Abstract
Membrane type-1 matrix metalloproteinase (MT1-MMP) is often activated and expressed in tumor cells with significant invasive properties, and is associated with poor prognosis of patients. This could partly be due to deregulated expression of microRNAs (miRNAs) which regulates the expression of MT1-MMP and PTEN (phosphatase and tensin homolog) contributing to tumor invasion and metastasis. We initially compared the expression profile of miR-200 family, PTEN and MT1-MMP expression in six pancreatic cancer (PC) cell lines by qRT-PCR and western blot analysis. We found loss of expression of miR-200a, b and c in chemo-resistant PC cell lines, which was correlated with loss of PTEN and over-expression of MT1-MMP. Based on our initial findings, we chose BxPC-3, MIAPaCa-2 and MIAPaCa-2-GR cells for further mechanistic studies We assessed the effect of two separate novel agents CDF (a synthetic analog of curcumin) and BR-DIM (a natural agent) on PC cells. The expression of miR-200 family and PTEN was significantly re-expressed whereas the expression of MT1-MMP was down-regulated by CDF and BR-DIM treatment. Forced over-expression or silencing of miR-200c, followed by either CDF or BR-DIM treatment of MIAPaCa-2 cells, altered the morphology of cells, wound-healing capacity, colony formation and the expression of MT1-MMP and PTEN. These results provide strong experimental evidence showing that the loss of miR-200 family and PTEN expression and increased level of MT1-MMP leads to aggressive behavior of PC cells, which could be attenuated through re-expression of miR-200c by CDF and/or BR-DIM treatment, suggesting that these agents could be useful for PC treatment.
Abbreviations:
- EMT
epithelial to mesenchymal transition
- miRNAs
microRNAs
- MT1-MMP
membrane type-1 matrix metalloproteinase
- PC
pancreatic cancer
- PTEN
phosphatase and tensin homolog.
Introduction
It has been estimated that 43 920 people will be newly diagnosed with pancreatic cancer (PC) in 2012, and it is the fourth leading cause of cancer-related deaths in the USA (1). Although research effort has advanced toward targeted therapies, late diagnosis and/or diagnosis with metastatic therapy-resistant disease has made PC the leading cause of high mortality. Hence, better understanding of the underlying mechanisms involved in therapeutic resistance and findings ways to overcome drug resistance is critical for improving the dismal survival statistics and therapeutic efficacies in PC patients.
Amongst the multiple matrix metalloproteinases activated and expressed in a wide range of tumors, matrix metalloproteinase 14 (MMP-14), also known as membrane type-1 matrix metalloproteinase (MT1-MMP), is believed to play a crucial role in facilitating the tumor cells’ penetration through extracellular matrix with significant potential for angiogenesis and invasive characteristics (2,3). MT1-MMP has also been associated to promote tumor invasion by the activation of proMMP-2, and is also directly associated with increased potential for angiogenesis and metastasis (4–6). According to another report, binding of endothelial cells to extracellular matrix shows the existence of two phases of MMP regulation, one through rapid inhibition of pro-MMP-2 activation, through inhibition of MT1-MMP, and the other by slower response of cell spreading and changes in the cytoskeleton to suppress the levels of MT1-MMP mRNA and protein (7). The over-expression of MT1-MMP has also been associated with metastatic behavior of virtually all types of cancers (8,9), including PC in K-Ras transgenic mouse model (10) and in biopsies from triple-negative breast cancer (11). However, little is known about the expression of MT1-MMP and the underlying mechanisms involved in human PC, suggesting that understanding the regulation of MT1-MMP and finding ways to inhibit its expression would be important for designing novel therapies for PC.
Besides the deregulation of MT1-MMP, the loss of expression of phosphatase and tensin homolog (PTEN), which is a ubiquitous tumor suppressor gene, has been shown to correlate with tumor aggressiveness and was also associated with up-regulation of miRNA expression such as miR-21 (12,13). Additionally, over-expression of MT1-MMP was found to be associated with loss of PTEN expression in prostate cancer cells derived from mice through the activation of the PI3K/Akt pathway (14); whereas in renal cell carcinoma, loss of PTEN induces HIF-2α transcriptional activity through antagonism of PI3K signaling (15). Moreover, in H-59 cells of lung carcinoma, IGF-1 receptor controls tumor cell invasion through MT1-MMP activation mediated by activation of PI3K/Akt/mTOR signaling (16). Thus, the loss of PTEN expression and increased MT1-MMP expression could have a significant impact on the regulation of cell growth, invasion, migration and aggressiveness of PC cells. Therefore, it is vital to find novel agents that could mechanistically regulate MT1-MMP and PTEN expression in PC, which would likely advance our knowledge in designing novel and improved therapies for the treatment of PC.
Emerging evidence suggest that microRNAs (miRNAs), highly conserved and small non-coding regulatory RNAs, play a major role in the regulation of gene expression through post-transcriptional repression, and appear to be important in PC. The expression of miR-200 family has been established by us and others both in vitro and in vivo as one of the most intensively studied epithelial to mesenchymal transition (EMT)-related miRNAs that target multiple genes (12,17–20). Recent evidence has also shown down-regulation of miR-200 family by ZEB1 due to suppression of stemness-inhibiting miRNAs in the 38 different carcinoma cell lines of the NCI-60 cell line panel (20). In lung cancer cell lines derived from mice, miR-200 altered the tumor environment, inhibiting the processes of EMT and metastasis (21). These findings suggest that the expression of miR-200 in PC is closely correlated with stemness, metastasis and EMT, which is due to targeting multiple genes. Hence, re-expression of miR-200 family either by transfection with its precursors or treatment by novel agents (natural agents) could have the potential for the inhibition of EMT and stemness markers, suggesting that such a strategy could become a novel therapeutic approach for the treatment of PC.
Although the loss of PTEN has been shown to regulate miR-21 expression, the extent to which it is affected through modulation of miR-200 and its role in the deregulation of MT1-MMP and PTEN has not been previously examined. Therefore, the aim of the current study was to investigate the interplay between the expression of MT1-MMP and PTEN deregulation mediated through the expression of miR-200 in PC cell lines. We further mechanistically investigated the putative role of miR-200c and its effects on the expression of MT1-MMP and PTEN by transfecting pre-miR-200c (precursor) or ASO-miR-200c (inhibitor) in human PC MIAPaCa-2 cell line. We found that the re-expression of pre-miR-200c led to decreased cell migration and clonogenicity, which was associated with down-regulation of MT1-MMP and re-expression of PTEN. Moreover, instead of transfection, our novel agents, BR-DIM and CDF, were able to cause re-expression of miR-200c and down-regulated the expression of MT1-MMP, which was consistent with the up-regulation of PTEN expression.
Materials and methods
Cells culture, drugs and reagents
Human PC cell lines AsPC-1, BxPC-3, COLO-357, MIAPaCa-2, MIAPaCa-GR (gemcitabine resistant) and PANC-1 were chosen for this study. The cell lines have been tested and authenticated using the core facility–Applied Genomics Technology Center at Wayne State University, on 13 March 2009. The method used for testing was short tandem repeat profiling using the PowerPlex® 16 System from Promega (Madison, WI, USA). These cells were stored in multiple vials in liquid nitrogen for our use. CDF was synthesized as described in our earlier publications (22,23), and BR-DIM, a formulated DIM with higher bioavailabilty (24), was obtained from Dr. Michael Zeligs (BioResponse, LLC, Boulder, CO, USA). Both of these novel agents have been extensively used in our laboratory for the treatment of most cancer cells in vitro and in vivo including PC, as shown by many of our published papers (12,24–31).
Protein extraction and western blot analysis
We initially tested a range of concentrations for BR-DIM (10–50 µM) and CDF (0.5–2 µM), and found that 25 µM of BR-DIM and 0.5–1 µM of CDF was optimal for further studies. Based on these initial results, MIAPaCa-2, MIAPaCa-2-GR and BxPC-3 cells were treated with BR-DIM (25 µM) or CDF (0.5–1 µM) for all subsequent assays for 24, 48 and 72h. Light micrographic pictures were taken at every time point with both BR-DIM and CDF treatment. Total protein was extracted from untreated and both BR-DIM- and CDF-treated cells at 24, 48 and 72h and were loaded with 50 µg of protein and subjected to western blot analysis as described previously (32) to evaluate the expression of PTEN and MT1-MMP. The data were adjusted against loading control using β-actin expression.
Real-time reverse transcriptase-polymerase chain reaction (RT-PCR)
To determine the basal level of miR-200 family (miR-200a, miR-200b, miR-200c) in all six PC cell lines, and also cells treated with BR-DIM (25 µM) or CDF (0.5–1 µM) for 24h, we used TaqMan MicroRNA (miRNA) Assay kit (Applied Biosystems) and the available primer and probe for miR-200 family from Applied Biosystems, following manufacturer’s protocol. Total RNA was extracted and 10ng from each sample were reverse transcribed as described earlier (33). The expression of miR-200a, 200b and 200c of untreated and treated with BR-DIM and CDF were then carried out in a total volume of 10 µl reaction mixture by qRT-PCR as described earlier (33). All reactions, including controls and the experiments, were performed in triplicate, using StepOnePlus Real-Time PCR (Applied Biosystems). Relative expression of miRNAs was analyzed using Ct method and was normalized by RNU48 expression.
Pre-miR-200c and antisense miR-200c oligonucleotide transfection
MIAPaCa-2 cells were plated in 6-well plates and incubated overnight. Cells were transfected with either control miRNA or pre-miR-200c or ASO-miR-200c (Ambion, Austin, TX, USA) at a final concentration of 20nM, using DharmaFECT transfection reagent (Dharmacon), followed by BR-DIM or CDF treatment for 48–72h. After 24h of transfection, the medium was changed to avoid cell death during transfection. Transfected cells were then tested for wound healing and clonogenic assay, and also harvested for the extraction of total RNA and protein using standard methods.
Wound healing assay of transfected cells
Wound healing assay was performed to examine the capacity of cell migration and invasion, as described previously (13). Briefly, after the cells grew in about 80–90% confluence in 6-well plates, the wound was generated by scratching the surface of the plates with a 200 µL pipette tip. The cells were then transfected with either control miRNA, pre-miR-200c or ASO-miR-200c, followed by either BR-DIM or CDF treatment for 18h. Photographic images were taken at 0h and 18h using a microscope (Nikon ECLIPSE TS100).
Clonogenic assay of transfected cells
Transfected cells as described above were trypsinized and 1000 viable cells were plated in 100-mm petri dishes. The cells were then incubated for about 10–12 days at 37°C in a 5% CO2/5% O2/90% N2 incubator. Colonies were stained with 2% crystal violet and scanned for images.
Statistical methods
Comparisons of treatment outcome were tested for statistical difference by the paired t-test. Statistical significance was assumed at a P value of <0.05.
Results
MT1-MMP and PTEN expression in PC cells was associated with deregulated expression of miRNAs
MT1-MMP is often expressed in tumor cells with significant invasive properties, and is associated with poor prognosis of patients. In contrast, PTEN, a well-known tumor suppressor gene, has been reported to be lost in tumors (12). We examined the basal level of MT1-MMP and PTEN expression in six human PC cell lines. MT1-MMP expression was highly elevated in AsPC-1, PANC-1 and MIAPaCa-2-GR cells, and moderately elevated in MIAPaCa-2 cells compared with BxPC-3 and COLO-357 cells (Figure 1A). In contrast, the expression of PTEN was significantly lower in PANC-1 and MIAPaCa-2-GR cells. Similarly, miRNA-200 family were differentially expressed in all six cell lines of which MIAPaCa-2, MIAPaCa-2-GR and PANC-1 cells showed significantly lower expression of miR-200a, miR-200b and miR-200c, compared with AsPC-1, BxPC-3 and COLO-357 cells (Figure 1B). The loss of expression of miR-200 family was correlated with the level of PTEN expression. For our subsequent studies, three cell lines (BxPC-3, MIAPaCa-2 and MIAPaCa-2-GR) were chosen as documented below.
Fig. 1.
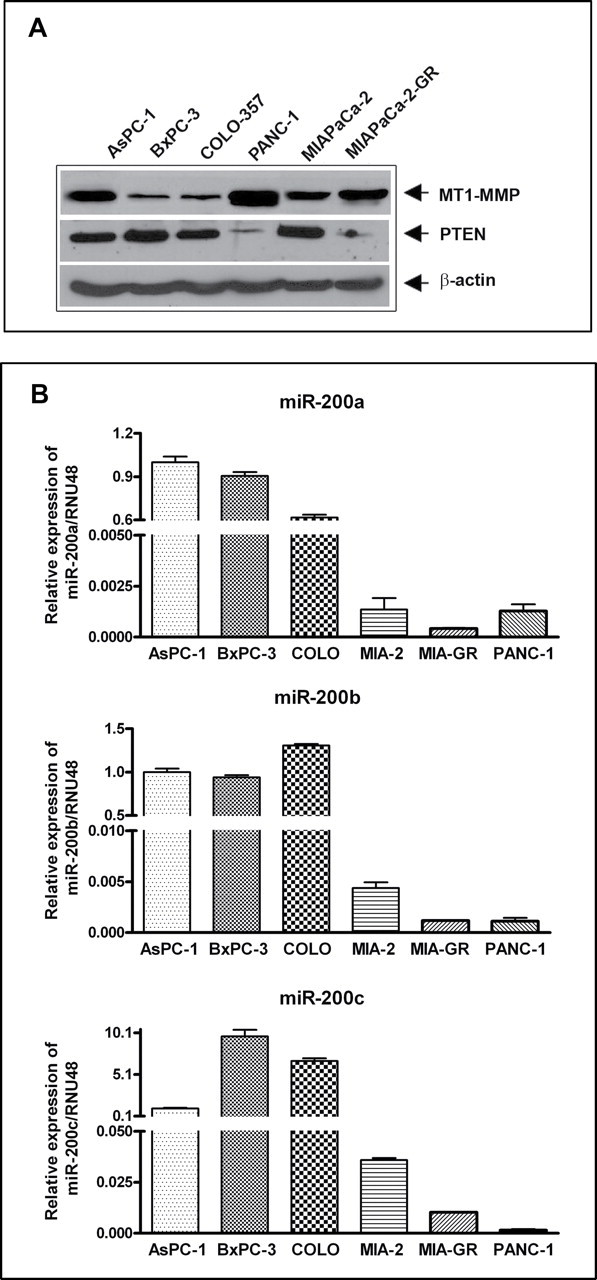
Basal levels of MT1-MMP and PTEN expression in human PC cell lines AsPC-1, BxPC-3, COLO-357, PANC-1, MIAPaCa-2 and MIAPaCa-2-GR cells. MT1-MMP expression was significantly higher in AsPC-1, PANC-1 and MIAPaCa-2-GR cells while PTEN expression was significantly lower in PANC-1 and MIAPaCa-2-GR cells (A). Relative expressions of miR-200a, miR-200b and miR-200c (B), as assessed by qRT-PCR in the above six PC cell lines. The expression of miR-200a, miR-200b and miR-200c was significantly lower in MIAPaCa-2, MIAPaCa-2-GR and PANC-1 cells. Each experiment was repeated, at least, three times independently, and the bar represents standard deviation from all three experiments.
Light micrographic pictures
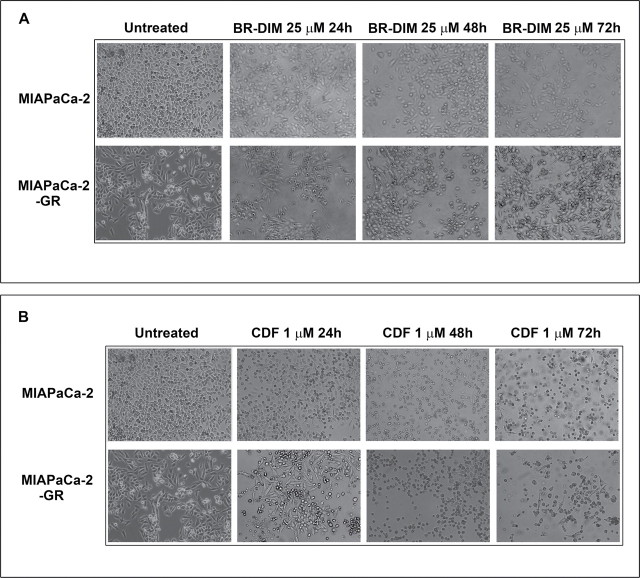
Figure 2A and 2B demonstrate the morphological differences in MIAPaCa-2 and MIAPaCa-2-GR cells. The MIAPaCa-2 cells were exposed to gemcitabine every other week for a period of 6 months which led to this mesenchymal phenotype as reported earlier (12,34). MIAPaCa-2 and MIAPaCa2-GR cells treated with either BR-DIM or CDF for 24, 48 and 72h (Figure 2A and 2B) were photographed and were subsequently used for all our experiments. BxPC-3 cells were also treated similarly (images not shown) and were used for all our subsequent experiments as shown below.
Fig. 2.
The time-dependent effect of treatment with both BR-DIM (A) and CDF (B). Light photomicrographs of MIAPaCa-2 and MIAPaCa-2-GR cell lines untreated and treated with BR-DIM (25 µM) for 24, 48 and 72h and untreated and treated with CDF 1 µM for 24, 48 and 72h. MIAPaCa-2 cells were exposed to gemcitabine and the paired cell line was called MIAPaCa-2 and MIAPaCa-2-GR based on their changes in morphology from epithelial-like to mesenchymal-like phenotype. Each experiment was repeated, at least, three times independently.
Re-expression of miR-200 family was achieved by BR-DIM treatment
We determined the expression levels of miR-200 family (miR-200a, miR-200b and miR-200c) after treatment with 25 µM of BR-DIM for 24h in MIAPaCa-2, MIAPaCa-2-GR and BxPC-3 cells. The expression level was determined by real-time RT-PCR. We found a significant up-regulation in the expression of miR-200a, miR-200b and miR-200c in all three cell lines treated with BR-DIM (Figure 3A).
Fig. 3.
Effect of treatment on the expression level of miR-200 family (miR-200a, miR-200b and miR-200c) as determined by real-time RT-PCR on MIAPaCa-2, MIAPaCa-2-GR and BxPC-3 cells with 25 µM of BR-DIM (A), and 1 µM of CDF (B) for 24h. We found significant up-regulation in the expression of miR-200a, miR-200b and miR-200c in all three cell lines treated with both BR-DIM and CDF. P values represent comparison between untreated and BR-DIM or untreated and CDF-treated cells as calculated by the paired t test. Each experiment was repeated, at least, three times independently, and the bar represents standard deviation from all three experiments.
Re-expression of miR-200 family was achieved by CDF treatment
We also determined the expression levels of miR-200a, miR-200b and miR-200c after treatment of PC cells with 0.5–1 µM of CDF for 24h and assessed by real-time RT-PCR. We found a significant up-regulation in the expression of miR-200a, miR-200b and miR-200c in all three cell lines treated with CDF (Figure 3B). To further validate whether the protein expression of MT1-MMP and PTEN could be altered by either BR-DIM or CDF treatment, we investigated the effect of treatment in all three cell lines by western blot analysis as presented below.
Modulation of MT1-MMP and PTEN expression by BR-DIM and CDF
BxPC-3, MiaPaCa-2 and MiaPaCa-2-GR cells were used to evaluate the effects of BR-DIM and CDF treatment on the expression of MT1-MMP and PTEN. Cells were treated with 25 µM BR-DIM or 0.5–1 µM CDF in a time-dependent manner for 24, 48 and 72h. Expression of MT1-MMP proteins was significantly reduced in all three cell lines treated with either BR-DIM or CDF when compared with untreated control (Figure 4). In contrast, the expression of PTEN, a tumor suppressor gene, was found to be decreased in MIAPaCa-2-GR cells compared with BxPC-3 or MIAPaCa-2 cells and was significantly enhanced with both BR-DIM and CDF treatment in all three cell lines. These results suggest that BR-DIM and CDF could be effective for re-expression of PTEN. To further validate whether miR-200 indeed could target the MT1-MMP, or PTEN expression, we chose to investigate the effect of transfection of miR-200c with both precursor and antisense oligonucleotide in MIAPaCa-2 cells, and also treated the cells with BR-DIM and CDF as presented below.
Fig. 4.
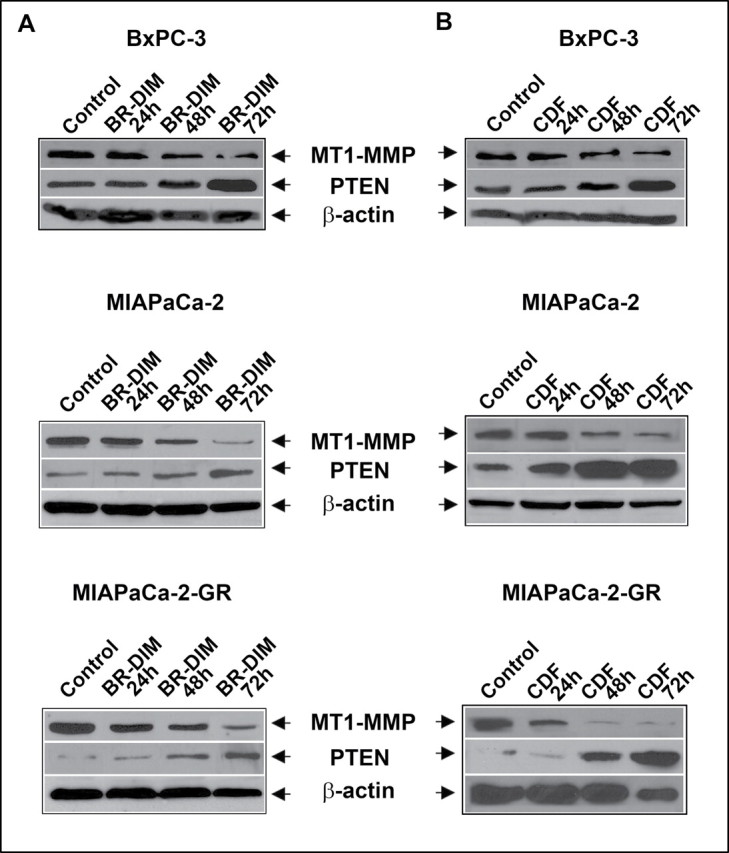
Effect of treatment on the expression level of MT1-MMP and PTEN as determined by western blot analysis of BxPC-3, MIAPaCa-2 and MIAPaCa-2-GR cells with 25 µM of BR-DIM for 72h (A) and 1 µM of CDF for 72h (B). We found significant down-regulation of MT1-MMP and up-regulation of PTEN in all three cell lines treated with either BR-DIM or CDF compared with untreated cells in a time-dependent manner.
MT1-MMP and PTEN expression are regulated by miR-200c and affecting wound-healing capacity and colony formation of MIAPaCa-2 cells
In order to test whether miR-200c expression could regulate MT1-MMP and PTEN expression, we over-expressed miR-200c with pre-miR-200c in MIAPaCa-2 cells, which express relatively low basal levels of miR-200c compared with BxPC-3 cells. MIAPaCa-2 cells were seeded in 6-well plates and were transfected with pre-miR-200c precursor for 48h followed by treatment with BR-DIM/CDF. Ectopic expression of miR-200c with miR-200c precursor led to changes in morphology of cells (Figure 5A), increased expression of miR-200c compared with control by qRT-PCR (Figure 5B) and significantly inhibited wound-healing capacity (Figure 5C). Interestingly, the re-expression of miR-200c led to decreased expression of MT1-MMP with simultaneous increase in PTEN expression (Figure 5D), and led to a significant decrease in colony formation (Figure 5E). These effects were further enhanced after BR-DIM/CDF treatment compared with control, suggesting the mechanistic role of miR-200c in the regulation of MT1-MMP and PTEN and that these effects could be easily achieved by treating the cells with CDF, which showed better effects than BR-DIM. These results further prompted us to conduct a reverse experiment by further inactivation in the expression of miR-200c using ASO-miR-200c transfection and assessed the expression of MT1-MMP and PTEN and correlated our findings with wound-healing capacity and clonogenic growth as presented below.
Fig. 5.
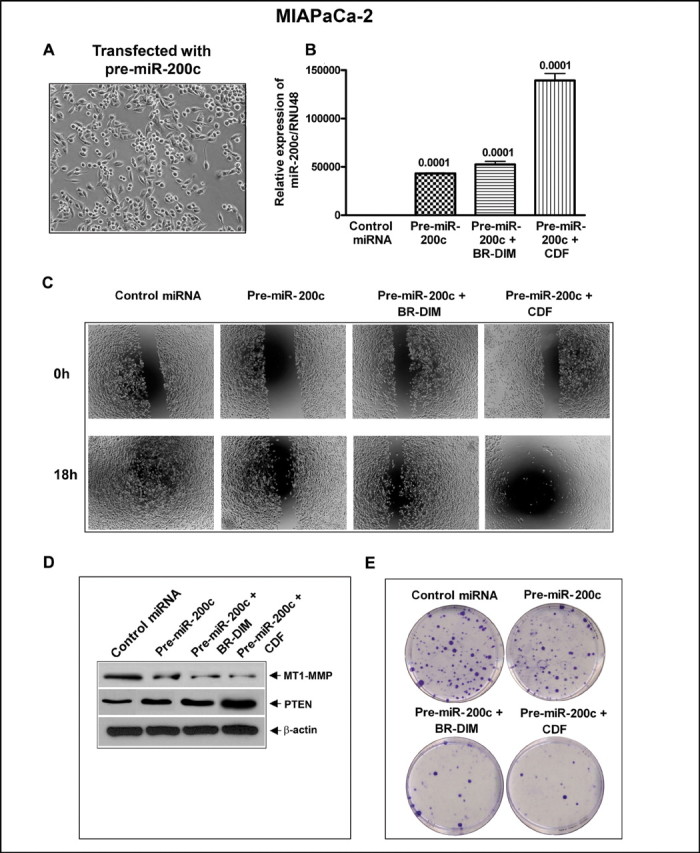
Re-expression of miR-200c by pre-miR-200c precursor transfection in MIAPaCa-2 cells led to change in morphology of cells (A). Transfection of miR-200c or treatment of MIAPaCa-2 cells by BR-DIM or CDF increased expression of miR-200c as assessed by qRT-PCR (B), decreased in cell migration (C), decreased levels of MT1-MMP, and up-regulation of PTEN by western blot analysis (D) decreased clonogenicity as determined by colony formation assay (E) as compared with control cells. Each experiment was repeated, at least, three times independently.
Transfection of antisense miR-200c in MIAPACa-2 cells increased cell migration and colony formation, and caused altered protein expression of MT1-MMP and PTEN
We investigated the consequence of inactivation of miR-200c expression by transfecting the cells with ASO-miR-200c on the expression of MT1-MMP and PTEN and also assessed cellular consequence on cell migration and colony formation. Interestingly, we found that the morphology of the cell changed to mesenchymal phenotype from epithelial phenotype after ASO-miR-200c transfection as shown in Figure 6A, which was correlated with decreased expression of miR-200c compared with control as assessed by qRT-PCR (Figure 6B). The inactivation of miR-200c using ASO-miR-200c transfection also led to an increased wound-healing capacity and clonogenicity in the transfected cells compared with control cells (Figure 6C and 6E), and these cellular characteristics were correlated with decreased expression of PTEN and increased expression of MT1-MMP (Figure 6D). Moreover, these changes were attenuated by treating the cells with CDF, which was superior than BR-DIM, as shown in Figure 6.
Fig. 6.
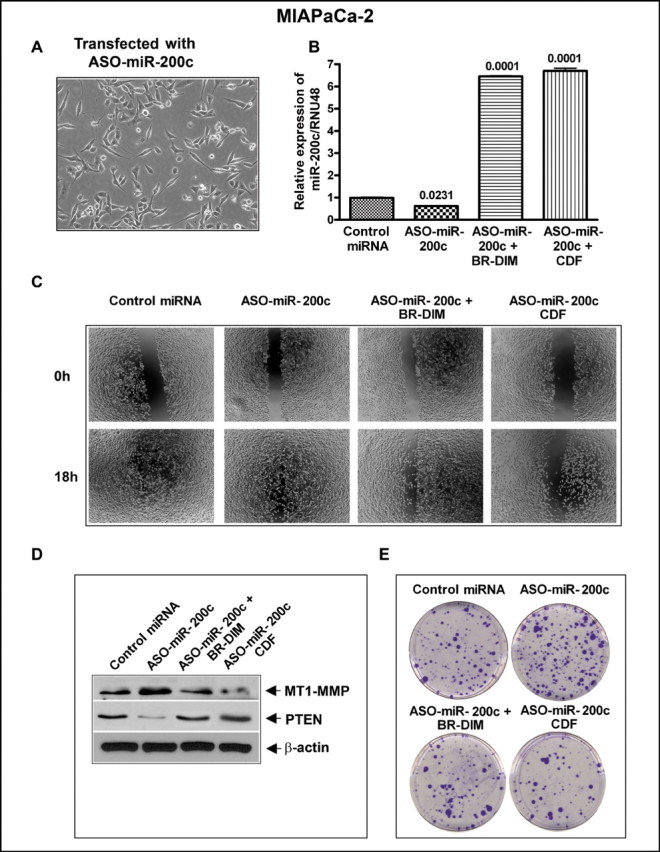
Inhibition of miR-200c by ASO-miR-200c transfection led to change in morphology of cells (A), decreased expression of miR-200c as assessed by qRT-PCR (B), increased in cell migration (C), increased levels of MT1-MMP, and inhibition of PTEN by western blot analysis (D) increased clonogenicity as determined by colony formation assay (E) as compared with control cells. All the above changes were rescued by BR-DIM or CDF treatment. Each experiment was repeated, at least, three times independently.
Discussion
MT1-MMP have been shown to be over-expressed in many human malignant tumors, including PC, and it is a crucial enzyme necessary during normal development but its activation as well as over-expression is also important in malignant processes (35,36). MT1-MMP is the only MMP shown to play a significant role in renal development both in vivo and in vitro (37). Messaritou et al. demonstrated that the collagen internalization receptor Endo180 is a novel regulator of MT1-MMP and uPA activity (38). In the present study, we have demonstrated that the over-expression of MT1-MMP was associated with reduced expression of PTEN in aggressive PC cell lines, which is consistent with previous studies in prostate, renal and lung cancer (14–16); however, to the best of our knowledge this is the first study to demonstrate the interplay between MT1-MMP and PTEN expression in PC. Since the majority of PC contains mutant K-Ras, the association between K-Ras mutation and PTEN expression has been reported especially in genetically engineered mouse model showing interactions of K-RasG12D and the loss of PTEN, resulting in increased metastasis through activation of NF-κB and its downstream cytokine pathway (39).
In the current study, we investigated the role of miRNAs especially because there is considerable interest in the mechanistic understanding of the role of miRNAs involved in tumor growth and metastasis, and their deregulation either by over-expression or knockdown by precursors and inhibitors are an emerging area of research. Computational algorithms have been the major methods in predicting miRNA targets based on the base pairing of miRNA and target gene 3’-UTR (40). Based on our experimental evidence and TargetScanHuman 5.2, we found miR-200b and miR-200c to possess a match for base pairing with the 3’-UTR of PTEN. Based on our initial finding, we chose miR-200c for further mechanistic studies in MIAPaCa-2 cells. We found that the loss of PTEN was directly correlated with low expression of miR-200, and that the forced over-expression of miR-200c with precursor or treatment of cells with our novel agents especially CDF resulted in increased PTEN expression, suggesting that miR-200c could regulate the expression of PTEN by translational regulation, and thus we believe that deregulation of miRNAs could become a newer strategy for the treatment of PC.
Previous studies have shown that PC cell lines exhibit significantly lower levels of expression of miR-200 family, which was associated with increased EMT, suggesting that miR-200 play an important role in several key aspects of tumor initiation and progression (12,41). Others have reported down-regulation of miR-200 family expression through Smad signaling-dependent manner during the progression of renal fibrosis (42). Moreover, the loss of p53 was correlated with decrease in miR-200c expression and an increase in EMT and stemness markers in a cohort of breast tumors (43). These evidences clearly suggest the role of miR-200 expression in tumor aggressiveness. In our current study, we found that miR-200 expression was drastically down-regulated in aggressive PC cell lines, and that the re-expression of miR-200c using pre-miR-200c transfection of MIAPaCa-2 cells led to decreased expression of MT1-MMP with concomitantly increased expression of PTEN. Interestingly, these effects were further pronounced by treatment of cells with both BR-DIM and/or CDF treatment, suggesting that these agents especially CDF could be useful for deregulation of important molecular events that are associated with tumor aggressiveness. In contrast, following further knockdown of miR-200c by ASO-miR-200c, we found a marked increase in MT1-MMP expression, which resulted in the down-regulation of tumor suppressor gene PTEN in PC cell lines. Moreover, the inactivation in the expression of miR-200c resulted in the acquisition of EMT phenotype and tumor cell aggressiveness, which is consistent with our previous findings (12). Based on our observations, we conclude the importance of the miR-200c miRNA because it may serve as the key regulators of MT1-MMP and PTEN expression, and that the restoration of PTEN expression and down-regulation of MT1-MMP could be easily achieved with both CDF and BR-DIM treatment, which was mediated through re-expression of miR-200c although CDF was found to be superior than BR-DIM.
We have previously demonstrated an increase in MT1-MMP expression in K-ras Cre-mediated activation of a mutant K-ras allele (KrasG12D) and deletion of a conditional Ink4a/Arf tumor suppressor allele in transgenic mouse model (10), which led to tumor initiation and progression. Another recent study reported that dimerization of MT1-MMP is required to promote cell invasion in a collagen-enriched environment (44). RNA silencing of endogenous MT1-MMP expression in fibrosarcoma and gastric carcinoma cell lines down-regulated only MT1-MMP expression, but not other MMPs which caused significant inhibition in the migration and invasion of tumor cells (45). Our observation in this study showed that over-expression of MT1-MMP increased cell migration in aggressive PC cell lines, which was significantly reduced by the treatment of cells with our novel agents BR-DIM and CDF. In conclusion, our current findings clearly suggest that increased expression of MT1-MMP and decreased expression of PTEN is in part due to loss of expression of miR-200c in PC. Moreover, we have provided experimental evidence, supporting that targeted re-expression of miR-200c by BR-DIM and CDF led to decreased expression of MT1-MMP and causing re-expression of PTEN, resulting in reducing tumor cell aggressiveness. Hence these agents, especially CDF, could serve as a novel approach for the treatment of PC.
Funding
National Cancer Institute (NIH 5R01CA131151, 3R01CA131151- 02S109, 1R01CA132794 and 1R01CA154321 to F.H.S.).
Acknowledgements
We also thank Puschelberg and Guido foundations for their generous contribution.
Conflict of Interest Statement: None declared.
References
- 1. Siegel R, et al. (2012). Cancer statistics, 2012 CA Cancer J. Clin., 62 10–29 [DOI] [PubMed] [Google Scholar]
- 2. Koike T, et al. (2000). Activation of MMP-2 by clostridium difficile toxin B in bovine smooth muscle cells Biochem. Biophys. Res. Commun. 277 43–46 [DOI] [PubMed] [Google Scholar]
- 3. Seiki M., et al. (2003). Roles of pericellular proteolysis by membrane type-1 matrix metalloproteinase in cancer invasion and angiogenesis Cancer Sci. 94 569–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Al-Raawi D., et al. (2011). Membrane type-1 matrix metalloproteinase (MT1-MMP) correlates with the expression and activation of matrix metalloproteinase-2 (MMP-2) in inflammatory breast cancer Int. J. Clin. Exp. Med. 4 265–275 [PMC free article] [PubMed] [Google Scholar]
- 5. Furuichi K., et al. (2011). Matrix metalloproteinase-2 (MMP-2) and membrane-type 1 MMP (MT1-MMP) affect the remodeling of glomerulosclerosis in diabetic OLETF rats Nephrol. Dial. Transplant. 26 3124–3131 [DOI] [PubMed] [Google Scholar]
- 6.Kachgal S., et al. Bone marrow stromal cells stimulate an angiogenic program that requires endothelial MT1-MMP. J. Cell Physiol. (2012) doi: 10.1002/jcp.24056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yan L., et al. (2000). Adhesion-dependent control of matrix metalloproteinase-2 activation in human capillary endothelial cells J. Cell Sci. 113(Pt 22)3979–3987 [DOI] [PubMed] [Google Scholar]
- 8. Ottaviano A.J., et al. (2006). Extracellular matrix-mediated membrane-type 1 matrix metalloproteinase expression in pancreatic ductal cells is regulated by transforming growth factor-beta1 Cancer Res. 66 7032–7040 [DOI] [PubMed] [Google Scholar]
- 9. Stetler-Stevenson W.G., et al. (2001). Proteases in invasion: matrix metalloproteinases Semin. Cancer Biol. 11 143–152 [DOI] [PubMed] [Google Scholar]
- 10. Ali S., et al. (2011). Inactivation of Ink4a/Arf leads to deregulated expression of miRNAs in K-Ras transgenic mouse model of pancreatic cancer J. Cell Physiol. 227, 3373–3380 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11. Perentes J.Y., et al. (2011). Cancer cell-associated MT1-MMP promotes blood vessel invasion and distant metastasis in triple-negative mammary tumors Cancer Res. 71 4527–4538 [DOI] [PubMed] [Google Scholar]
- 12. Ali S., et al. (2010). Gemcitabine sensitivity can be induced in pancreatic cancer cells through modulation of miR-200 and miR-21 expression by curcumin or its analog CDF Cancer Res. 70 3606–3617 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Bao B., et al. Anti-tumor activity of a novel compound-CDF is mediated by regulating miR-21, miR-200, and PTEN in pancreatic cancer. PLoS One. (2011);6:e17850. doi: 10.1371/journal.pone.0017850. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14. Kim S., et al. (2010). Posttranslational regulation of membrane type 1-matrix metalloproteinase (MT1-MMP) in mouse PTEN null prostate cancer cells: enhanced surface expression and differential O-glycosylation of MT1-MMP Biochim. Biophys. Acta 1803 1287–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Petrella B.L., et al. (2009). PTEN suppression of YY1 induces HIF-2 activity in von-Hippel-Lindau-null renal-cell carcinoma Cancer Biol. Ther. 8 1389–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang D., et al. (2003). Type 1 insulin-like growth factor regulates MT1-MMP synthesis and tumor invasion via PI 3-kinase/Akt signaling Oncogene 22 974–982 [DOI] [PubMed] [Google Scholar]
- 17. Ahmad A., et al. (2011). Phosphoglucose isomerase/autocrine motility factor mediates epithelial-mesenchymal transition regulated by miR-200 in breast cancer cells Cancer Res. 71 3400–3409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brabletz S., et al. (2011). The ZEB1/miR-200 feedback loop controls Notch signalling in cancer cells EMBO J. 30 770–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kong D., et al. Epithelial to mesenchymal transition is mechanistically linked with stem cell signatures in prostate cancer cells. PLoS One. (2010);5:e12445. doi: 10.1371/journal.pone.0012445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wellner U., et al. (2009). The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs Nat. Cell Biol. 11 1487–1495 [DOI] [PubMed] [Google Scholar]
- 21. Schliekelman M.J., et al. (2011). Targets of the tumor suppressor miR-200 in regulation of the epithelial-mesenchymal transition in cancer Cancer Res. 71 7670–7682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Padhye S., et al. (2009). New difluoro Knoevenagel condensates of curcumin, their Schiff bases and copper complexes as proteasome inhibitors and apoptosis inducers in cancer cells Pharm. Res. 26 1874–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Padhye S., et al. (2009). Fluorocurcumins as cyclooxygenase-2 inhibitor: molecular docking, pharmacokinetics and tissue distribution in mice Pharm. Res., 26, 2438–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ali S., et al. (2008). Apoptosis-inducing effect of erlotinib is potentiated by 3,3'-diindolylmethane in vitro and in vivo using an orthotopic model of pancreatic cancer Mol. Cancer Ther. 7 1708–1719 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25. Ali S., et al. (2009). Sensitization of squamous cell carcinoma to cisplatin induced killing by natural agents Cancer Lett. 278 201–209 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26. Ali S., et al. (2012). Increased Ras GTPase activity is regulated by miRNAs that can be attenuated by CDF treatment in pancreatic cancer cells Cancer Lett. 319 173–181 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27. Banerjee S., et al. (2009). 3,3'-Diindolylmethane enhances chemosensitivity of multiple chemotherapeutic agents in pancreatic cancer Cancer Res. 69 5592–5600 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28. Bao B., et al. (2012). Curcumin analog CDF inhibits pancreatic tumor growth by switching on suppressor microRNAs and attenuating EZH2 expression Cancer Res. 72 335–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kong D., et al. Loss of Let-7 Up-Regulates EZH2 in prostate cancer consistent with the acquisition of cancer stem cell signatures that are attenuated by BR-DIM. PLoS One. (2012);7:e33729. doi: 10.1371/journal.pone.0033729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kong D., et al. (2012). Epigenetic silencing of miR-34a in human prostate cancer cells and tumor tissue specimens can be reversed by BR-DIM treatment Am. J. Transl. Res. 4 14–23 [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y., et al. Targeting bone remodeling by isoflavone and 3,3'-diindolylmethane in the context of prostate cancer bone metastasis. PLoS One. (2012);7:e33011. doi: 10.1371/journal.pone.0033011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ali S., et al. (2005). Simultaneous targeting of the epidermal growth factor receptor and cyclooxygenase-2 pathways for pancreatic cancer therapy Mol. Cancer Ther. 4 1943–1951 [DOI] [PubMed] [Google Scholar]
- 33. Ali S., et al. (2010). Differentially expressed miRNAs in the plasma may provide a molecular signature for aggressive pancreatic cancer Am. J. Transl. Res. 3 28–47 [PMC free article] [PubMed] [Google Scholar]
- 34. Wang Z., et al. (2009). Acquisition of epithelial-mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathway Cancer Res. 69 2400–2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nagakawa O., et al. (2000). Expression of membrane-type 1 matrix metalloproteinase (MT1-MMP) on prostate cancer cell lines Cancer Lett. 155 173–179 [DOI] [PubMed] [Google Scholar]
- 36. ngi-Garimella S., et al. (2011). Collagen regulation of let-7 in pancreatic cancer involves TGF-beta1-mediated membrane type 1-matrix metalloproteinase expression Oncogene 30 1002–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Riggins K.S., et al. (2010). MT1-MMP-mediated basement membrane remodeling modulates renal development Exp. Cell Res. 316 2993–3005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Messaritou G., et al. (2009). Membrane type-1 matrix metalloproteinase activity is regulated by the endocytic collagen receptor Endo180 J Cell Sci. 122 4042–4048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ying H., et al. (2011). Pten is a major tumor suppressor in pancreatic ductal adenocarcinoma and regulates an NF-kappaB-cytokine network Cancer Discov. 1 158–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Witkos T.M., et al. (2011). Practical aspects of microRNA target prediction Curr. Mol. Med. 11 93–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Burk U., et al. (2008). A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells EMBO Rep. 9 582–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xiong M., et al. (2011). MiR-200 family regulates TGF-{beta}1-induced renal tubular epithelial to mesenchymal transition through Smad pathway by targeting ZEB1 and ZEB2 expression Am. J. Physiol. Renal. Physiol. 302, F369–F379 [DOI] [PubMed] [Google Scholar]
- 43. Chang C.J., et al. (2011). p53 regulates epithelial-mesenchymal transition and stem cell properties through modulating miRNAs Nat. Cell Biol. 13 317–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Itoh Y., et al. (2011). Dimerization of MT1-MMP during cellular invasion detected by fluorescence resonance energy transfer Biochem. J. 440 319–326 [DOI] [PubMed] [Google Scholar]
- 45. Ueda J., et al. (2003). Sequence-specific silencing of MT1-MMP expression suppresses tumor cell migration and invasion: importance of MT1-MMP as a therapeutic target for invasive tumors Oncogene 22 8716–8722 [DOI] [PubMed] [Google Scholar]