Abstract
The major obstacles in human prostate cancer (PCA) treatment are the development of resistance to androgen ablation therapy leading to hormone-refractory state and the toxicity associated with chemotherapeutic drugs. Thus, the identification of additional non-toxic agents that are effective against both androgen-dependent and androgen-independent PCA is needed. In the present study, we investigated the efficacy of a novel phytochemical poly[3-(3, 4-dihydroxyphenyl)glyceric acid] (p-DGA) from Caucasian species of comfrey (Symphytum caucasicum) and its synthetic derivative syn-2, 3-dihydroxy-3-(3, 4-dihydroxyphenyl) propionic acid (m-DGA) against PCA LNCaP and 22Rv1 cells. We found that both p-DGA and m-DGA suppressed the growth and induced death in PCA cells, with comparatively lesser cytotoxicity towards non-neoplastic human prostate epithelial cells. Furthermore, we also found that both p-DGA and m-DGA caused G1 arrest in PCA cells through modulating the expression of cell cycle regulators, especially an increase in CDKIs (p21 and p27). In addition, p-DGA and m-DGA induced apoptotic death by activating caspases, and also strongly decreased AR and PSA expression. Consistent with in vitro results, our in vivo study showed that p-DGA feeding strongly inhibited 22Rv1 tumors growth by 76% and 88% at 2.5 and 5mg/kg body weight doses, respectively, without any toxicity, together with a strong decrease in PSA level in plasma; and a decrease in PCNA, AR and PSA expression but increase in p21/p27 expression and apoptosis in tumor tissues from p-DGA-fed mice. Overall, present study identifies p-DGA as a potent agent against PCA without any toxicity, and supports its clinical application.
Introduction
Carcinoma of the prostate gland is the most common non-cutaneous malignancy (1). Surgical or chemical ablation of androgen had been the frontline therapy for treating patients with androgen-dependent or locally advanced PCA (2,3). Although most patients initially respond to such treatments, the disease eventually progresses to hormone-refractory state, and thereafter chemotherapy, radiotherapy or subsequent hormonal therapy provide little survival benefit(2-–4). Moreover, the side effects or toxicity associated with these treatments seriously compromise PCA patients’ quality of life. Thus, the identification of non-toxic agents that are effective against both androgen-dependent and androgen-independent PCA would be a better and viable therapeutic approach.
In general, cancer cells have a deregulated cell cycle that provide them an unrestrained potential to proliferate (5). Cell cycle de-regulation in cancer cells have been associated with overexpression/activity of cyclins, cyclin-dependent kinases (CDKs), cell division cycle 25 (Cdc25) phosphatases and/or decreased expression/mutations of cyclin-dependent kinase inhibitors (CDKIs) (6). Beside deregulated cell cycle, cancer cells develop mechanisms to evade apoptosis, and there is a lot of overlapping in the molecular regulation of cell cycle and apoptosis (7). Several studies have reported the role of androgen receptor (AR) in regulating the cell cycle as well as apoptosis in PCA cells (8,9). Therefore, the agent/s that could simultaneously target the deregulated cell cycle, apoptosis resistance mechanisms and AR would be effective in inhibiting PCA cells proliferation.
Research interest in the specific bioactivity and potential translational applications of plant compounds is increasing rapidly. Here, we assessed the anti-cancer efficacy of a novel water-soluble phenolic polymer namely p-DGA (poly[3-(3, 4 dihydroxyphenyl) glyceric acid]) (Figure 1A) isolated from the roots of the Caucasian species of comfrey (S.caucasicum, Boraginaceae). p-DGA has been extensively tested for its efficacy to heal wounds and burns as well as for its antioxidant and anti-inflammatory effects (10–12). These beneficial properties of p-DGA are relevant to cancer prevention and/or treatment as tissue injuries, inflammation and oxidative stress are integral components of carcinogenesis (13). Already, there are few preliminary evidences supporting the notion about potential anti-cancer efficacy of p-DGA (14,15). For example, Barbakadze et al. showed that p-DGA treatment affects cell cycle progression and induces apoptosis in β-chronic lymphocyte leukemia cells in vitro (14). p-DGA was also reported to abrogate adhesion of murine B16 melanoma cells to tumor-activated hepatic sinusoidal endothelium (15). However, mechanism based anti-cancer efficacy studies with p-DGA in PCA have not been performed. Therefore, in the present study we examined detailed efficacy and molecular mechanisms of p-DGA using androgen-dependent (LNCaP) and androgen-independent (22Rv1) PCA cells. We also compared p-DGA efficacy with its synthetic monomer syn-2, 3-dihydroxy-3-(3, 4-dihydroxyphenyl) propionic acid [m-DGA] (Figure 1A). We report that p-DGA is more effective than its synthetic derivative, and strongly inhibits PCA cells growth both in vitro and in vivo through modulating AR expression as well as regulators of cell cycle and apoptosis.
Fig. 1.
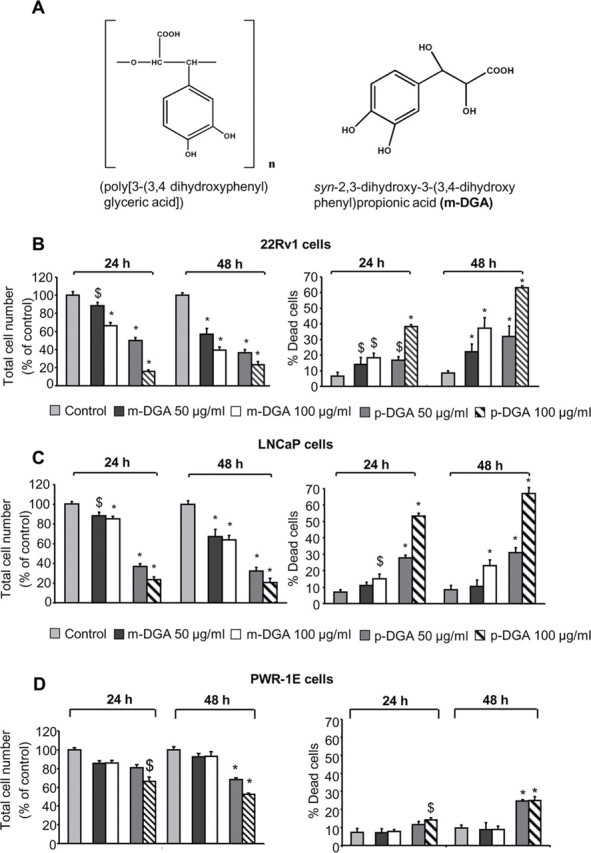
p-DGA and m-DGA selectively inhibit growth and induce death in human PCA cells. (A) The chemical structure of p-DGA and m-DGA. (B-D) 22Rv1, LNCaP and PWR-1E cells were treated with vehicle (sterile DI water) or two different concentrations of m-DGA or p-DGA (50 and 100 µg/mL) for 24 and 48h. Afterwards, cells were collected and total cell number (viable plus dead cells) as well as dead cell population were determined by trypan blue exclusion assay as described in the Materials and Methods section. The data are presented as mean (n=3) ± standard error of mean (SEM) and represent at least three independent experiments. *, P<0.001; $, P<0.05.
Materials and Methods
Reagents
p-DGA was isolated and purified from the roots of S.caucasicum as described earlier (14,16,17); and m-DGA was synthesized following various chemical steps reported previously (18). Antibodies for cyclin D1, cyclin D3, cyclin E, Cdk2, Cdk4, Cdk6, Cdc25c, AR and histone-H1 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). p21 antibody was from Millipore (Charlottesville, VA) and the antibody for p27 was from Neomarker (Fremont, CA). Antibodies for cleaved caspases 3, cleaved caspases 9, cleaved poly (ADP ribose) polymerase [cPARP] were from Cell Signaling (Beverly, MA). Antibodies for PSA and AR (used for IHC) were from Dako A/S (Glostrup, Denmark). Antibody for β-actin was from Sigma-Aldrich (St Louis, MO). Enhanced Chemiluminescence (ECL) detection system and anti-mouse peroxidase-conjugated secondary antibody were from GE Healthcare (Buckinghamshire, UK). Antibody for α-tubulin was from Lab Vision (Fremont, CA). Annexin V/propidium iodide (PI) apoptosis kit was from Molecular probes (Eugene, OR) and Dead End Colorimetric TUNEL kit was purchased from Promega (Madison, MI).
Cell lines, cell culture and treatment
PCA androgen-independent 22Rv1 and androgen-dependent LNCaP cells as well as immortalized non-neoplastic prostate epithelial PWR-1E cells were purchased from ATCC (Manassas, VA). 22Rv1 and LNCaP cells were cultured in RPMI-1640 medium supplemented with 10% FBS, 100U/mL of penicillin and 100 µg/mL of streptomycin under standard cell culture conditions. PWR-1E cells were cultured in keratinocyte-serum free media supplemented with 10% fetal bovine serum, 5ng/ml recombinant human epidermal growth factor and 0.05mg/ml bovine pituitary extract (19). The stock solution (100mg/mL) of both p-DGA and m-DGA was prepared by dissolving in sterile distilled water overnight, followed by sonication and filtration through PVDF filter (size 0.22 µm) from Millipore (Charlottesville, VA). As needed, cells were treated with different doses of p-DGA and m-DGA (50 and 100 µg/mL effective concentrations in cell culture). At the end of desired treatment time points, different analyses were performed as described below.
Cell growth and cell death assay
Cell viability was determined by trypan blue exclusion assay. Briefly, LNCaP, 22Rv1 and PWR-1E cells were grown and treated with p-DGA or m-DGA (50 and 100 µg/mL in media) for 24 and 48h. At the end of experiment, cells were collected by brief trypsinization and trypan blue positive (dead cells) and negative cells (viable cells) were counted using hemocytometer as described earlier (20).
Cell cycle distribution and apoptosis assay by flow cytometry
Both cell cycle distribution and AnnexinV/PI positive cells were analyzed using flow cytometry as described before (20). In brief, cells were seeded and treated with 50 and 100 µg/mL of p-DGA or m-DGA for 24 and 48h. Cells were then trypsinized and stained with saponin (permeabilizing agent) and PI (to stain DNA) [0.3% saponin, (w/v); 25 µg/ml PI (w/v); and 20 µg/ml RNase A] overnight at 40C, and analyzed by flow cytometry for cell cycle distribution. Similarly, for apoptosis, cells were treated with p-DGA or m-DGA for 48h and AnnexinV/PI positive cells were analyzed using flow cytometry following the vendor’s protocol. FACS analysis was performed by University of Colorado Cancer Center flow cytometry core service.
In vivo tumor xenograft study
In vivo efficacy of p-DGA was examined using 22Rv1 xenograft model. In this study, 4 weeks old athymic (nu/nu) male mice were obtained from NCI (Frederick, MD) and housed in quarantine for two weeks. Mice were fed AIN-76A pellet diet from Dyet Inc. (Bethlehem, PA) and water ad libitum. All the protocols used were approved by the Institutional Animal care and Use Committee of the University of Colorado. 22Rv1 cells were collected, mixed with matrigel (1:1), and injected (approximately, 1x106 cells/mouse) subcutaneously on the right flank of each athymic nude mouse to initiate tumor growth. After the cells injection, mice were randomly divided into three groups, control (vehicle), p-DGA (2.5mg/kg body weight dose) and p-DGA (5mg/kg body weight dose) with 12–15 animals per group. The doses for p-DGA used in the study were deduced from earlier published study where p-DGA has shown in vivo anti-inflammatory efficacy when administered orally (10). The animals in the control group were gavaged only saline and treatment group animals were administered either 2.5 or 5.0mg/kg body weight dose of p-DGA 5 days/week for 5 weeks. Once the tumors started growing, their sizes were measured twice weekly in two dimensions using a digital caliper. The tumor volume was calculated by the formula: 0.5236 L1(L2)2, where L1 is the long diameter, and L2 is the short diameter. At the termination of the experiment, each tumor was carefully dissected and weighed. All small tumors and a piece of large tumors were fixed and processed for immunohistochemical (IHC) analysis. The rest of the tumor tissue samples were snap frozen in liquid nitrogen and stored at −800C for immunoblot analyses. Mice body weight and diet consumption were monitored weekly till the termination of the experiment.
PSA measurement by ELISA
To determine the circulating level of human PSA, blood was withdrawn from each mouse at the termination of the xenograft study and collected in heparin containing tubes. Blood was centrifuged and plasma was collected and stored at −80°C. The circulating PSA level in the plasma was quantified by ELISA as per vendor’s protocol (R&D Systems, Minneapolis, MN, USA, Cat # DKK300). Briefly, standards and plasma samples were pipetted into wells pre-coated with PSA specific monoclonal antibody. After washing, an enzyme-linked polyclonal antibody specific for PSA was added to the wells. Subsequently, substrate solution was added and color developed in proportion to the amount of PSA in each sample was measured at 450nm.
IHC analyses of tumor sections
For IHC analysis, tumor tissues were processed and paraffin-embedded 5 µm-thick tissue sections were stained for PCNA and AR following earlier published protocol with some modification in the antigen retrieval duration (retrieved for 30min) for AR immunostaining (21,22). Proliferating cells were quantified by counting the PCNA-positive cells and the total number of cells at five arbitrarily selected fields at 400× magnification. The proliferation index (per 400× microscopic field) was determined as (number of PCNA-positive cells × 100) / total number of cells. AR immunoreactivity (represented by brown staining) was analyzed in five random areas for each tumor tissue and was scored as 0+ (no staining), 1+ (weak staining), 2+ (moderate staining), 3+ (strong staining), 4+ (very strong staining).
In situ apoptosis detection by TUNEL staining
Paraffin-embedded 5 µm-thick sections were also used to identify apoptotic cells by staining using TUNEL assay kit as per vendor’s protocol. The extent of apoptosis was evaluated by counting the TUNEL-positive cells (brown-stained) as well as the total number of cells in five randomly selected fields at 400× magnification. The apoptotic index was calculated as (number of apoptotic cells × 100) / total number of cells.274
Western blot analysis
As required, total cell/tissue lysates or nuclear and cytosolic lysates were prepared following procedures published earlier (23,20). Equal amounts of protein from desired samples were separated on SDS-PAGE and immunoblotting was performed for desired proteins as described earlier (23). β-actin, α-tubulin and histone-H1 were used as loading controls for total cell lysates, cytoplasmic and nuclear fraction, respectively. For each protein, immunoblotting was performed at least twice in lysates prepared from independent cell culture experiments and representative blots are shown.
Statistical analysis
All the data from in vitro and in vivo study were analyzed using sigma stat 2.03 software from Jandel Scientific (San Rafael, CA). Immunoblots were scanned using Adobe Photoshop 6.0 (Adobe system Inc, San Jose, CA), and band intensity was measured using Scion Image program (NIH, Bethesda, MD). The statistical differences between groups were analyzed using one way ANOVA (Tukey test) and considered significant at P<0.05.
Results
p-DGA and m-DGA cause strong cell growth inhibition and death selectively in human PCA, but not in non-neoplastic prostate epithelial PWR-1E, cells
Our first objective was to examine as well as compare the effects of p-DGA and its synthetic derivative m-DGA on the growth of human PCA 22Rv1 and LNCaP cells. As shown in Figure 1B, m-DGA (50 and 100 µg/mL) decreased 22Rv1 cell growth by 12–34% and 43–61% (P<0.05-0.001) after 24 and 48h of treatment, and concomitantly caused 14–18% and 22–37% (P<0.05-0.001) cell death at these time-points, respectively. At similar concentrations; however, p-DGA showed much prominent effect, and decreased 22Rv1 cell growth by 50–84% and 64–77% (P<0.001–<0.001) after 24 and 48h, whereas increasing cell death to 17–38% and 32–63% (p<0.05–0.001) at these time-points, respectively. In case of LNCaP cells, under similar treatment conditions, m-DGA decreased cell growth by 12–15% and 33–36% (P<0.05–0.001) and induced 11–15% and 10–23% (P<0.05-0.001) cell death after 24 and 48h of treatment, respectively (Figure 1C). Again, similar to 22Rv1 cells, in LNCaP cells also, the p-DGA treatment caused much stronger effect compared to its synthetic derivative, and decreased cell growth by 63–76% and 68–80% (P<0.001) and caused 28–53% and 31–67% (P<0.001) cell death after 24 and 48h of treatment, respectively (Figure 1C).
PWR-1E is an immortalized cell line developed from the non-neoplastic adult human prostatic epithelium using adenovirus-12/simian virus-40 (Ad12-SV40) hybrid virus. As shown in Figure 1D, m-DGA treatment (50 and 100 µg/mL) had no statistically significant effect on the total cell number as well as cell death after 24 and 48h of treatment in PWR-1E cells. However, the p-DGA treatment (50 and 100 µg/mL) decreased PWR-1E cells growth by 19–34% and 32–47% (P<0.05–0.001), but caused only 11–14 and 24.6-25% (P<0.05–0.001) cell death after 24 and 48h of p-DGA treatment, respectively (Figure 1D). Even though, p-DGA showed some growth inhibition in PWR-1E cells, but compared to PCA cells, these growth inhibitory effects were lesser.
We conducted another experiment to identify and compare the IC50 of p-DGA in different cell lines. We used 25, 50 and 100 µg/ml p-DGA in LNCaP and 22Rv1 cells, whereas in PWR-1E cells we used 50, 100, 150, 200 and 300 µg/ml p-DGA. Results revealed that p-DGA IC50 was 47 and 60 µg/ml in 22Rv1 and LNCaP cells, respectively; whereas its IC50 was much higher (155 µg/ml) in PWR-1E cells (data not shown). Overall, these results confirmed that p-DGA is more cytotoxic towards PCA cells compared to non-neoplastic PWR-1E cells.
p-DGA and m-DGA induce G1 phase arrest by modulating the cell cycle regulatory molecules in human PCA cells
To understand the mechanism involved in the growth inhibition of PCA cells by m-DGA and p-DGA, we next examined their effect cell cycle distribution. As shown in Figure 2A, both m-DGA and p-DGA treatments (50 and 100 µg/mL) induced G1-phase arrest in both 22Rv1 and LNCaP cells, and similar to cell growth inhibition, p-DGA was more effective than m-DGA at both 24 and 48h time-points in both cell lines. Next, we analyzed the effects of m-DGA and p-DGA on cell cycle regulators to identify the molecular mechanism involved in the induction of G1-phase arrest. Results clearly showed that m-DGA treatment has no or slight effect on the levels of cyclin D1, cyclin D3, Cdk4, Cdk6, Cdk2, cyclin E and Cdc25A but strongly enhanced the levels of CDKIs p21 and p27 (Figure 2B). The p-DGA treatment, however, resulted in a moderate to strong decrease in the levels of cyclin D1, cyclin D3, Cdk4, Cdk6, Cdk2, cyclin E and Cdc25A; and strongly increased the levels of p21 and p27 in both 22Rv1 and LNCaP cells (Figure 2B). These molecular effects resemble the cell cycle distribution results where p-DGA was more potent with larger percentage of cells arrested in the G1-phase compared to m-DGA both LNCaP and 22Rv1 cells.
Fig. 2.
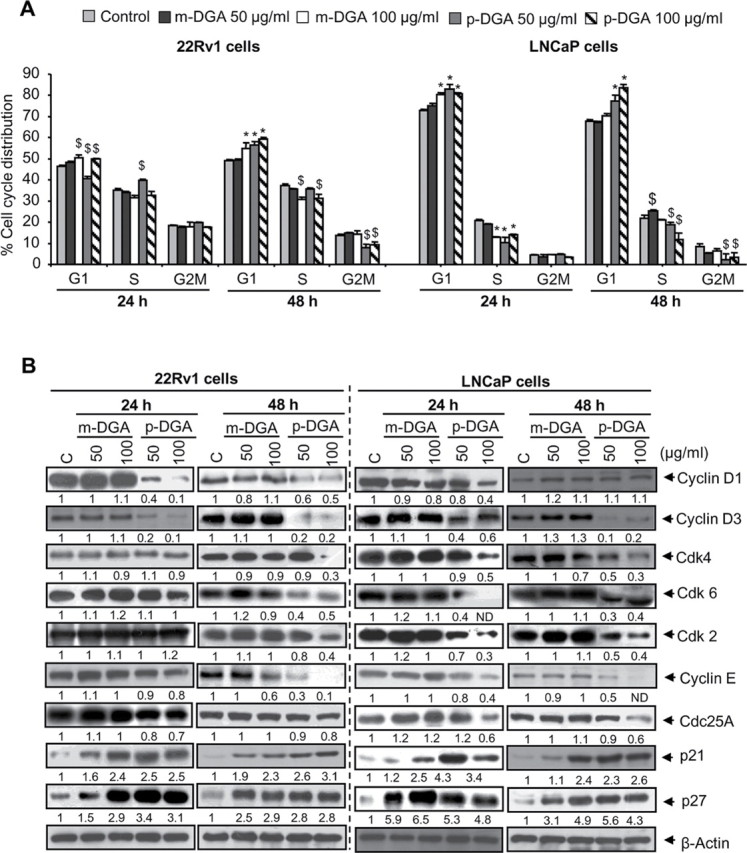
p-DGA and m-DGA induce cell cycle arrest by modulating cell cycle regulatory molecules in human PCA cells. Human PCA 22Rv1 and LNCaP cells were treated with vehicle or m-DGA or p-DGA (50 and 100 µg/mL) for 24 and 48h. At the end of each time point, cells were collected and (A) Stained using saponin/PI and analyzed for cell cycle distribution by flow cytometry; (B) Whole cell lysates were prepared and western blotting was performed for desired cell cycle regulatory proteins as detailed in the Materials and Methods section. Membranes were re-probed with β-actin to check equal protein loading. The data are presented as mean (n=3) ± SEM and represents three independent experiments. *, P<0.001; $, P<0.05. The densitometry data presented below the bands are “fold change” as compared with control after normalization with respective loading control (β-actin). ND: Not detectible.
p-DGA and m-DGA induce apoptotic cell death in human PCA cells
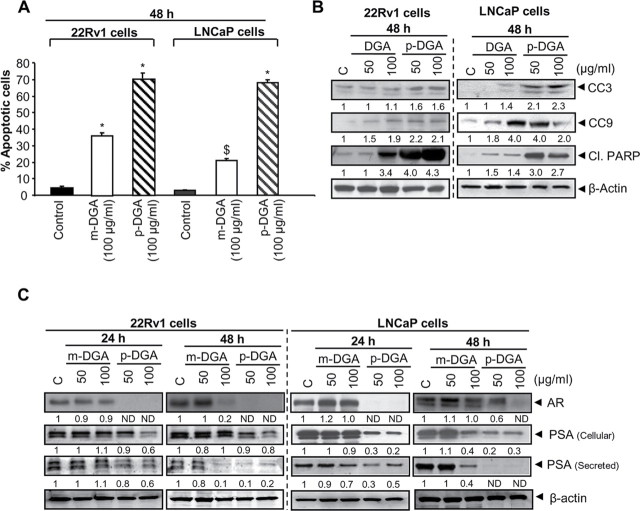
Our cell viability data clearly showed that both m-DGA and p-DGA cause a significant cell death, and therefore, we next examined their potential to induce apoptotic death in 22Rv1 and LNCaP cells. As shown in Figure 3A, m-DGA (100 µg/mL) and p-DGA (100 µg/mL) treatment for 48h in 22Rv1 cells increased the apoptotic cell population by 34% (P<0.001) and 70% (P<0.001) respectively. Under similar treatment conditions in LNCaP cells, m-DGA (100 µg/mL) and p-DGA (100 µg/mL) increased the apoptotic cell population by 18% (P< 0.05) and 65% (P<0.001) respectively compared to vehicle treated controls (Figure 3A). To get further insight into the molecular mechanism involved in the apoptotic cell death, we studied the effect of m-DGA and p-DGA on the activation of caspases and level of PARP cleavage, the established molecular markers for apoptotic death. As shown in Figure 3B, m-DGA and p-DGA (100 µg/mL) increased the expression of cleaved caspases (3 and 9) and cleaved PARP in both LNCaP and 22Rv1 cells, and again, compared to m-DGA, we observed a stronger effect of p-DGA on the apoptotic cell population as well as apoptosis regulatory molecules in both the cell lines.
Fig. 3.
Effect of p-DGA and m-DGA on apoptosis and AR in human PCA cells. Human PCA 22Rv1 and LNCaP cells were treated with vehicle or m-DGA or p-DGA (50 and 100 µg/mL) for 24 and 48h. (A) After 48h of treatment, both adherent and non-adherent cells were collected, stained with annexinV/PI and analyzed by flow cytometry for the apoptotic cell population. The data are presented as mean (n=3) ± SEM and represents two independent experiments. *, P<0.001; $, P<0.05 (B) Whole cell lysate were prepared after treating 22Rv1 and LNCaP cells with m-DGA or p-DGA for 48h and used to analyze the protein expression of cleaved caspase 3 (CC3), cleaved caspase 9 (CC9), and cleaved PARP (Cl. PARP) by western blotting. (C) Western blotting was performed for AR and PSA; and membranes were re-probed with β-actin to check equal protein loading. For the secreted PSA expression, media was collected and analyzed for PSA expression by immunoblotting. In each case, the media loading volume was normalized with the respective protein value of the cell lysate. The densitometry data presented below the bands are “fold change” as compared to control after normalization with respective loading control (β-actin). ND: Not detectible.
p-DGA and m-DGA decrease AR and PSA in human PCA cells
AR plays a critical role in the growth and progression of androgen-dependent as well as androgen-independent PCA (3,24,4). Therefore, next, we examined the effect of both m-DGA and p-DGA treatment on the protein expression of AR, and its target gene PSA. As shown in Figure 3C, m-DGA decreased AR and PSA level (both intracellular and secreted) in 22Rv1 and LNCaP cells but only at the higher dose (100 µg/mL) after 48h of treatment; while p-DGA exerted a strong effect on decreasing AR and PSA levels (both intracellular and secreted) at both doses (50 and 100 µg/mL) after 24 and 48h of treatment. These results clearly suggested that compared to m-DGA, p-DGA has a stronger inhibitory effect on AR signaling in PCA cells and that matches with its overall stronger biological effect in PCA cells.
p-DGA strongly inhibits human PCA 22Rv1 xenograft growth together with a decrease in circulating PSA level in nude mice
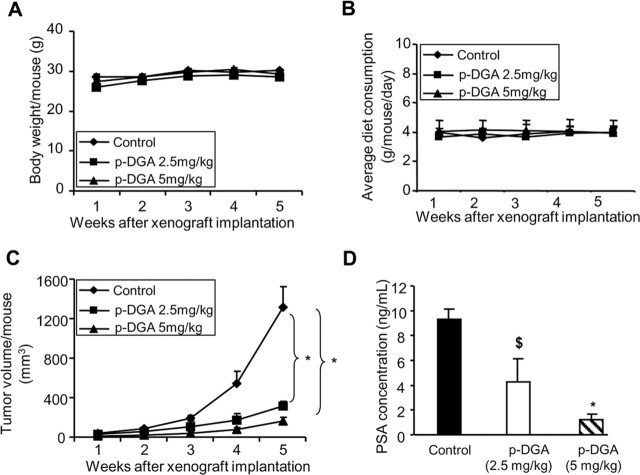
The result from our in vitro study clearly showed that p-DGA has much better anti-PCA activity compared to m-DGA, and therefore, we studied the in vivo efficacy of only p-DGA using 22Rv1 xenograft model. In this study, male athymic nude mice with 22Rv1 xenografts were administered orally 2.5 or 5.0mg/kg dose of p-DGA for five weeks. The administration of p-DGA through oral gavage did not cause any significant change in the body weight of mice or their diet consumption (Figure 4A and 4B). Furthermore, at the time of necropsy, all animals were examined for gross pathology, and we did not observe any signs of abnormality in all the vital organs examined in p-DGA-fed mice in both groups. Also, we did not observe any adverse effect in terms of general behavior of animals, suggesting an overall safe nature of this natural agent. Regarding its anti-tumor efficacy, as shown in Figure 4C, tumor volume was lesser in p-DGA treated groups compared to vehicle control. After 5 weeks, the tumor volume per mouse was decreased by 76% (control versus p-DGA 2.5mg/kg) (P<0.001) and 88% (control versus p-DGA 5mg/kg) (P<0.001) (Figure 4C). Plasma analyses revealed that p-DGA administration at 2.5 and 5.0mg/kg doses caused a strong dose-dependent decrease in PSA levels by 55% (P<0.05) and 87% (P<0.001), respectively, compared to control (Figure 4D). Together, these results clearly showed the anti-PCA efficacy of p-DGA in vivo.
Fig. 4.
Effect of p-DGA oral administration on the growth of human PCA 22Rv1 tumors and secreted PSA in athymic nude mice. 22Rv1 cells at the density of 1×106 were injected subcutaneously on the right flank of each male athymic nude mouse; and p-DGA (2.5mg/kg or 5.0mg/kg body weight) was administered through oral gavage route 5 days/week for 5 weeks as described in the Material and Methods section. (A) The body weight of the animals was monitored throughout the experiment duration and presented as body weight/mouse in grams (g). (B) The diet consumption of the animals was also monitored throughout the experiment duration and presented as average diet consumption/mouse/day in grams (g). (C) Tumor volume was measured as described in the Material and Methods section and presented as tumor volume/mouse (mm3). (D) At the end of the study, blood was collected from mice, plasma was isolated and PSA level was determined by ELISA as described in the Materials and Methods section. Data are presented as mean ± SEM, where n=12 to 15 animals in each group for the data in panels A–C; and 4 animal samples for each group for the data shown in panel D. *, P<0.001; $, P<0.05
Anti-proliferative and pro-apoptotic effect of p-DGA in 22Rv1 tumor tissues
Next, we analyzed 22Rv1 tumors for proliferation and apoptosis biomarkers. In IHC analysis, compared to controls, 22Rv1 tumor tissues from 5.0mg/kg p-DGA fed mice showed a 48% decrease (P<0.05, Figure 5A) in PCNA positive cells, which was accompanied with a strong increase (6–7 folds) in the TUNEL positive cells with p-DGA treatment (Figure 5A). Together, these findings suggested that p-DGA possesses in vivo efficacy through inhibiting the proliferation and promoting the apoptosis in PCA tumors.
Fig. 5.
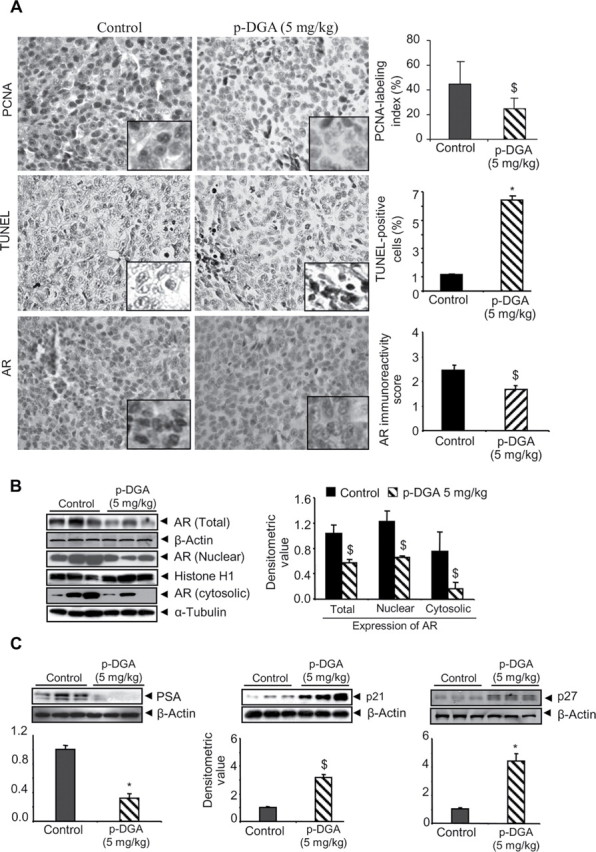
In vivo effect of p-DGA feeding on the proliferation and apoptosis markers as well as AR, PSA and CDKIs expression in 22Rv1 tumors. (A) IHC was performed in 22Rv1 xenograft tissues and immunoreactivity for PCNA, TUNEL and AR was quantified following the procedure described in the Materials and Methods section. Representative photographs are presented at 400×; insets represent further magnification of a part of the photographs. (B–C) Total cell lysates or nuclear and cytoplasmic fraction lysates were prepared from 22Rv1 xenograft tissues and analyzed for AR, PSA, p21 and p27 expression by western blotting. In each case, three random samples were analyzed from control and p-DGA treatment group (5mg/kg body weight). Membranes were reprobed with β-actin (total cell lysates), histone H1 (for nuclear fractions) or α-tubulin (for cytosolic fractions) to check protein loading. The densitometric values were normalized with respective loading control values (β-actin, histone H1 or α-tubulin) and presented as mean ± SEM.
p-DGA targets AR, PSA and CDKIs expression in vivo
We next analyzed 22Rv1 tumors to determine the effect of p-DGA treatment on AR and PSA level in vivo. IHC analyses clearly showed a decreased AR levels in 22Rv1 tumor tissues from p-DGA-fed versus control mice (Figure 5A). Immunoblot analyses of tumors further confirmed IHC results, and we observed a strong decrease in total, nuclear and cytoplasmic levels of AR in tumor tissues from p-DGA-treated versus control mice (Figure 5B). Furthermore, p-DGA treatment also decreased the PSA level in 22Rv1 tumors (Figure 5C). As we observed a strong effect of p-DGA on CDKIs expression in cell culture, we also examined p-DGA effect on the expression of CDKIs (p21 and p27) in 22Rv1 tumors. As shown in Figure 5C, the tumor tissues from p-DGA-treated mice showed a strong up-regulation of both p21 and p27, compared to control mice.
Discussion
PCA is one of the most frequently diagnosed cancers among men in the Western world and is the second leading cause of cancer-related deaths among American men (1), suggesting that additional strategies are needed for the treatment of PCA. Natural compounds have been an important source of anti-cancer drugs and close to 100 natural product-derived drugs are at different stages of pre-clinical or clinical testing for their therapeutic utility (25–29). Hence, the study of natural agents for the treatment of PCA seems to have great prospect in improving the quality of life in patients. In the present study, we tested the anti-PCA efficacy of a novel polyether compound isolated from the roots of Caucasian comfrey (14,18).
The roots and leaves of Comfrey species have been used therapeutically for various ailments for centuries (30). The extract from Comfrey species has been reported to possess strong anti-oxidative, anti-inflammatory, anti-lipoperoxidant and anti-complementary activity without any cytotoxicity towards polymorphonuclear leukocytes and human fibroblasts (11). However, mechanism based efficacy studies with p-DGA have not been performed. The present study, therefore, is the first in the direction of establishing the anti-cancer efficacy of p-DGA and its synthetic derivative in androgen-dependent (LNCaP) and -independent (22Rv1) human PCA cells. Even though, our results clearly showed that p-DGA is more effective compared to its synthetic derivative, synthesis effort would still be important in future studies to further improve the anti-cancer efficacy of p-DGA using medicinal chemistry tools.
AR plays a crucial role at all stages of PCA development (3,24). In the past, various strategies have been attempted to inhibit AR function including double-stranded RNA interference, antisense oligonucleotides, hammerhead ribozymes, heat shock protein inhibitors etc (31–35). Even though these approaches have shown promise in terms of inhibiting AR signaling and PCA growth, the debilitating side effects have dampened much of the enthusiasm (36). Results from the present study are quite exciting as we observed that p-DGA effectively decreases AR level in LNCaP and 22Rv1 cells in cell culture and 22Rv1 tumors in vivo with strong inhibition in PSA level. However, more studies are required in future to delineate the exact mechanism(s) of AR decrease by p-DGA.
Several studies in the past have elicited the direct role of AR in regulating the cell cycle progression especially the G1-S phase transition (8,37). Blocking of AR expression has been reported to result in cell cycle arrest in PCA cells (31,37–39). Androgen ablation in PCA cells has also been reported to induce strong cell cycle arrest (40,38). It is important to mention here that increased AR expression and a decrease in CDKIs expression are considered important events for the hormone-refractory progression of PCA (40,8,41). Furthermore, AR has been reported to negatively regulate CDKIs expression (8,41); and together, AR hyperactivity and loss of p21/p27 confer adverse prognosis in PCA patients (40,41). Results from the present study showed that p-DGA decreases AR expression whereas strongly induces p21 and p27 expression in PCA cells both in vitro and in vivo, but further studies are warranted to understand whether the increase in CDKIs by p-DGA is AR-dependent or -independent.
In the present study, we also observed that p-DGA induces strong apoptotic death in PCA cells in cell culture and in 22Rv1 xenografts. Earlier studies have shown a direct link between AR expression and apoptosis in prostate cancer cells (9). In this regard, the blocking of AR expression with specific hammerhead ribozyme or siRNA has been shown to induce apoptosis in PCA cells (9,39). Moreover, androgen deprivation has been reported to induce apoptosis in PCA cells, which is overcome by AR over-expression during progression towards hormone-refractory stage (42,43,36). But whether the strong induction of apoptosis with p-DGA treatment is directly related to its inhibitory effects on AR remains unknown and requires detailed mechanistic molecular studies using genetic approaches.
Currently, we lack the understanding about p-DGA biotransformation; however it is clear that a molecule of such a size and mass as p-DGA could not be passively absorbed from the gastrointestinal tract or interact directly with cell membrane receptors. Therefore, we cannot exclude that the observed biological effects are possibly caused by fragments of the p-DGA with much smaller size/mass. However, detailed pharmacokinetics studies are required in future to understand p-DGA absorption, distribution, metabolism, excretion and the liberation (if any) of fragments (or oligomers). The results from the present study also showed that p-DGA is extremely potent in vivo compared to its efficacy in cell culture studies. This could be due to its strong effect on tumor microenvironment (which is absent in cell culture studies) components such as endothelial cells, fibroblasts and so on. Therefore, we need to assess p-DGA effects on tumor microenvironment. Further, in vitro cells were exposed only once to p-DGA, whereas in vivo mice were orally administered p-DGA (5 days/week) for 5 weeks. Therefore comparatively stronger effect in vivo could also be due to the repetitive dosing over a period of time. Together, more studies in future would identify the mechanism(s) and targets for strong efficacy of p-DGA in vivo.
In summary, our studies for the first time revealed that p-DGA inhibits the growth of androgen-dependent and androgen-independent PCA cells both in vitro and in vivo. Results also revealed the broad spectrum effects of p-DGA on AR and PSA levels, cell cycle, and apoptosis revealing some of the plausible underlying mechanisms. Nevertheless, convincing proof for the notion that p-DGA is a promising new tool in PCA management requires a potency comparison with other naturally occurring phenols exemplified by fisetin/quercetin and AR signaling-modulating drugs such as finasteride/dutasteride. In conclusion, present study is significant as we identified a natural non-toxic compound with efficacy against PCA that supports its further pre-clinical and clinical testing as well as its translational applicability in near future.
Funding
RO1 grants CA91883, CA102514, Cancer Center Core Grant P30 CA046934, and GEB2-3344-TB-06.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations:
- AR
androgen receptor
- ATCC
American type culture collection
- Cdc25C
cell division cycle 25C
- CDKs
cyclin-dependent kinases
- Cdk2/4/6
cyclin-dependent kinase 2/4/6
- CDKIs
cyclin-dependent kinase inhibitors
- ECL
enhanced chemiluminescence
- ELISA
enzyme-linked immunosorbent assay
- FACS
fluorescence activated cell sorting
- IHC
immunohistochemical
- m-DGA
syn-2, 3-dihydroxy-3-(3, 4-dihydroxyphenyl) propionic acid
- PCA
human prostate cancer
- PCNA
proliferating cell nuclear antigen
- p-DGA
poly[3-(3, 4-dihydroxyphenyl)glyceric acid]
- PI
propidium iodide
- PSA
prostate specific antigen
- PVDF
polyvinylidene fluoride
- TUNEL:
terminal deoxynucleotidyl transferase dUTP nick end labeling
References
- 1. Jemal A., et al. (2010). Cancer statistics, 2010. CA. Cancer J. Clin. 60 277–300 [DOI] [PubMed] [Google Scholar]
- 2. Chen C.D., et al. (2004). Molecular determinants of resistance to antiandrogen therapy. Nat. Med. 10 33–39 [DOI] [PubMed] [Google Scholar]
- 3. Grossmann M.E., et al. (2001). Androgen receptor signaling in androgen-refractory prostate cancer. J. Natl. Cancer Inst. 93 1687–1697 [DOI] [PubMed] [Google Scholar]
- 4. Sharifi N., et al. (2006). Androgen receptor as a therapeutic target for androgen independent prostate cancer. Am. J. Ther. 13 166–170 [DOI] [PubMed] [Google Scholar]
- 5. Hanahan D., et al. (2000). The hallmarks of cancer. Cell 100 57–70 [DOI] [PubMed] [Google Scholar]
- 6. Deep G., et al. (2008). New combination therapies with cell-cycle agents. Curr. Opin. Investig. Drugs 9 591–604 [PMC free article] [PubMed] [Google Scholar]
- 7. Kastan M.B., et al. (1995). P53, cell cycle control and apoptosis: implications for cancer. Cancer Metastasis Rev. 14 3–15 [DOI] [PubMed] [Google Scholar]
- 8.Balk S.P., et al. AR, the cell cycle, and prostate cancer. Nucl. Recept. Signal. (2008);6:e001. doi: 10.1621/nrs.06001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liao X., et al. (2005). Small-interfering RNA-induced androgen receptor silencing leads to apoptotic cell death in prostate cancer. Mol. Cancer Ther. 4 505–515 [DOI] [PubMed] [Google Scholar]
- 10. Abuladze G.V, et al. (1995). Isolation and pharmacological investigation of polysaccharides from Symphytum asperum Proc. Georg. Acad. Sci. Biol. Series 21 129–132 [Google Scholar]
- 11. Barthomeuf C.M., et al. (2001). Evaluation of the dietetic and therapeutic potential of a high molecular weight hydroxycinnamate-derived polymer from Symphytum asperum Lepech. Regarding its antioxidant, antilipoperoxidant, antiinflammatory, and cytotoxic properties. J. Agric. Food Chem. 49 3942–3946 [DOI] [PubMed] [Google Scholar]
- 12. Mulkijanyan K, et al. (2009). Burn healing compositions from Caucasian speceies of Comfrey (Symphytum L.). Bulletin of the Georgian National Academy of Sciences 3 114–117 [Google Scholar]
- 13. Schäfer M., et al. (2008). Cancer as an overhealing wound: an old hypothesis revisited. Nat. Rev. Mol. Cell Biol. 9 628–638 [DOI] [PubMed] [Google Scholar]
- 14. Barbakadze V., et al. (2005). Poly[3-(3,4-dihydroxyphenyl)glyceric acid], a new biologically active polymer from Symphytum asperum Lepech. and S. caucasicum Bieb. (Boraginaceae). Molecules 10 1135–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barbakadze V, et al. Effects of Poly[3-(3,4-dihydroxyphenyl)glyceric acid] on the Inflammatory Response of Tumor-Activated Hepatic Sinusoidal Endothelium. Bulletin of the Georgian National Academy of Sciences. (2008);2:108–112. [Google Scholar]
- 16. Barbakadze V, et al. (2002). Poly[3-(3,4-dihydroxyphenyl)glyceric acid]: a new biologically active polymer from two comfrey species Symphytum asperum and S. caucasicum (Boraginaceae). Russ. J. Bioorg. Chem 28 362–330 [Google Scholar]
- 17. Barbakadze V, et al. (2000). Structure of a new anticomplementary dihydroxycinnamate-derived polymer from Symphytum asperum (Boraginaceae). Mendeleev Communications 10 148–149 [Google Scholar]
- 18. Merlani M., et al. (2010). Enantioselective synthesis and antioxidant activity of 3-(3,4-dihydroxyphenyl)-glyceric acid–basic monomeric moiety of a biologically active polyether from Symphytum asperum and S. caucasicum. Chirality 22 717–725 [DOI] [PubMed] [Google Scholar]
- 19. Kaur M., et al. (2009). Gallic acid, an active constituent of grape seed extract, exhibits anti-proliferative, pro-apoptotic and anti-tumorigenic effects against prostate carcinoma xenograft growth in nude mice. Pharm. Res. 26 2133–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaur M., et al. (2008). Grape seed extract induces cell cycle arrest and apoptosis in human colon carcinoma cells. Nutr. Cancer 60 Suppl 1 2–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Faith D.A., et al. (2004). Trefoil factor 3 overexpression in prostatic carcinoma: prognostic importance using tissue microarrays. Prostate 61 215–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Singh R.P., et al. (2004). Oral silibinin inhibits lung tumor growth in athymic nude mice and forms a novel chemocombination with doxorubicin targeting nuclear factor kappaB-mediated inducible chemoresistance. Clin. Cancer Res. 10 8641–8647 [DOI] [PubMed] [Google Scholar]
- 23. Gu M., et al. (2005). Silibinin inhibits ultraviolet B radiation-induced mitogenic and survival signaling, and associated biological responses in SKH-1 mouse skin. Carcinogenesis 26 1404–1413 [DOI] [PubMed] [Google Scholar]
- 24. Li T.H., et al. (2007). A promoting role of androgen receptor in androgen-sensitive and -insensitive prostate cancer cells. Nucleic Acids Res. 35 2767–2776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aggarwal B.B., et al. (2006). Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol. 71 1397–1421 [DOI] [PubMed] [Google Scholar]
- 26. Deep G., et al. (2010). Antimetastatic efficacy of silibinin: molecular mechanisms and therapeutic potential against cancer. Cancer Metastasis Rev. 29 447–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gullett N.P., et al. (2010). Cancer prevention with natural compounds. Semin. Oncol. 37 258–281 [DOI] [PubMed] [Google Scholar]
- 28. Harvey A.L. (2008). Natural products in drug discovery. Drug Discov. Today 13 894–901 [DOI] [PubMed] [Google Scholar]
- 29. Singh R.P., et al. (2004). A cancer chemopreventive agent silibinin, targets mitogenic and survival signaling in prostate cancer. Mutat. Res. 555 21–32 [DOI] [PubMed] [Google Scholar]
- 30. Predel H.G., et al. (2005). Efficacy of a comfrey root extract ointment in comparison to a diclofenac gel in the treatment of ankle distortions: results of an observer-blind, randomized, multicenter study. Phytomedicine 12 707–714 [DOI] [PubMed] [Google Scholar]
- 31. Cheng H., et al. (2006). Short hairpin RNA knockdown of the androgen receptor attenuates ligand-independent activation and delays tumor progression. Cancer Res. 66 10613–10620 [DOI] [PubMed] [Google Scholar]
- 32. Eder I.E., et al. (2002). Inhibition of LNCaP prostate tumor growth in vivo by an antisense oligonucleotide directed against the human androgen receptor. Cancer Gene Ther. 9 117–125 [DOI] [PubMed] [Google Scholar]
- 33. Solit D.B., et al. (2003). Hsp90 as a therapeutic target in prostate cancer. Semin. Oncol. 30 709–716 [DOI] [PubMed] [Google Scholar]
- 34. Solit D.B., et al. (2002). 17-Allylamino-17-demethoxygeldanamycin induces the degradation of androgen receptor and HER-2/neu and inhibits the growth of prostate cancer xenografts. Clin. Cancer Res. 8 986–993 [PubMed] [Google Scholar]
- 35. Zegarra-Moro O.L., et al. (2002). Disruption of androgen receptor function inhibits proliferation of androgen-refractory prostate cancer cells. Cancer Res. 62 1008–1013 [PubMed] [Google Scholar]
- 36. Scher H.I., et al. (2004). Targeting the androgen receptor: improving outcomes for castration-resistant prostate cancer. Endocr. Relat. Cancer 11 459–476 [DOI] [PubMed] [Google Scholar]
- 37. Hååg P., et al. (2005). Androgen receptor down regulation by small interference RNA induces cell growth inhibition in androgen sensitive as well as in androgen independent prostate cancer cells. J. Steroid Biochem. Mol. Biol. 96 251–258 [DOI] [PubMed] [Google Scholar]
- 38. Knudsen K.E., et al. (1998). Multiple G1 regulatory elements control the androgen-dependent proliferation of prostatic carcinoma cells. J. Biol. Chem. 273 20213–20222 [DOI] [PubMed] [Google Scholar]
- 39. Tong Q., et al. (2003). Effects of blocking androgen receptor expression with specific hammerhead ribozyme on in vitro growth of prostate cancer cell line. Chin. Med. J. 116 1515–1518 [PubMed] [Google Scholar]
- 40. Agus D.B., et al. (1999). Prostate cancer cell cycle regulators: response to androgen withdrawal and development of androgen independence. J. Natl. Cancer Inst. 91 1869–1876 [DOI] [PubMed] [Google Scholar]
- 41. Wang L.G., et al. (2001). Overexpressed androgen receptor linked to p21WAF1 silencing may be responsible for androgen independence and resistance to apoptosis of a prostate cancer cell line. Cancer Res. 61 7544–7551 [PubMed] [Google Scholar]
- 42. Feldman B.J., et al. (2001). The development of androgen-independent prostate cancer. Nat. Rev. Cancer 1 34–45 [DOI] [PubMed] [Google Scholar]
- 43. Kerr J.F., et al. (1973). Deletion of cells by apoptosis during castration-induced involution of the rat prostate. Virchows Arch. B. Cell Pathol. 13 87–102 [DOI] [PubMed] [Google Scholar]