Abstract
Studies of body weight regulation have focused almost entirely on caloric intake and energy expenditure. However, a number of recent studies in animals linking energy regulation and the circadian clock at the molecular, physiological and behavioral levels raise the possibility that the timing of food intake itself may play a significant role in weight gain. The present study focused on the role of the circadian phase of food consumption in weight gain. We provide evidence that nocturnal mice fed a high fat diet only during the 12 hour light phase gain significantly more weight than mice fed only during the 12 hour dark phase. A better understanding of the role of the circadian system for weight gain could have important implications for developing new therapeutic strategies for combating the obesity epidemic facing the human population today.
Introduction
The reduction or prevention of obesity is expected to alleviate a number of debilitating health problems including diabetes, cardiovascular disease, stroke, infertility, depression and Alzheimer's disease (1–3). Attempts to understand the causes of obesity and develop new therapeutic strategies have mostly focused on caloric intake and energy expenditure. A number of recent studies in animals have linked energy regulation and the circadian clock at the molecular, physiological and behavioral levels, findings that raise the possibility that the timing of food intake itself may play a significant role in weight gain. For example, it has been shown that genetic mutation of the Clock gene causes metabolic syndrome in mice (4) and that a high fat diet can directly affect circadian behavior and circadian patterns of metabolic gene expression (5). Additionally, human studies on non-breakfast eaters and Night-Eating Syndrome patients are also consistent with the timing of food intake being a determining factor in weight gain (6, 7). Therefore, the present study sought to determine whether the circadian phase of feeding could directly affect body weight. Body weights, food intake, and locomotor activity of mice fed a high fat diet either during the 12 hour circadian light phase or 12 hour circadian dark phase were measured for 6 weeks. Here we report that mice fed a high fat diet only during the 12 hour light phase gained significantly more weight than mice fed the same diet only during the 12 hour dark phase.
Methods and Procedures
Male C57BL/6J mice were maintained on a 12 light:12 dark light cycle since birth and were fed a regular diet (Lab Diet, #5K52, Richmond, IN) containing 6% Kcal from fat after weaning at three weeks of age. At nine weeks of age, the mice were divided into two groups (N=6 per group) and fed a high fat diet (60% Kcal from fat, Research Diets, #D12492, New Brunswick, NJ) either only during the 12 hour light phase or the 12 hour dark phase, for 6 weeks. During the remaining 12 hours per day, animals were not provided any food. Diet conditions were maintained by manually switching the mice between two habituated cages (a light cage and a dark cage) only one of which contained food at lights on and lights off. Water was available ad libitum. Caloric intake was determined by providing pre-weighed food in access to the feeding cage and weighing leftover food after one week. Body weights were measured twice a week during both cage switch times. Locomotor activity as measured by infrared beam breaks as previously described (5).
Results
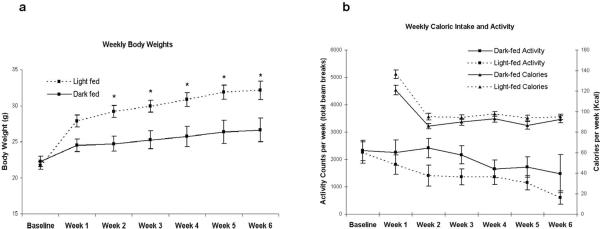
Mice provided the high fat diet only during the light phase gained significantly more weight than mice fed only during the dark phase over the 6 week period (Figure 1a, F(1,20)=10.78, p<0.004). Both groups consumed equivalent amount of calories (Figure 1b, F(1,10)=2.56, p>0.10) and exhibited similar levels of locomotor activity over the 6 week period (Figure 1b, F(1,10)=1.95, p>0.10). Additionally, analysis of body composition showed a tendency for the light-fed group to have higher fat percentage (average 7.8% more, p = 0.06) than dark-fed mice.
Figure 1.
(a) The Effect of Light or Dark Phase Feeding on Body Weight. Body weight (mean ± standard error) of B6 mice fed 60% high fat only during the 12 hour light phase (dashed line) or only during the 12 hour dark phase (solid line). Body weights were taken at the end of the 12 hour feeding phase in all animals. Similar significant differences were also observed when weights were taken at the end of the 12 hour fasting phase (data not shown). Within two weeks of maintenance on the high-fat diet, the light-fed animals weighed significantly more than the dark-fed animals (* = p < 0.05 light vs. dark-fed) and remained significantly heavier over the next 4 weeks (F (1,20) = 10.78, p < 0.004). (b) Weekly Activity Counts and Caloric Intake. Total weekly activity counts (squares) and calories (Kcal, triangles) are depicted for both light fed (dashed line) and dark fed (solid line) groups. Note that while over the 6 week period neither activity nor caloric intake differed significantly (p>0.10), the light fed group appears to be consuming more calories and moving less than the dark fed group. This raised the possibility that the additive effect of a small increase in caloric intake and a small decrease in activity can together contribute to specific differences in body weight.
Discussion
Like other inbred strains of laboratory mice, C57BL/6J mice are nocturnal, being more active and consuming most of their calories (80%) during the dark phase when maintained on a light:dark cycle (5, 8). The present results show that simply modifying the time of feeding alone can greatly affect body weight. Mice fed a high fat diet only during the “right” feeding time (i.e., during the dark) weigh significantly less than mice fed only during the time when feeding is normally reduced (i.e., during the light). While statistically the caloric intake and locomotor activity are not significantly different between the light and dark fed conditions, the light-fed mice on average were consistently less active than the dark fed group overtime (Figure 1b), indicating a decrease in energy expenditure. Furthermore, the light-fed group also consumed slightly more than the dark fed group. The impact of these two effects may be additive, and combine to contribute to differences in body weights between the two groups.
These results are consistent with previous studies (5), supporting the hypothesis that light phase feeding may be a critical component of weight gain. While the mechanism behind light-fed weight gain in mice is unknown, body temperature, satiety hormones and sleep could be contributing to the phenotype. Light phase feeding has been noted to cause a decrease in dark phase body temperature (9) which could have contributed to the increased fat storage and weight gain observed in the present study. Circulating satiety hormones, such as leptin, may have a circadian variation independent of meal timing and be contributing to food intake levels as seen with humans on a circadian misaligned schedule (10). Sleep restriction or poor sleep quality could also be leading to weight gain (11), although our preliminary data (12) indicate no overall sleep differences between light and dark fed mice.
In the past few years there have been a number of genetic and environmental studies linking circadian clock and metabolic gene networks with one another. For example, a mutation of the circadian clock gene, Clock, can lead to obesity and the metabolic syndrome and alterations in the circadian expression of metabolic genes (4), while mice fed a high-fat diet show alterations in circadian behavior as well as the levels and timing of the expression of clock and metabolic genes in both central and peripheral tissues involved in energy regulation (5, 8). In humans, phase delayed eating patterns of non-breakfast eaters or patients with Night-Eating Syndrome, have suggested an association with increased body mass index (6, 7, 13). Additionally, studies of patients with circadian:behavior misalignment develop indictors of the metabolic syndrome, specifically an increase in circulating glucose and insulin levels (10). These findings, taken together with the present results indicate that the synchrony between circadian and metabolic processes plays an important role in the regulation of energy balance and body weight control. Importantly, this study is the first to show causal evidence that feeding at the “wrong” time can lead to weight gain.
The present results indicate that a better understanding of the role of the circadian system in weight regulation could have important implications for combating the obesity epidemic facing the human population today. Once gained, losing body weight by controlling energy intake through dieting and/or energy expenditure has proven to be difficult over a prolonged period of time, making significant and sustained weight loss challenging (14). A preventive strategy based on behavior modification (e.g. the timing of food intake),possibly without a significant change in caloric intake or physical activity, could be a critical element needed to slow the ever-increasing incidence of obesity and associated cardiometabolic disorders facing the world today.
Acknowledgements
This research was supported by NIH/NIA Grant P01 AG114212 and NIH/NHLBI Grant T32 HL007909.
Footnotes
Disclosure Statement: We have no conflicts of interest to declare.
References
- 1.Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 2.Fabricatore AN, Wadden TA. Obesity. Annu Rev Clin Psychol. 2006;2:357–377. doi: 10.1146/annurev.clinpsy.2.022305.095249. [DOI] [PubMed] [Google Scholar]
- 3.Pasinetti GM, Eberstein JA. Metabolic syndrome and the role of dietary lifestyles in Alzheimer's disease. J Neurochem. 2008;106:1503–1514. doi: 10.1111/j.1471-4159.2008.05454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turek FW, Joshu C, Kohsaka A, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kohsaka A, Laposky AD, Ramsey KM, et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6:414–421. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Ma Y, Bertone ER, Stanek EJ, 3rd, et al. Association between eating patterns and obesity in a free-living US adult population. Am J Epidemiol. 2003;158:85–92. doi: 10.1093/aje/kwg117. [DOI] [PubMed] [Google Scholar]
- 7.Colles SL, Dixon JB, O'Brien PE. Night eating syndrome and nocturnal snacking: association with obesity, binge eating and psychological distress. Int J Obes (Lond) 2007;31:1722–1730. doi: 10.1038/sj.ijo.0803664. [DOI] [PubMed] [Google Scholar]
- 8.Laposky AD, Bass J, Kohsaka A, Turek FW. Sleep and circadian rhythms: key components in the regulation of energy metabolism. FEBS Lett. 2008;582:142–151. doi: 10.1016/j.febslet.2007.06.079. [DOI] [PubMed] [Google Scholar]
- 9.Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol. 2009;5:253–261. doi: 10.1038/nrendo.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arble DM, Goldschmidt C, Vitaterna MH, Turek FW. Sleep during phase restricted feeding in C57BL/6 mice. Sleep. 2009;32:A61. [Google Scholar]
- 13.O'Reardon JP, Ringel BL, Dinges DF, et al. Circadian eating and sleeping patterns in the night eating syndrome. Obes Res. 2004;12:1789–1796. doi: 10.1038/oby.2004.222. [DOI] [PubMed] [Google Scholar]
- 14.Elfhag K, Rossner S. Who succeeds in maintaining weight loss? A conceptual review of factors associated with weight loss maintenance and weight regain. Obes Rev. 2005;6:67–85. doi: 10.1111/j.1467-789X.2005.00170.x. [DOI] [PubMed] [Google Scholar]