Abstract
Epidemiological studies suggest that trichloroethylene (TCE) exposure may be associated with renal cancer. The biological mechanisms involved are not exactly known although nephrotoxicity is believed to play a role. Studies on TCE nephrotoxicity among humans, however, have been largely inconsistent. We studied kidney toxicity in Chinese factory workers exposed to TCE using novel sensitive nephrotoxicity markers. Eighty healthy workers exposed to TCE and 45 comparable unexposed controls were included in the present analyses. Personal TCE exposure measurements were taken over a 2-week period before urine collection. Ninety-six percent of workers were exposed to TCE below the current US Occupational Safety and Health Administration permissible exposure limit (100 ppm 8h TWA), with a mean (SD) of 22.2 (35.9) ppm. Kidney injury molecule-1 (KIM-1) and Pi-glutathione S transferase (GST) alpha were elevated among the exposed subjects as compared with the unexposed controls with a strong exposure-response association between individual estimates of TCE exposure and KIM-1 (P < 0.0001). This is the first report to use a set of sensitive nephrotoxicity markers to study the possible effects of TCE on the kidneys. The findings suggest that at relatively low occupational exposure levels a toxic effect on the kidneys can be observed. This finding supports the biological plausibility of linking TCE exposure and renal cancer.
Abbreviations:
- GST
glutathione-S-transferase
- KIM-1
kidney injury molecule-1
- NAG
N-acetyl-beta-(d)-glucosaminidase
- OVM
organic vapour monitoring
- TCE
trichloroethylene
- VEGF
vascular endothelial growth factor.
Introduction
Trichloroethylene (TCE) is a volatile chlorinated organic compound commonly used in industrial settings as a degreaser for metal parts and general-purpose solvent for lipophilic compounds. In 1995, it was estimated that more than 400 000 workers were exposed to TCE on an annual basis in the United States (1). Further, as a consequence of its presence in workplaces for many years and relative stability, TCE has become a widespread environmental water contaminant and one of the most frequently observed chemicals at Superfund sites.
TCE has recently been classified by the US EPA as carcinogenic in humans by all routes of exposure, whereas the National Toxicology Program classified TCE as reasonably anticipated to be a human carcinogen based on limited evidence of carcinogenicity from studies in humans, and sufficient evidence of carcinogenicity from studies in experimental animals and information suggesting TCE acts through mechanisms that indicate it would probably cause cancer in humans (2,3). Evidence for the carcinogenicity of TCE in humans is strongest for liver and kidney cancers and non-Hodgkin’s lymphoma (4). A recent meta-analysis of 23 studies on kidney carcinogenicity of TCE in humans showed a probable relationship between TCE exposure and kidney cancer (5). TCE is bioactivated to reactive intermediates through the renal glutathione S transferase (GST) and cysteine conjugate beta-lyase enzymes to form cysteine S conjugates, which are believed to be responsible for its nephrotoxic and nephrocarcinogenic effects (6,7). Therefore, the recent findings of an increased risk among TCE-exposed subjects especially in those subjects carrying polymorphisms in GST and cysteine conjugate beta-lyase genes has provided strong biological plausibility of an association between TCE and renal cancer in humans (7).
Long-term animal studies have shown low incidences of renal adenoma and adenocarcinoma in a number of rat strains (8). Rats given TCE orally or by inhalation also developed non-neoplastic lesions in the kidneys at relatively low dose levels. It has been suggested that kidney tumours in rats arise as a result of persistent cytotoxicity and regeneration. However, although nephrotoxicity occurs also in mice they do not seem to develop renal neoplasms. As such, kidney toxicity seems to be a possible but insufficient contributing factor for rodent renal carcinogenesis. It is worthy to note that mutagenicity has been proposed to contribute, independently of cytotoxicity and regeneration, to renal tumorigenesis in the rat (7). Data from human studies on TCE and nephrotoxicity are limited but have suggested that there might be a toxic effect of TCE on the kidneys at relatively high exposure levels (>35 ppm) (9–12). Information on nephrotoxicity at lower TCE doses is generally lacking.
Recently, several new sensitive markers of kidney toxicity have been developed including glutathione S transferase alpha and pi (Alpha-GST and Pi-GST) and kidney injury molecule-1 (KIM-1), which enables the identification of low level kidney toxicity. To address questions about TCE’s potential to cause kidney cancer, we carried out a cross-sectional study to evaluate the impact of occupational exposure to TCE on kidney injury using a panel of nephrotoxicity markers (i.e. Alpha- and Pi-GST, KIM-1, vascular endothelial growth factor (VEGF), N-acetyl-beta-(d)-glucosaminidase (NAG) activity).
Methods
Study design
To select factories for study, we conducted an initial screening of more than 40 potential study factories over a 1 year period using Dräger tubes and 3M organic vapour monitoring (OVM) badges to measure TCE and other chemicals including benzene, styrene, ethylene oxide, formaldehyde, methylene chloride, chloroform, perchloroethylene and epichlorohydrin. Factories were included if they used TCE in manufacturing processes, had no detectable benzene, styrene, ethylene oxide, formaldehyde or epichlorohydrin levels, and low to negligible levels of methylene chloride, chloroform or perchloroethylene. Ultimately, six study factories with metal (n = 4), optical lenses (n = 1) and circuit boards (n = 1) cleaning processes were identified that fulfilled the above selection criteria (13).
In June and July, 2006, we carried out a cross-sectional study of 80 workers currently exposed to TCE in the six study factories with TCE cleaning operations and 45 unexposed controls. Control subjects were enrolled from a clothing manufacturing factory and a food production factory that did not use TCE and were in the same geographic region as the factories that used TCE. Controls were frequency matched by age (±5 years) to exposed workers. Exclusion criteria for both TCE-exposed and unexposed workers included history of cancer, chemotherapy and radiotherapy, as well as previous occupations with notable exposure to benzene, butadiene, styrene and/or ionizing radiation. The study was approved by Institutional Review Boards at the US National Cancer Institute and the Guangdong National Poison Control Center, China. Participation was voluntary and all subjects gave written informed consent.
Exposure measurement and sample collection
For TCE-exposed workers (n = 80), full-shift personal air exposure measurements, 2–3 per subject resulting in 235 measurements, were taken in a 2-week time period in the factories using 3M OVM badges before biological sample collection. For control workers (n = 45), a single OVM badge was collected before biological sample collection. All OVM badges were analysed for TCE by GC-MS (LOD 0.12 ppm) and a subset (48 from TCE-exposed workers) was analysed for a panel of organic hydrocarbons including benzene (LOD 0.08 ppm), methylene chloride (LOD 0.14 ppm), perchloroethylene (LOD 0.1 ppm) and epichlorohydrin (LOD 0.1 ppm) by GC-MS. As part of the quality control procedures, duplicate badges were analysed for TCE in Guangdong, China and the United States and showed similar results with a Pearson correlation of 0.99.
Subjects were interviewed using a questionnaire that requested information about demographic and lifestyle characteristics and occupational history. They were also asked to provide a peripheral blood, buccal cell mouth rinse and urine samples, and undergo a brief physical exam that included measurement of blood pressure, height, weight and temperature.
Urinary analyses
Post-shift urine samples were stored at 4°C until being processed within 10h of collection. Samples were centrifuged and 1.4ml of urine supernatant was then mixed with 0.3ml freezing buffer (NEPHKIT® Urine Stabilizing Buffer; Argutus Medical) to stabilize proteins for storage and freezing. Samples were subsequently stored at −80°C.
Post-shift spot urine samples were analysed for creatinine, Alpha-GST, Pi-GST, VEGF, KIM-1 and NAG concentrations. Creatinine was determined by automated Jaffé reaction. Alpha-and Pi-GSTs were determined by a commercially available ELISA kit (Argutus Medical). Urinary VEGF was determined by commercially available ELISA kit (Quantikine Human VEGF Immunoassay; R&D Systems). KIM-1 was determined by luminex-based XMAP technology as described by Han et al. (14). Urinary NAG concentration was determined by an enzyme substrate–based colorimetric assay. Assay CVs were 5% for Pi-GST, 10% for NAG, 15% for Alpha-GST and KIM-1, and 20% for VEGF.
Statistical analysis
Levels of TCE exposure and urinary markers were natural log transformed to normalize their distributions. Student t-test was used to test for difference in the natural logarithm (ln) of each endpoint between unexposed control and exposed workers. In addition, exposure response analyses were performed by linear regression. In these analyses, current TCE air levels in part per million (ppm) were based on the arithmetic mean of an average of two to three measurements per subject. Cumulative exposure in ppm years was calculated by multiplying the individual arithmetic mean TCE levels with duration of employment at the current job. Linear regression models included in addition to the natural log–transformed exposure variables the frequency-matching factor age (as a continuous variable) and were corrected for ln(creatinine) (as a continuous variable). In addition, potential confounders that have been shown previously to influence one or more of the studied markers in this report were included in a model for a given marker if the regression coefficient was altered by ±15%, and included sex, current smoking (yes/no), current alcohol consumption (yes/no) and body mass index (BMI). As current smoking and sex were largely collinear (i.e. only men smoked) we only included sex in the final models. All analyses were carried out using SAS version 9.2 software (SAS Institute, Cary, NC, USA).
Results
Demographic characteristics including age, sex, BMI, current smoking and alcohol status were comparable between the unexposed control and exposed subjects (Table 1). Mean TCE exposure among the exposed was 22 ppm (SD 35.9) while TCE exposure was negligible in the control factories. On average, the exposed subjects worked for 2 years in the TCE facilities while unexposed subjects worked for 2.3 years in the control factories.
Table I.
Demographic characteristics and TCE exposure level
| Subjects | Unexposed (n = 45) | Exposed (n = 80) |
|---|---|---|
| Demographic characteristics | ||
| Age, mean ± SD | 24.9±6.0 | 25.2±6.6 |
| BMI, mean ± SD | 21.5±2.8 | 21.5±2.8 |
| Sex | ||
| Male n (%) | 29 (64.4) | 57 (71.3) |
| Female n (%) | 16 (35.6) | 23 (28.8) |
| Current smoking | ||
| Yes n (%) | 15 (33.3) | 34 (42.5) |
| No n (%) | 30 (66.7) | 46 (57.5) |
| Current alcohol use | ||
| Yes n (%) | 19 (42.2) | 26 (32.5) |
| No n (%) | 26 (57.8) | 54 (67.5) |
| TCE exposure | ||
| Current (ppm) mean ± SD | <0.03 | 22.2±35.9 |
| Cumulative (ppm. years), mean ± SD | <0.1 | 35.8±68.2 |
| Employment in current job | ||
| Duration, Years ± SD | 2.3±2.8 | 2.0±2.0 |
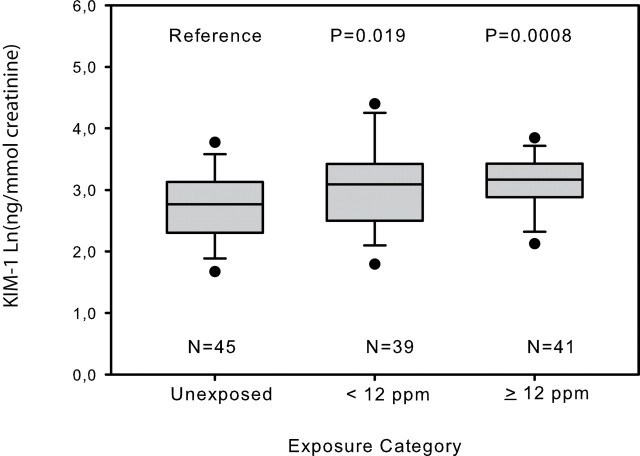
Urinary markers of kidney toxicity were comparable between the unexposed and the exposed workers except for KIM-1, which was statistically significantly elevated among the exposed subjects compared with controls (t-test P = 0.01). Pi-GST was borderline statistically significantly elevated among the exposed workers (t-test P = 0.09; Table 2). Linear exposure-response modelling based on the natural logarithm (Ln) of current and cumulative TCE exposure, corrected for covariates [i.e. ln(creatinine), sex, current alcohol use and BMI], showed significant associations with Ln(KIM-1) and borderline significant associations with Ln(Pi-GST) (Table 3). Furthermore, TCE-exposed individuals exposed at relatively low levels of TCE (<12 ppm; the median TCE concentration among the exposed) had significant elevated KIM-1 levels as compared with the unexposed controls (P = 0.02; Figure 1).
Table II.
Renal toxicity markers by trichloroethylene exposure status
| Parameter | Unexposed(n = 45)a | Exposed(n = 80)a | P for exposed versus unexposedb | ||||
| Urinary markers | AM | GM | GSD | AM | GM | GSD | |
| Creatinine [mmol L−1] | 11.6 | 10.3 | 1.72 | 13.7 | 11.6 | 1.86 | 0.27 |
| VEGF [ng l−1]c | 302.7 | 210.6 | 2.15 | 257.0 | 210.4 | 1.91 | 0.99 |
| AlphaGST [µg l−1] | 8.1 | 6.1 | 2.20 | 8.3 | 6.1 | 2.35 | 0.98 |
| PiGST [µg l−1] | 26.5 | 18.2 | 2.78 | 31.2 | 23.9 | 2.15 | 0.09 |
| KIM-1 [ng l−1] | 211.7 | 162.7 | 2.19 | 311.2 | 254.1 | 1.87 | 0.01 |
| NAG [units l−1] | 2.5 | 1.8 | 2.49 | 2.6 | 1.8 | 3.06 | 0.94 |
aAM, arithmetic mean; GM, Geometric mean; GSD, Geometric standard deviation
b t-Test of difference in mean between ln(urinary marker) between unexposed and exposed individuals.
cSamples of one control and three exposed subjects were not analysed for VEGF.
Table III.
Results of linear regressiona analysis of renal toxicity markers by current and cumulative trichloroethylene exposure
| Ln(Current TCE exposure levels)(ppm) | Ln(Cumulative TCE exposure levels)(ppm. years) | |||||
|---|---|---|---|---|---|---|
| Parameter | Estimate | 95% CI | P-value | Estimate | 95% CI | P-value |
| Urinary markers | ||||||
| Ln(VEGF) [ng l−1]b | −0.01 | −0.04 to 0.01 | 0.34 | −0.02 | −0.05 to 0.009 | 0.19 |
| Ln(AlphaGST) [µg l−1] | −0.003 | −0.04 to 0.03 | 0.87 | −0.0005 | −0.04 to 0.04 | 0.71 |
| Ln(PiGST) [µg l−1] | −0.03 | −0.00 to 0.05 | 0.06 | −0.03 | −0.00 to 0.06 | 0.05 |
| Ln(KIM-1) [ng l−1] | −0.05 | −0.03 to 0.08 | 0.0001 | −0.06 | −0.03 to 0.08 | 0.0001 |
| Ln(NAG) [units l−1] | −0.006 | −0.05 to 0.04 | 0.81 | −0.01 | −0.06 to 0.04 | 0.68 |
a Adjusted for ln(creatinine), sex, current alcohol use, and BMI.
b Samples of one control and three exposed subjects were not analysed for VEGF.
Fig. 1.
Box and whisker plot, demonstrating the median (line), lower and upper interquartile range (IQR; box) and whiskers to the highest and lowest values, excluding outliers (>1.5 times IQR; open rounds) of KIM-1 concentration corrected for urinary creatinine by trichloroethylene exposure category; 12 ppm was the median trichloroethylene concentration of the exposed subjects. P-values are from a linear model testing the pair wise difference in Ln(KIM-1) concentration corrected for ln(creatinine), sex, current alcohol use and BMI between the unexposed and the two exposure categories.
To evaluate the influence of exposure to other chlorinated solvents that were present at relatively low levels in some factories, we excluded one factory at a time from the analyses, and found that results were similar and conclusions unchanged (data not shown).
Discussion
We found that exposure to relatively low levels of TCE (i.e. average 22 ppm) was associated with a dose-dependent increase in nephrotoxicity markers KIM-1 and possibly Pi-GST. The association was seen for both current TCE exposure (ppm) and cumulative TCE exposure (ppm years). However, given the relatively short tenure of most exposed workers (average 2 years) cumulative TCE exposure was mostly driven by exposure intensity. An analysis with duration among the exposed workers indeed did not show an effect with any of the markers suggesting that the observed effect was driven predominantly by current TCE exposure levels. Altogether, these data suggest that kidney toxicity is found at occupational levels of TCE exposure below the current Occupational Safety and Health Administration permissible exposure limit of 100 ppm and below the current National Institute of Occupational Safety and Health recommended exposure limit of 25 ppm.
TCE exposure has been shown to be related to nephrotoxicity in animal studies. These studies suggest that kidney damage due to TCE can occur due to persistent cytotoxicity and regeneration (6). As in the rats, kidney toxicity is believed to be a contributing factor to the development of renal cancer in humans following exposure to TCE (6). TCE-induced nephrotoxicity in humans, however, has not been demonstrated conclusively partly due to methodological limitations on exposure assessment and the use of insufficient sensitive markers (10). In the present study, we collected extensive exposure information and used novel sensitive nephrotoxicity markers. Our findings of kidney toxicity at relatively low TCE levels are of importance as it indicates that toxic metabolites are formed at these concentration levels adding to the plausibility of the epidemiological findings linking TCE and kidney cancer.
We did not observe an association between TCE exposure and NAG or VEGF. NAG is mainly found in the proximal tubular brush border cells and is shed into the urine in case of kidney damage (15,16). VEGF is a protein involved in wound healing (16). Both biomarkers are indicative of severe kidney damage resulting in functional loss. The absence of an association between TCE exposure and VEGF and NAG indicates that TCE exposure at the levels and duration encountered in this study does not lead to severe loss of function of the kidneys.
Among the more sensitive nephrotoxicity markers (Pi- and Alpha-GST and KIM-1), we found a positive association with KIM-1 and possibly Pi-GST. KIM-1 is a kidney-specific membrane protein. In healthy cells, KIM-1 is expressed at a very low level, mostly in the proximal tubules. KIM-1 is strongly upregulated in injured kidney cells throughout the kidneys (17). When kidney cells are damaged, the KIM-1 ectodomain is cleaved and shed into the urine (18). Recently, urinary KIM-1 has been shown to outperform traditional biomarkers of kidney injury in preclinical biomarker qualification studies (19). In addition, in a variety of acute and chronic rodent kidney injury models resulting from drugs and environmental toxicants (i.e. metals), KIM-1 has been shown to be a very sensitive and early diagnostic indicator of kidney injury (19,20). KIM-1 has not been previously measured in studies on nephrotoxicity among TCE-exposed subjects. PiGST is a glutathione‑S‑transferase expressed in the distal tubules and collecting duct cells. PiGST is shed into the urine in case of distal tubular damage (21). Pi-GST has been measured in a retrospective study among subjects with symptoms and controls by Bruning et al. (22). In contrast to our study, a positive association with Alpha-GST was found but not with Pi-GST. However, given the retrospective design of this study, it is difficult to compare the results of our cross-sectional study with the study of Bruning et al. Green et al. (10) measured Alpha-GST but not Pi-GST in a study among current-exposed subjects and did not observe a difference in urinary Alpha-GST levels between exposed and unexposed subjects. As such, the results on Alpha- and Pi-GSTs among TCE-exposed subjects are largely inconsistent and therefore, our borderline statistical finding of Pi-GST being related to TCE exposure should be interpreted with caution.
We corrected our exposure-response models by including ln(creatinine) as a continuous variable in the model. This allows for a correction of urinary flow that is not necessarily directly proportional to the urinary creatinine concentration and which can vary by marker. We repeated our analyses by expressing the biological parameters without creatinine correction and per millimole creatinine and obtained essentially the same results.
This study shows a toxic effect, as expressed by KIM-1 and possibly Pi-GST, of inhaled TCE on the kidney at concentrations below the US Occupational Safety and Health Administration exposure limit, which is 100 ppm. This finding supports the biological plausibility between TCE exposure and renal cancer.
Funding
This work was supported by intramural funds from the National Institutes of Health, National Cancer Institute, and grants from the National Institute of Environmental Health Sciences (P42ES04705 and P30ES01896), the Northern California Center for Occupational and Environmental Health, and the Department of Science and Technology of Guangdong Province, China (2007A050100004), and a grant from the Department of Science and Technology of Guangdong Province, P.R. China (2007A050100004 to XT). JVB was supported by US NIH grant DK072381.
Acknowledgements
We would like to thank the participants for taking part in this study. We thank Jackie King and the other members of the BioReliance BioRepository (Bioreliance, Rockville, MD) for all aspects of biological sample handling, storage and shipping, and for assisting with laboratory analysis monitoring.
Disclosure
JVB is the co-inventor on KIM-1 patents assigned to Partners Healthcare, which has licensed them to a number of companies including Genzyme, Johnson and Johnson, BiogenIdec, BioassayWorks and R and D systems. He is a consultant to Genzyme.
References
- 1. Gist G.L, et al. (1995). Trichloroethylene—a review of the literature from a health effects perspective. Toxicol. Ind. Health 11 253–307 [DOI] [PubMed] [Google Scholar]
- 2.NTP (2000). Report on Carcinogens; Background Document for Trichloroethylene. National Toxicology Program; Research Triangle Park, NC: [Google Scholar]
- 3.US-EPA Integrated Risk Information System; Trichloroethylene (CASRN 79-01-6) [Google Scholar]
- 4. Wartenberg D. (2009). Environmental factors in cancer: trichloroethylene and related solvents: science, regulation, and cancer prevention. Rev. Environ. Health 24 297–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kelsh M.A., et al. (2010). Occupational trichloroethylene exposure and kidney cancer: a meta-analysis. Epidemiology 21 95–102 [DOI] [PubMed] [Google Scholar]
- 6. Bruning T., et al. (2000). Renal toxicity and carcinogenicity of trichloroethylene: key results, mechanisms, and controversies. Crit. Rev. Toxicol. 30 253–85 [DOI] [PubMed] [Google Scholar]
- 7. Moore L.E., et al. (2010). Occupational trichloroethylene exposure and renal carcinoma risk: evidence of genetic susceptibility by reductive metabolism gene variants. Cancer Res. 70 6527–6536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.IARC (1995). Dry Cleaning, Some Chlorinated Solvents and Other Industrial Chemicals IARC; Lyon, France. [PMC free article] [PubMed] [Google Scholar]
- 9. Chia S.E., et al. (1997). Endocrine profiles of male workers with exposure to trichloroethylene. Am. J. Ind. Med. 32 217–22 [DOI] [PubMed] [Google Scholar]
- 10. Green T., et al. (2004). Biological monitoring of kidney function among workers occupationally exposed to trichloroethylene. Occup. Environ. Med. 61 312–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nagaya T., et al. (1989). Urinary total protein and beta-2-microglobulin in workers exposed to trichloroethylene. Environ. Res. 50 86–92 [DOI] [PubMed] [Google Scholar]
- 12. Selden A., et al. (1993). Trichloroethylene exposure in vapour degreasing and the urinary excretion of N-acetyl-beta-D-glucosaminidase. Arch. Toxicol. 67 224–226 [DOI] [PubMed] [Google Scholar]
- 13. Lan Q., et al. (2010). Occupational exposure to trichloroethylene is associated with a decline in lymphocyte subsets and soluble CD27 and CD30 markers. Carcinogenesis 31 1592–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Han W.K., et al. (2002). Kidney injury molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 62 237–244 [DOI] [PubMed] [Google Scholar]
- 15. Gibey R., et al. (1981). Predictive value of urinary N-acetyl-beta-D- glucosaminidase (NAG), alanine-aminopeptidase (AAP) and beta-2- microglobulin (beta 2M) in evaluating nephrotoxicity of gentamicin. Clin. Chim. Acta 116 25–34 [DOI] [PubMed] [Google Scholar]
- 16. Vaidya V.S., et al. (2008). Urinary biomarkers for sensitive and specific detection of acute kidney injury in humans. Clin. Transl. Sci. 1 200–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ichimura T., et al. (1998). Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J. Biol. Chem. 273 4135–4142 [DOI] [PubMed] [Google Scholar]
- 18. Bailly V., et al. (2002). Shedding of kidney injury molecule-1, a putative adhesion protein involved in renal regeneration. J. Biol. Chem. 277 39739–39748 [DOI] [PubMed] [Google Scholar]
- 19. Vaidya V.S., et al. (2010). Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat. Biotechnol., 28 478–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matheis K.A., et al. (2011). Cross-study and cross-omics comparisons of three nephrotoxic compounds reveal mechanistic insights and new candidate biomarkers. Toxicol. Appl. Pharmacol. 252 112–122 [DOI] [PubMed] [Google Scholar]
- 21. Kharbanda R., et al. (1991). Heterogeneity of glutathione S-transferase isoenzyme expression in renal disease. Nephrol. Dial. Transplant. 6 695–700 [DOI] [PubMed] [Google Scholar]
- 22. Bruning T., et al. (1999). Pathological excretion patterns of urinary proteins in renal cell cancer patients exposed to trichloroethylene. Occup. Med. (Lond) 49 299–305 [DOI] [PubMed] [Google Scholar]