et al. (1999) and from Meyler et al. (2008) for left inferior occipital and for left angular, respectively. Mean activation (β estimates) within each region for each participant was extracted. An Independent t-test was then used to compare activation difference between groups at the threshold of p<0.05.
Results
Behavioral results of the fMRI experiment
Mixed-model ANOVAs were carried out with task (orthography/phonology) as the within subjects factor and group (typical/impaired English readers) as the between subjects factor. For accuracy, there was a significant main effect of task (F(1,26)=29.60, p<0.001), a marginally significant main effect of group (F(1,26)=4.16, p=0.052) and a group×task interaction (F(1,26)=5.12, p <0.05). Typical children’s performance was better (91%) than children with reading impairment (81%) for the phonological task but they did not differ for the orthographical task. For reaction time, a significant main effect of task indicated that the orthographical task (731 ms) was performed faster than the phonological one (1245 ms) across groups (F(1,26)=157.03, p<0.001). Neither the main effect of group nor the task×group interaction was significant for reaction time.
We also conducted a Pearson correlation between the accuracy of the rhyme task and the behavioral evaluations. Except for WRAT-words spelling and the self-developed spelling test, each of the evaluations is significantly correlated with the in-scanner performance (word identification: r=0.48, p<0.05; word attack: r=0.55, p<0.01; word reading: r=0.50, p<0.01; rhyme detection: r=0.55, p<0.01; oral cloze: r=0.42, p<0.05; syllable identification: r=0.42, p<0.05; initial phoneme deletion: r=0.53, p<0.01).
fMRI results
Orthographical processing
Activation patterns in typical and impaired English readers
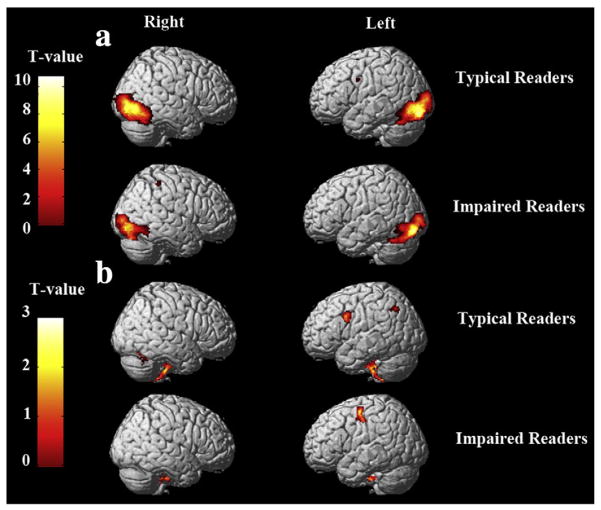
Orthographic processing was defined as letter-match vs. fixation. Whole-brain analyses were conducted for the typical and impaired group. Compared with fixation, the typical readers showed activation in bilateral lingual, bilateral inferior occipital, left calcarine, several regions in the frontal lobe and left thalamus. Impaired English readers exhibited activation in bilateral lingual, bilateral inferior occipital, right inferior parietal and left SMA regions (Table 2, pFig. 2a). Increased activations for the typical readers than impaired ones in bilateral temporal lobe, right precentral and bilateral occipital (Table 4) were observed. Notably, the strongest difference was shown in the left lingual (cluster size=197, <0.001), which is extended to the left calcarine (BA 17).
Table 2.
Coordinates of activation peaks, match letters vs. fixation.
| Region | Cluster size | BA | x | y | Z | Z | p(FDR) |
|---|---|---|---|---|---|---|---|
| Typical English readers (n=16) | |||||||
| Occipital lobe | |||||||
| R lingual | 3955 | 18 | 18 | −88 | −8 | inf | 0.000 |
| R occipital inf | 19 | 42 | −72 | −8 | inf | 0.000 | |
| R occipital inf | 19 | 40 | −86 | −10 | 7.75 | 0.000 | |
| L lingual | 4052 | 18 | −24 | −86 | −16 | Inf | 0.000 |
| L occipital inf | 19 | −44 | −80 | −8 | 7.80 | 0.000 | |
| L calcarine | 18 | −12 | −92 | −6 | 7.18 | 0.000 | |
| Frontal lobe | |||||||
| L supp motor area | 1192 | 32 | −2 | 10 | 50 | 6.51 | 0.000 |
| L supp motor area | 6 | −6 | 2 | 66 | 4.73 | 0.000 | |
| L precentral | 16 | 6 | −46 | 2 | 34 | 4.21 | 0.000 |
| Subcortical | |||||||
| L thalamus | 33 | N/A | −4 | −24 | 8 | 4.45 | 0.000 |
| Impaired English readers (n=12) | |||||||
| Occipital lobe | |||||||
| R lingual | 2292 | 18 | 18 | −90 | −8 | 7.75 | 0.000 |
| R occipital inf | 19 | 40 | −86 | −10 | 6.98 | 0.000 | |
| R occipipal inf | 19 | 42 | −74 | −10 | 6.15 | 0.000 | |
| L lingual | 2511 | 18 | −24 | −86 | −16 | 6.91 | 0.000 |
| L occipital inf | 19 | −38 | −84 | −12 | 6.66 | 0.000 | |
| L occipital mid | 18 | −26 | −94 | 4 | 6.57 | 0.000 | |
| Frontal lobe | |||||||
| L supp motor area | 170 | 6 | −8 | 6 | 52 | 4.90 | 0.000 |
| Parietal lobe | |||||||
| R parietal inf | 24 | 40 | 54 | −38 | 56 | 4.43 | 0.000 |
L, left; R, right; N/A, not applicable.
Fig. 2.
(a) Whole brain activation for the contrast letter match > fixation. The group activation difference is rendered on a 3D brain (FDR p<0.001 ET, 10). The activation map is based on T-value in SPM5. (b) Whole brain activation for the contrast rhyme>letter match. The group activation difference is rendered on a 3D brain (p<0.025, unc. ET, 80). The activation map is based on T-value in SPM5.
Table 4.
Group difference, typical English readers > impaired English readers, n=28.
| Region | Cluster size | BA | x | y | z | Z | p(unc.) |
|---|---|---|---|---|---|---|---|
| Letter-match vs. fixation | |||||||
| Occipital lobe | |||||||
| L linguala | 197 | 17 | −8 | −54 | 4 | 3.71 | 0.000 |
| R calcarine | 11 | 17 | 6 | −64 | 14 | 2.70 | 0.003 |
| Temporal lobe | |||||||
| R temporal inf | 22 | 19 | 44 | −68 | −4 | 3.02 | 0.001 |
| L temporal mid | 26 | 37 | −58 | −58 | 10 | 2.81 | 0.002 |
| Frontal lobe | |||||||
| R precentral | 24 | 6 | 48 | −6 | 56 | 3.07 | 0.001 |
| Rhyme judgment vs. letter match | |||||||
| Parietal lobe | |||||||
| L angular | 42 | 19 | −40 | −72 | 42 | 2.99 | 0.001 |
Region that survived through alphasim correction.
Activation after multiple comparison correction
Alphasim correction was conducted for the between-group contrast across the whole brain. Results showed that the left lingual/calcarine gyrus was the only cluster that survived the multiple comparison correction.
ROI analysis
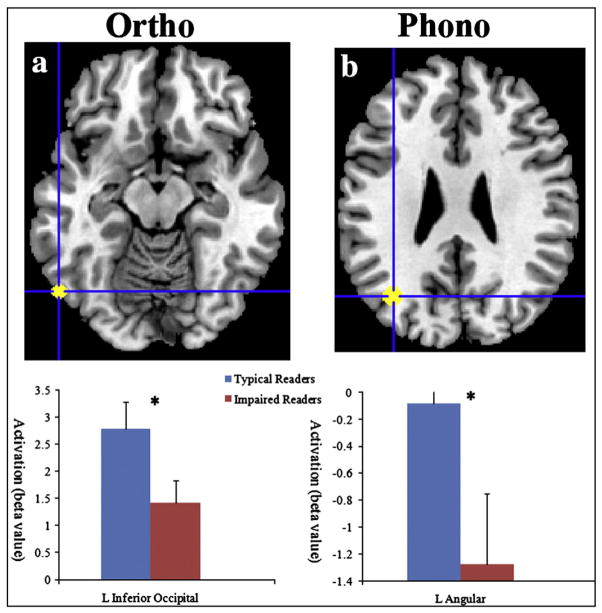
To illustrate the group activation differences within the left inferior occipital region (occipitotemporal region, BA 37), Fig. 3a shows the mean activation (β estimates) in this region for each group for orthographic processing greater than rest, which was calculated by averaging the Beta value of all voxels within the defined ROIs (βTR =2.79;βIR =1.42; t(26) =2.06, p < 0.05).
Fig. 3.
Brain activation patterns comparing impaired English readers (IR) and Typical English readers (TR). The location map, based on previous literature, was generated in Marsbar. (a) Increased activation (β estimates) for TR compared to IR for orthographic processing (letter match vs. rest) with the left inferior occipital region (BA 37). (b) Increased activation for TR compared to IR for phonological processing (rhyme vs. letter match). Bar graphs represent the mean contrast β values and error bars represent SEM. *<0.05.
Phonological processing
Activation patterns in typical and impaired English readers
For the phonological task contrasted with letter matching, typical readers exhibited neural activation in several regions within the frontal lobe, left inferior parietal and the cerebellum. Impaired English readers exhibited activity in left precentral areas, left postcentral areas and the cerebellum (Table 3). Direct comparison between these two groups indicated increased activation in typical compared to the impaired readers in left angular regions (Table 4).
Table 3.
Coordinates of activation peaks, rhyme judgment vs. letter match.
| Region | Cluster size | BA | X | Y | z | Z | p(unc.) |
|---|---|---|---|---|---|---|---|
| Typical English readers(n=16) | |||||||
| Frontal lobe | |||||||
| L frontal inf oper | 171 | 44 | −46 | 8 | 30 | 2.97 | 0.002 |
| L supp motor area | 168 | 6 | −2 | 8 | 60 | 2.79 | 0.003 |
| Parietal lobe | |||||||
| L parietal inf | 149 | 7 | −32 | −62 | 42 | 2.50 | 0.006 |
| Cerebellum | |||||||
| R cerebellum 3 | 2034 | 30 | 14 | −32 | −22 | 3.90 | 0.000 |
| R cerebellum 6 | 284 | 19 | 26 | −66 | −20 | 3.04 | 0.001 |
| Impaired English readers (n=12) | |||||||
| Frontal lobe | |||||||
| L precentral | 169 | 6 | −42 | −10 | 60 | 2.67 | 0.004 |
| L supp motor area | 89 | 6 | −4 | 0 | 64 | 2.65 | 0.004 |
| Parietal lobe | |||||||
| L postcentral | 4 | −50 | −14 | 46 | 2.31 | 0.010 | |
L, left; R, right; N/A, not applicable.
Activation after multiple comparison correction
Alphasim correction was conducted for between-group contrast across the whole brain. No cluster survived the threshold.
ROI analysis
Fig. 3b shows the mean activation (β estimates) within the left angular gyrus (parietotemporal region, BA 7). The mean activation was calculated by averaging the Beta values of all voxels within the defined ROI for phonological processing>orthographic processing (βTR = −0.08; βIR = −1.28; t(26) =2.14, p < 0.05).
In order to confirm that the observed group differences in Fig. 3b are due to group differences during the rhyming condition rather than the letter matching condition (the baseline), we further examined the group activation differences (β estimates) for rhyming vs. rest, and letter matching vs. rest. Mean activation in the left angular for each subject was extracted for the rhyming vs. rest contrast and for the letter matching vs. rest contrast respectively. For letter matching vs. rest, there was no significant difference between the two participant groups (βIR = −0.25, βTR = −0.46, t(26) = −0.48, p =0.63); while for the rhyme condition, the two groups differed significantly in β value from each other (βIR = −1.53, βTR = −0.54, t(26) = −2.05, p =0.06). We conclude that the group differences reported in Fig. 3b are more likely due to group differences in the rhyming and not the letter matching task.
Correlations between brain activations and reading measures
To further investigate the relations between brain activations and reading measures, a correlation analysis was conducted between scores of all language behavioral tests and the magnitudes of activation at the left angular and left inferior occipital region for all 28 participants. First, the activations within the regions were calculated by averaging the Beta value of all voxel within the range that was derived from previously defined ROIs based on literature reports. Then the correlation of the activation in each brain region with the scores in behavioral tests was calculated over all participants. For the left angular gyrus, the results showed significantly positive correlations with Spelling and Word Reading while the left inferior occipital region exhibited significantly positive correlations with multiple reading tests, such as word identification, non-word decoding, spelling and phonological awareness (Table 5).
Table 5.
Correlations between brain activations in two conditions and reading measurements.
| WRAT-spelling | Spelling | Word reading | Rhyme detection | Oral cloze | Syllable identification | Initial phoneme deletion | Word identification | Word attack | |
|---|---|---|---|---|---|---|---|---|---|
| Left angular | 0.286 | 0.389* | 0.374* | 0.237 | 0.337 (p=0.08) | 0.233 | 0.338 (p=0.079) | 0.373 (p=0.051) | 0.356 (p=0.063) |
| Left inferior occipital | 0.436* | 0.388* | 0.427* | 0.29 | 0.421* | 0.120 | 0.353 | 0.509** | 0.594*** |
Notes: β for left angular came from typical reader vs. impaired reader for rhyming vs. letter match; β for left inferior occipital came from typical reader vs. impaired reader for letter match vs. rest (fixation).
<0.05.
<0.01.
<0.005.
Discussion
To our knowledge, this is the first functional brain imaging study investigating brain activations for both orthographic and phonological processing in reading impaired and typical English second language learners. Investigating the neural substrates and deficits of English orthographic and phonological processing in Chinese school children with and without reading impairments who learn English as a second language, the present study explored whether there is a common neural mechanism in English learning as a second and as the native language. Results showed that impaired readers of English and Chinese are less accurate on a rhyming task which correlated with their reading scores. Compared to the typical control children, impaired readers exhibited decreased left parietotemporal activation during a phonological task (via an independent ROI analysis) which indicates that the neural mechanisms within parieto-temporal regions of impaired readers in second language learning are similar to that of the impaired reading in a mother language. Hypoactivation in reading impaired children were also observed in occipitotemporal regions during orthographic processing using an independent ROI analysis and lingual gyrus using a whole brain analysis with a multiple comparison correction. Furthermore, the present study has a relatively small sample size and a strong gender bias. Follow-up studies with larger gender matched sample sizes are needed in order to replicate the findings in a more representative subject group and in whole brain analyses.
Phonological processing
Compared to the typical reading group, the impaired English readers performed with less accuracy on the in-scanner rhyming task. They also exhibited impaired performance on behavioral tests of phonological awareness, spelling, WRAT-spelling, word identification and word attack in comparison with the control group. Additionally, significant correlation between rhyme accuracy and behavioral scores of English reading measures suggested that phonological processing involved in the rhyme judgment task is related to reading ability. For neural activation, the impaired English readers showed reduced left parietotemporal (e.g. left angular) activation in phonological processing when compared to the controls in an independent ROI analysis.
Our findings are consistent with many functional neuroimaging studies investigating English phonological processing in impaired adult readers (Horwitz et al., 1998; Shaywitz et al., 1998; Brunswick et al., 1999) and reading impaired children (Temple et al., 2001; Shaywitz et al., 2002). These studies all reported reduced left parietotemporal activation. Additionally, converging evidence showed that neural deficit in this region for impaired English reading can be improved by behavioral remediation in adults (Eden et al., 2004) and children (Temple et al., 2003). Moreover, with both age-matched and reading-matched children as control groups, Hoeft et al. (2006) found that functional disruption in this region was primarily attributed to a distinct developmental abnormality in dyslexia’s phonological processing rather than delayed reading development. Therefore, the left parietotemporal region, which has been related to mapping orthographic onto phonological representations (Hoeft et al., 2007) and vice versa, seems to be critical in phonological processing, regardless of whether English is the mother language or the second language.
Notably, the activation is negative in the left parietotemporal area during phonological processing (Fig. 3b). This region has been identified as part of the brain default network, which is active during wakeful rest and is deactivated during goal-oriented activity (Buckner et al., 2008). The default network in humans has been thought to be responsible for generation of spontaneous thoughts during mind-wandering (Buckner et al., 2008), and activity of this network may represent underlying physiological processes in the brain that are not related to any particular thought or thoughts (Raichle and Snyder, 2007). Reduced default network activity has been associated with autism and over activity in patients with schizophrenia (Whitfield-Gabrieli et al., 2009). In previous reading studies, negative activation within or near the left angular gyrus was reported in both typical readers (Rumsey et al., 1997; Sakurai et al., 1992, 1993) and in people with English reading difficulties (Hoeft et al., 2006; Ruff et al., 2002). But positive activation patterns in this area have also been reported by many studies of native English speakers (Shaywitz et al., 1998; Temple et al., 2001). Our findings may imply that impaired readers suffer an abnormal default network. However, few studies have been done to investigate this issue. The negative activation, differential activation levels with reading abilities, as well as inconsistent findings between studies at these areas deserve future study. Nevertheless, the significant correlations between activation in the angular gyrus and phonological skills (e.g. Spelling and Word Reading) suggests that this region is involved in phonological processing and that the observed effect is not due to overall differences in the default networks between children with and without reading impairments.
Orthographic processing
When performing orthographic processing of single letter pairs, typically developing children compared to impaired readers revealed increased activation within the lingual gyrus despite equal performance accuracy and reaction time. Greater activation in occipitotemporal regions were also observed in typical compared to impaired readers using an independent ROI analysis. Furthermore, correlation analyses also indicated significant correlations between activation within an occipitotemporal region and reading, spelling and phonological processing performance.
The impaired readers showed significantly reduced activation within the left lingual/calcarine cortex. Many studies investigating the neural correlates of dyslexia have reported reduced activation as well as reduced gray matter volume indices within lingual gyrus or calcarine cortex for individuals with dyslexia compared to typical readers (e.g. Hoeft et al., 2007; Horwitz et al., 1998; Brunswick et al., 1999; Eckert et al., 2005). Furthermore, lesions within the lingual gyrus can lead to alexia (e.g., Feinberg et al., 1994) and previous studies have demonstrated activation within lingual gyrus for processing single words (Moore and Price, 1999). Additionally, Demb et al. (1998) reported decreased activation for subjects with compared to without dyslexia within the lingual gyrus during the presentation of moving grating stimuli. However, a recent study found hyperactivation in the left lingual gyrus in readers with dyslexia compared to non impaired readers (Kronbichler et al., 2006), which suggests that the role of the left lingual gyrus in reading disorders warrants further investigation.
Furthermore, we observed neural disruption in the left occipito-temporal cortex in impaired readers who learn English as a second language. This regional hypoactivation is in concert with studies of individuals with dyslexia who learn English as their native language. For example, using a similar orthographic task, Temple et al. (2001) reported decreased neural activation for this region in dyslexia. Such functional disruptions can also be observed in Chinese children with Chinese reading difficulty. Siok et al. (2004) reported significant differences in occipital lobe (BA37) activation between Chinese typical children and children with reading impairment in Chinese orthographic processing. Thus, it is possible that the underlying neural mechanisms for orthographic processing are universal across different language systems.
However, there is still a debate regarding the role of the left occipitotemporal region for typical reading. Some researchers suggested that the left occipitotemporal region is involved in general orthographic processing (Uchida et al., 1999). More specifically, some researchers proposed that this area is utilized for retrieval of visual graphic images through writing (Nakamura et al., 2000; Kuo et al., 2004). However, others postulated that activity in this region can be explained by the integration of phonology and visual information rather than orthographic decoding (McCrory et al., 2005). In the current study we observed no left occipitotemporal activation in any of the groups in phonological processing, suggesting that this area may not be involved in phonological processing. Moreover, less activation in this region for impaired English readers during orthographic processing indicated that the left occipitotemporal region may play a specific role in efficient orthographic processing. However, in our current data set we cannot rule out the possibility that the occipitotemporal region reflects the integration of phonology and orthography and that the absence of activation in this region when contrasting phonological with orthographic processing is due to the automatic phonological processing during the orthographic task (Kherif et al., 2010).
Implications for language development and second language education
Phonological skills have been shown to be important in reading development and are a powerful predictor of the speed and efficiency of reading acquisition (Wagner and Torgesen, 1987; Bradley and Bryant, 1983; Lukatela and Turvey, 1994; Share et al., 1984). Several research studies have suggested a cross-language transfer of phonological awareness from the native to the second language (e.g., Chiappe and Siegel, 1999; Cisero and Royer, 1995; Durgunoglu et al., 1993), but it remains unclear how phonological skills develop in children with a non-alphabetic first language (e.g.; Chinese) and some studies even suggested no predictive value for phonological processing when predicting English reading comprehension among adolescence (Wang et al., 2002; Yu and Wang, 2001). Based on these results, it remains challenging to develop a research-based ESL curriculum for Chinese speaking children. Previous research has suggested that educational variables such as program type, method of instruction or characteristics of the native language of the bilingual child may impact literacy proficiency in ESL children (August and Hakuta, 1997; Fitzgerald, 1995; Hakuta, 1999; Tabors and Snow, 2001). The results presented here significantly add to the behavioral literature. They are suggestive of a similar neural mechanism for phonological and orthographic processing in typically developing Chinese speaking ESL children, and similar neural deficits in ESL children as found in children with reading impairments in English. This suggests that learning a non-alphabetic language as the first language does not influence the underlying brain network engaged in phonological processing in Chinese ESL learners, whether they are impaired readers or not. This suggests that an ESL curriculum should target the same neural network as the curriculum for L1 children, and that reading remediation programs for ESL children can be developed based on existing intervention programs in L1 children. However, future research projects investigating larger sample sizes and targeting reading sub skills as well as reading fluency and comprehension in ESL children are needed. Furthermore, remediation programs for ESL children with reading impairments need to be evaluated on the behavioral as well as neural level. This line of research will have important implications for curriculum design and educational policy and may help reduce the higher incidence of school drop outs among students from ESL backgrounds (e.g.; Gunderson and Clarke, 1998).
Acknowledgments
This study was supported by a research grant from The Joint PekingU–PolyU Center for Child Development and Learning, a grant from NSFC (30870757) and the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT). We thank two anonymous reviewers for their constructive comments and suggestions, and the children and the schools for participating in the experiment.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric press; 1994. p. 80. (DSM-IV) [Google Scholar]
- August D, Hakuta K, editors. Improving Schooling for Language-Minority Children: A Research Agenda. National Academy Press; Washington, DC: 1997. [Google Scholar]
- Berninger V. Varieties of Orthographic Knowledge: Theoretical and Developmental. 1994. [Google Scholar]
- Binder JR, McKiernan KA, Parsons ME, Westbury CF, Possing ET, Kaufman JN, Buchanan L. Neural correlates of lexical access during visual word recognition. J Cogn Neurosci. 2003;15:372–393. doi: 10.1162/089892903321593108. [DOI] [PubMed] [Google Scholar]
- Binder JR, Medler DA, Westbury CF, Liebenthal E, Buchanan L. Tuning of the human left fusiform gyrus to sublexical orthographic structure. Neuroimage. 2006;33:739–748. doi: 10.1016/j.neuroimage.2006.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger DJ, Perfetti CA, Schneider W. Cross-cultural effect on the brain revisited, universal structures plus writing system variation. Hum Brain Mapp. 2005;25:92–104. doi: 10.1002/hbm.20124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Van Santen FW, Harasaki Y, Gitelman DR, Parrish TR, Mesulam MM. The development of specialized brain systems in reading and oral-language. Child Neuropsychol. 2001;7:119–141. doi: 10.1076/chin.7.3.119.8740. [DOI] [PubMed] [Google Scholar]
- Bradley L, Bryant PE. Difficulties in auditory organisation as a possible cause of reading backwardness. Nature. 1978;271 (5647):746–747. doi: 10.1038/271746a0. [DOI] [PubMed] [Google Scholar]
- Bradley L, Bryant PE. Categorizing sounds and learning to read: a causal connection. Nature. 1983;301:419–421. [Google Scholar]
- Brem S, Bach S, Kucian K, Guttorm TK, Martin E, Lyytinen H, Brandeis D, Richardson U. Brain sensitivity to print emerges when children learn letter-speech sound correspondences. Proc Natl Acad Sci USA. 2010;107 (17):7939–7944. doi: 10.1073/pnas.0904402107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunswick N, McCroy E, Price CJ, Frith CD, Frith U. Explicit and implicit processing of words and pseudowords by adult developmental dyslexics: a search for Wernicke’s Wortschatz? Brain. 1999;122:1901–1917. doi: 10.1093/brain/122.10.1901. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network. Anatomy, function, and relevance to disease. Ann NY Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Cao F, Bitan T, Chou TL, Burman DD, Booth JR. Deficient orthographic and phonological representations in children with dyslexia revealed by brain activation pattens. J Child Psychol Psychiatry. 2006;47 (10):1041–1050. doi: 10.1111/j.1469-7610.2006.01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HC, Juola JF. Dimensions of lexical coding in Chinese and English. Mem Cogn. 1982;10:216–224. doi: 10.3758/bf03197632. [DOI] [PubMed] [Google Scholar]
- Chiappe P, Siegel LS. Phonological awareness and reading acquisition in English- and Punjabi-speaking Canadian children. J Educ Psychol. 1999;91:20–28. [Google Scholar]
- Cisero CA, Royer JM. The development and cross-language transfer of phonological awareness. Contemp Educ Psychol. 1995;20:275–303. [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehericy S, Dehaene-Lambertz G, Henaff MA, Michel F. The visual word form area—spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123:291–307. doi: 10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- Coltheart M, Curtis B, Atkins P, Haller M. Models of reading aloud: dual-route and parallel-distributed-processing approaches. Psychol Rev. 1993;100:589–608. [Google Scholar]
- Cunningham AE, Stanovich KE. Assessing print exposure and orthographic processing skill in children: a quick measure of reading experience. J Educ Psychol. 1990;82:733–740. [Google Scholar]
- Cunningham AE, Perry K, Stanovich KE. Converging evidence for the concept of orthographic processing. Read Writ Interdiscip J. 2001;14:549–568. [Google Scholar]
- Dehaene S, Cohen L, Sigman M, Vinckier F. The neural code for written words: a proposal. Trends Cogn Sci. 2005;9:335–341. doi: 10.1016/j.tics.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Demb JB, Boynton GM, Heeger DJ. Functional magnetic resonance imaging of early visual pathways in dyslexia. J Neurosci. 1998;18:6939–6951. doi: 10.1523/JNEUROSCI.18-17-06939.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding GS, Perry C, Peng DL, Ma L, Li DJ, Xu SY, Luo Q, Xu D, Yang J. Neural mechanisms underlying semantic and orthographic processing in Chinese–English bilinguals. NeuroReport. 2003;14 (12):1557–1562. doi: 10.1097/00001756-200308260-00003. [DOI] [PubMed] [Google Scholar]
- Durgunoglu A, Nagy W, Hancin-Bhatt B. Cross-language transfer of phonological awareness. J Educ Psychol. 1993;85:453–465. [Google Scholar]
- Eckert MA, Leonard CM, Wilke M, Eckert M, Richards T, Richards A, Berninger V. Anatomical signatures of dyslexia in children: unique information from manual and voxel based morphometry brain measures. Cortex. 2005;41:304–315. doi: 10.1016/s0010-9452(08)70268-5. [DOI] [PubMed] [Google Scholar]
- Eden GF, Jones KM, Cappell K, Gareau L, Wood FB, Zeffiro TA, Dietz NAE, Agnew JA, Flowers DL. Neural changes following remediation in adult developmental dyslexia. Neuron. 2004;44 (3):411–422. doi: 10.1016/j.neuron.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Feinberg TE, Schindler RJ, Ochoa E, Kwan PC, Farah MJ. Associative visual agnosia and alexia without prosopagnosia. Cortex. 1994;30 (3):395–411. doi: 10.1016/s0010-9452(13)80337-1. [DOI] [PubMed] [Google Scholar]
- Fitzgerald J. English-as-a-second-language learners’ cognitive reading processes: a review of research in the United States. Rev Educ Res. 1995;65:145–190. [Google Scholar]
- Friston KJ, Zarahn E, Josephs O, Henson RNA, Dale AM. Stochastic designs in event-related fMRI. Neuroimage. 1999;10:607–619. doi: 10.1006/nimg.1999.0498. [DOI] [PubMed] [Google Scholar]
- Gunderson L, Clarke D. In: Shanahan T, Rodriguez-Brown FV, Worthman C, Burnison JC, Cheung A, editors. An exploration of the relationship between ESL students’ backgrounds and their English and academic achievement; 47th Yearbook of the National Reading Conference. National Reading Conference; Chicago. 1998. pp. 264–273. [Google Scholar]
- Hakuta K. The debate on bilingual education. J Dev Behav Pediatr. 1999;20:36–37. doi: 10.1097/00004703-199902000-00006. [DOI] [PubMed] [Google Scholar]
- Ho CSH, Fong KM. Do Chinese dyslexic children have difficulties learning English as a second language? J Psycholinguist Res. 2005;34 (6):603–618. doi: 10.1007/s10936-005-9166-1. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Hernandez A, McMillon G, Taylor-Hill H, Martindale JL, Meyler A, Keller TA, et al. Neural basis of dyslexia: a comparison between dyslexic and nondyslexic children equated for reading ability. J Neurosci. 2006;26 (42):10700–10708. doi: 10.1523/JNEUROSCI.4931-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Meyler A, Hernandez A, Juel C, Taylor-Hill H, Martindale JL, McMillon G, et al. Functional and morphometric brain dissociation between dyslexia and reading ability. Proc Natl Acad Sci USA. 2007;104 (10):4234–4239. doi: 10.1073/pnas.0609399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz B, Rumsey JM, Donohue BC. Functional connectivity of the angular gyrus in normal reading and dyslexia. Proc Natl Acad Sci USA. 1998;95:8939–8944. doi: 10.1073/pnas.95.15.8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Lee HL, Zhang Q, Liu T, Geng LB, Seghier ML, Shakeshaft C, Twomey T, Green DW, Yang YM, Price CJ. Developmental dyslexia in Chinese and English populations: dissociating the effect of dyslexia from language differences. Brain. 2010 May 20; doi: 10.1093/brain/awq106. (published online) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kherif F, Josse G, Price CJ. Automatic top-down processing explains common left occipito-temporal responses to visual words and objects. Cerebral Cortex. 2010 Apr 22; doi: 10.1093/cercor/bhq063. (published online) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronbichler M, Hutzler F, Staffen W, Mair A, Ladurner G, Wimmer H. Evidence for a dysfunction of left posterior reading areas in German dyslexic readers. Neuropsychologia. 2006;44:1822–1832. doi: 10.1016/j.neuropsychologia.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Kronbichler M, Bergmann J, Hutzler F, Staffen W, Mair A, Ladurner G, Wimmer H. Taxi vs. Taksi: on orthographic word recognition in the left ventral occipitotemporal cortex. J Cogn Neurosci. 2007;19 (10):1584–1594. doi: 10.1162/jocn.2007.19.10.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronbichler M, Hutzler F, Wimmer H, Mair A, Staffen W, Ladurner G. The visual word form area and the frequency with which words are encountered: evidence from a parametric fMRI study. Neuroimage. 2004;21:946–953. doi: 10.1016/j.neuroimage.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Kuo WJ, Yeh TC, Lee JR, Chen LF, Lee PL, Chen SS, Ho LT. Orthographic and phonological processing of Chinese characters: an fMRI study. Neuroimage. 2004;21:1721–1731. doi: 10.1016/j.neuroimage.2003.12.007. [DOI] [PubMed] [Google Scholar]
- LaBerge D, Samuels SJ. Towards a theory of automatic information processing in reading. Cogn Psychol. 1974;6:293–323. [Google Scholar]
- Leck KJ, Weekes BS, Chen MJ. Visual and phonological pathways to the lexicon: evidence from Chinese readers. Mem Cogn. 1995;23:468–476. doi: 10.3758/bf03197248. [DOI] [PubMed] [Google Scholar]
- Liu WL, Shu H, Yang YF. Speech perception deficits by Chinese children with phonological dyslexia. J Exp Child Psychol. 2009;103:338–354. doi: 10.1016/j.jecp.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Lukatela G, Turvey MT. Visual lexical access is initially phonological I: evidence from associative priming words, homophones, and pseudohomophones. J Exp Psychol. 1994;123:107–128. doi: 10.1037//0096-3445.123.2.107. [DOI] [PubMed] [Google Scholar]
- Lundberg I, Frost J, Petersen O. Effects of an extensive program for stimulating phonological awareness in preschool children. Read Res Q. 1988;23:263–284. [Google Scholar]
- Maurer U, Brem S, Bucher K, Kranz F, Benz R, Steinhausen HC, Brandeis D. Impaired tuning of a fast occipito-temporal response for print in dyslexic children learning to read. Brain. 2007;130:3200–3210. doi: 10.1093/brain/awm193. [DOI] [PubMed] [Google Scholar]
- McClelland J, Rumelhart E. An interactive activation model of context effects in letter perception: Part 1. An account of Basic findings. Psychol Rev. 1981;88:375–407. [PubMed] [Google Scholar]
- McCrory EJ, Mechelli A, Frith U, Price CJ. More than words: a common neural basis for reading and naming deficits in developmental dyslexia? Brain. 2005;128:261–267. doi: 10.1093/brain/awh340. [DOI] [PubMed] [Google Scholar]
- Meyler A, Keller TA, Cherkassky VL, Gabrieli JDD, Just MA. Modifying the brain activation of poor readers during sentence comprehension with extended remedial instruction: a longitudinal study of neuroplasticity. Neuropsychologia. 2008;46:2580–2592. doi: 10.1016/j.neuropsychologia.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CJ, Price CJ. Three distinct ventral occipitotemporal regions for reading and object naming. Neuroimage. 1999;10 (2):181–192. doi: 10.1006/nimg.1999.0450. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Honda M, Okada T, Hanakawa T, Toma K, Fukuyama H, Konishi J, Shibaski H. Participation of the left posterior inferior temporal cortex in writing and mental recall of kanji orthography: a functional MRI study. Brain. 2000;123:954–967. doi: 10.1093/brain/123.5.954. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Demonet JF, Fazio F, McCrory E, Chanoine V, Brunswick N, Cappa SF, Cossu G, Habib M, Frith CD, Frith U. Dyslexia-cultural diversity and biological unity. Science. 2001;291:2165–2167. doi: 10.1126/science.1057179. [DOI] [PubMed] [Google Scholar]
- Price CJ, Wise RJ, Frackowiak RS. Demonstrating the implicit processing of visually presented words and pseudowords. Cereb Cortex. 1996;6:62–70. doi: 10.1093/cercor/6.1.62. [DOI] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: contributions from functional neuroimaging. J Anat. 2000;197:335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puce A, Allison T, Asgari M, Gore JC, McCarthy G. Differential sensitivity of human visual cortex to faces, letterstrings, and textures: a functional magnetic resonance imaging study. J Neurosci. 1996;16:5205–5215. doi: 10.1523/JNEUROSCI.16-16-05205.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee Jun Ren, Shaywitz SE, Shaywitz BA. Functional neuroimaging studies of reading and reading disability (developmental dyslexia) Ment Retard Dev Disabil Res Rev. 2000;6:207–213. doi: 10.1002/1098-2779(2000)6:3<207::AID-MRDD8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37 (4):1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Ramus F, Rosen S, Dakin SC, Day BL, Castellote JM, White S, Frith U. Theories of developmental dyslexia: insights from a multiple case study of dyslexic adults. Brain. 2003;126:841–865. doi: 10.1093/brain/awg076. [DOI] [PubMed] [Google Scholar]
- Ruff S, Cardebat D, Marie N, Demonet JF. Enhanced response of the left frontal cortex to slowed down speech indyslexia: an fMRI study. NeuroReport. 2002;13:1285–1289. doi: 10.1097/00001756-200207190-00014. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Horwitz B, Donohue BC, Nace K, Maisog JM, Andreason P. Phonological and orthographic components of word recognition: a PET-rCBF study. Brain. 1997;120:739–759. doi: 10.1093/brain/120.5.739. [DOI] [PubMed] [Google Scholar]
- Sakurai Y, Momose T, Iwata M, Watanabe T, Ishikawa T, Kanazawa I. Semantic process in kana word reading: activation studies with positron emission tomography. NeuroReport. 1993;4:327–330. doi: 10.1097/00001756-199303000-00026. [DOI] [PubMed] [Google Scholar]
- Sakurai Y, Momose T, Iwata M, Watanabe T, Ishikawa T, Takeda K, Kanazawa I. Kanji word reading process analysed by positron emission tomography. NeuroReport. 1992;3:445–448. doi: 10.1097/00001756-199205000-00017. [DOI] [PubMed] [Google Scholar]
- Seki A, Koeda T, Sugihara S, Kamba M, Hirata Y, Ogawa T, Takeshita K. A functional magnetic resonance imaging study during sentence reading in Japanese dyslexic children. Brain Dev. 2001;23:312–316. doi: 10.1016/s0387-7604(01)00228-5. [DOI] [PubMed] [Google Scholar]
- Share D, Jorm A, Maclean R, Matthews R. Sources of individual differences in reading acquisition. J Educ Psychol. 1984;76:1309–1324. [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Mencl WE, Fulbright RK, Skudlarski P, Todd-Constable R, Marchione KE, Fletcher JM, Lyon GR, Gore JC. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biol Psychiatry. 2002;52:101–110. doi: 10.1016/s0006-3223(02)01365-3. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Fulbright RK, Skudlarski P, Mencl WE, Constable RT, Pugh KR, Holahan JM, Marchione KE, Fletcher JM, Lyon GR, Gore JC. Neural systems for compensation and persistence: young adult outcome of childhood reading disability. Biol Psychiatry. 2003;54:25–33. doi: 10.1016/s0006-3223(02)01836-x. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Constable RT, Mencl WE, Shankweiler DP, Liberman AM, Skudlarski P, Fletcher JM, et al. Functional disruption in the organization of the brain for reading in dyslexia. Proc Natl Acad Sci USA. 1998;95:2636–2641. doi: 10.1073/pnas.95.5.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA. Dyslexia (specific reading disability) Biol Psychiactry. 2005;57:1301–1309. doi: 10.1016/j.biopsych.2005.01.043. [DOI] [PubMed] [Google Scholar]
- Siok WT, Niu Z, Jin Z, Perfetti CA, Tan LH. A structural-functional basis for dyslexia in the cortex of Chinese readers. Proc Natl Acad Sci USA. 2008;105 (14):5561–5566. doi: 10.1073/pnas.0801750105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siok WT, Perfetti CA, Jin Z, Tan LH. Biological abnormality of impaired reading is constrained by culture. Nature. 2004;43:71–76. doi: 10.1038/nature02865. [DOI] [PubMed] [Google Scholar]
- Stanovich KE, West RF. Exposure to print and orthographic processing. Read Res Q. 1989;24:402–433. [Google Scholar]
- Tabors P, Snow C. Young bilingual children and early literacy development. In: Newman SB, Dickinson DK, editors. Handbook on Research in Early Literacy. Guilford Press; New York: 2001. pp. 159–178. [Google Scholar]
- Tagamets MA, Novick JM, Chalmers ML, Friedman RB. A parametric approach to orthographic processing in the brain: an fMRI study. J Cogn Neurosci. 2000;12:281–297. doi: 10.1162/089892900562101. [DOI] [PubMed] [Google Scholar]
- Tan LH, Spinks JA, Feng CM, Siok WT, Perfetti CA, Xiong JH, Fox PT, Gao JH. Neural systems of second language reading are shaped by native language. Hum Brain Mapp. 2003;18:158–166. doi: 10.1002/hbm.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple E, Deutsch GK, Poldrack RA, Miller SL, Tallal P, Merzenich MM, Gabrieli JDE. Neural deficits in children with dyslexia ameliorated by behavioral remediation: evidence from functional MRI. Proc Natl Acad Sci USA. 2003;100:2860–2865. doi: 10.1073/pnas.0030098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple E. Brain mechanisms in normal and dyslexia readers. Cogn Neurosci. 2002:178–183. doi: 10.1016/s0959-4388(02)00303-3. [DOI] [PubMed] [Google Scholar]
- Temple E, Poldrack RA, Salidis J, Deutsch GK, Tallal P, Merzenich MM, Gabrieli DE. Disrupted neural responses to phonological and orthographic processing in dyslexic children: an fMRI study. NeuroReport. 2001;12 (2):299–307. doi: 10.1097/00001756-200102120-00024. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Garaeu L, Flowers DL, Zefirro TA, Eden G. Development of the neural mechanisms for reading. Nat Neurosci. 2003;6:767–773. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- Uchida I, Kikyo H, Nakajima K, Konishi S, Sekihara K, Miyashita Y. Activation of lateral extrastriate areas during orthographic processing of Japanese characters studied with fMRI. Neuroimage. 1999;9:208–215. doi: 10.1006/nimg.1999.0400. [DOI] [PubMed] [Google Scholar]
- Wagner RK, Torgesen JK. The nature of phonological processing and its causal role in the acquisition of reading skills. Psychol Bull. 1987;101 (2):192–212. [Google Scholar]
- Wang Y, Lin CD, Yu GL. Reading comprehension ability among poor English learners. Acta Psychol Sin. 2002;34 (3):279–283. [Google Scholar]
- Wang XL, Tao BP. Chinese Character Recognition Test Battery and Assessment Scale for Primary School Children. Shanghai Education Press; Shanghai: 1996. [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Shenton ME, Green AI, Nieto-Castanon A, LaViolette P, Wojcik J, Gabrieli JDE, Seidman LJ. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci USA. 2009;106 (4):1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson GS. Wide Range Achievement Test-Revision 3. Jastak Associates; Wilmington, DE: 1993. [Google Scholar]
- Woodcock RW. Manual for the Woodcock Reading Mastery Test-Revised. American Guidance Service; Circle Pines, MN: 1987. [Google Scholar]
- Xue G, Jiang T, Chen CS, Dong Q. Language experience shapes early electrophysiological responses to visual stimuli: the effects of writing system, stimulus length, and presentation duration. Neuroimage. 2008;39:2025–2037. doi: 10.1016/j.neuroimage.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Xue G, Poldrack RA. The neural substrates of visual perceptual learning of words: implications for the visual word form area hypothesis. J Cogn Neurosci. 2007;19 (10):1643–1655. doi: 10.1162/jocn.2007.19.10.1643. [DOI] [PubMed] [Google Scholar]
- Xue G, Chen C, Jin Z, Dong Q. Language experience shapes fusiform activation when processing a logographic artificial language: an fMRI training study. Neuroimage. 2006;31:1315–1326. doi: 10.1016/j.neuroimage.2005.11.055. [DOI] [PubMed] [Google Scholar]
- Xue G, Dong Q, Chen K, Jin Z, Chen C, Zeng YW, Reiman ME. Cerebral asymmetry of children in reading Chinese characters. Cogn Brain Res. 2005;24:206–214. doi: 10.1016/j.cogbrainres.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Yan CG, Liu DQ, He Y, Zou QH, Zhu CZ, Zuo XN, Long XY, Zang YF. Spontaneous brain activity in the default mode network is sensitive to different resting-state conditions with limited cognitive load. PLoS ONE. 2009;4 (5):1–11. doi: 10.1371/journal.pone.0005743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu GL, Wang Y. Phonological awareness, pseudoword reading and word recognition ability in poor English learners. Psychol Sci. 2001;24 (6):683–686. [Google Scholar]
- Zhang HC, Wang XP. Raven Standard Progressive Matrices: Chinese city revision. Beijing: The National Revision Collaborative Group; 1985. [Google Scholar]