Abstract
GB virus C (GBV-C) is a member of the Flaviviridae family and the most closely related human virus to HCV. However, GBV-C does not replicate in hepatocytes, but rather in lymphocytes. GBV-C has a worldwide distribution and is transmitted sexually, parenterally and through mother-to-child transmission. Thus, co-infection with HCV and HIV is common. Until now, no human disease has been associated with GBV-C infection. However, there are several reports of a beneficial effect of GBV-C on HIV disease progression in vivo. Different mechanisms to explain these observations have been proposed, including modification of antiviral cytokine production, HIV co-receptor expression, direct inhibition of HIV-1 entry, T-cell activation and Fas-mediated apoptosis. Further understanding of these mechanisms may open new strategies for the treatment of HIV/AIDS.
Keywords: co-infection, GBV-C, HCV, HIV
In 1995, Abbott Laboratories reported experimental infection of tamarins with two transmissible agents – GB virus A (GBV-A) and GB virus B (GBV-B) – initially obtained from the serum of a surgeon (initials GB) with non-A, non-B hepatitis [1]. Using degenerate primers and human sera containing antibodies recognizing GBV-A and/or GBV-B recombinant proteins a novel virus was identified, tentatively named GBV-C [2]. Another independent research group discovered novel viral sequences extracted from the serum of a patient with cryptogenic hepatitis naming the virus hepatitis G (HGV) [3]. The genomes of GBV-C and HGV were found to share more than 95% homology, representing two variants of the same virus [4]. GBV-C/HGV was initially considered a possible cause of hepatitis [5]. However, no association between GBV-C/HGV and chronic hepatitis or any other human disease has been confirmed subsequently (reviewed in [6]). Therefore, in this review the virus will be referred to as GBV-C.
GBV-C: transmission, epidemiology & detection
The modes of transmission of GBV-C are through sexual contact [7–9], parenterally after exposure to blood or blood products [3,10,11] and less frequently vertically from mother-to-child [12,13]. GBV-C has a worldwide distribution and is commonly found in the general population [14]. Up to 4% of healthy blood donors in Western countries are GBV-C viremic [15–17], while in developing countries the prevalence of GBV-C approaches 5–18% in the general population [18–22]. Among populations at high risk for other parenterally transmitted viruses, such as HBV, HCV or HIV, the GBV-C prevalence approaches 50% [15,23–25]. The temporal relationship between GBV-C persistence and clearance of GBV-C RNA in the blood is unknown. Generally, immunocompetent individuals eliminate GBV-C within the first years following infection [26–28]. However, in some individuals, GBV-C replication may persist for decades [23]. Clearance of GBV-C is commonly associated with the development of antibodies against the E2 region [29–31]. GBV-C E2 antibodies are two- to six-times more prevalent than GBV-C RNA and protect against re-infection with GBV-C to some extent [26,31] (reviewed in [32]). However, there are cases in which GBV-C clearance does not result in the development of E2 antibodies [33–35]. In immunocompetent individuals, GBV-C RNA and E2 antibodies are rarely detected in the blood simultaneously, and when this does occur, it is usually a transient condition [24].
GBV-C replication is detected by nucleic acid amplification [15], and quantified by terminal dilution methods [36], branched chain DNA assays [37], real-time PCR [38] or competitive real-time PCR [39]. Unfortunately, no commercial assay for the detection of GBV-C E2 antibodies is currently available. Future development of a GBV-C E2 antibody assay is very much needed in order to provide better insight into GBV-C infection status and thereby enabling better analysis of the impact of GBV-C infection on other viral diseases [40,41].
GBV-C: virology
GBV-C shares approximately 30% amino acid homology with HCV [5,32]. GBV-C consists of a positive-sense, ssRNA genome of approximately 9400 nucleotides. The 5′ untranslated region contains an internal ribosomal entry site and is followed by a single long open reading frame encoding for a multifunctional polyprotein of approximately 3000 amino acids (reviewed in [42]). This polyprotein is co- and post-translationally cleaved by viral and cellular proteases resulting in several structural and nonstructural viral proteins [3,5,43,44]. The structural proteins of GBV-C include two envelope glycoproteins (E1 and E2), while the nonstructural proteins include NS2 (a protease), NS3 (a RNA helicase and trypsin-like serine protease), NS4, NS5A and NS5B (a RNA-dependant RNA polymerase) [5]. However, it should be noted that nearly all of these data are inferred by sequence alignment and comparison with the genomic structure of HCV; therefore, divergent functions are plausible.
The exact site(s) of GBV-C replication has not been established firmly. GBV-C RNA replication has been described in the liver and localized in hepatocytes [45,46]. In vitro replication of GBV-C in human hepatoma cell lines suggested hepatotropism [47,48]. By contrast, a study by Laskus et al. does not confirm these findings [49]. Moreover, GBV-C RNA has been detected in peripheral blood mononuclear cells (PBMCs), spleen and bone marrow suggesting lymphotropism [50–52]. More recently, GBV-C has been shown to replicate in primary T and B lymphocytes and in PBMCs in vitro [36,53]. Thus, GBV-C tropism is still a matter of debate, but appears to be primarily lymphotropic [54,55].
GBV-C: genotype diversity
On the basis of the predicted genomic structure and relatedness to other viruses, the GB viruses have been classified as members of the Flaviviridae family. As GBV-B induced hepatitis in experimentally infected tamarins it has been classified with the various hepatitis C genotypes within the Hepacivirus genus [56], while a more recent report suggests that GBV-A, GBV-C and the distantly related GBV-D in bats should be classified within the newly proposed Pegivirus genus [57].
Phylogenetic analysis of the 5′ untranslated region and the E2 gene suggests the existence of at least six distinct genotypes of GBV-C [58]. GBV-C genotypes show a worldwide geographical clustering, suggesting an evolutionary history paralleling prehistoric human migration [59,60]. Genotype 1 is common in Africa and North America, genotype 2 in North/South America and Europe, genotype 3 in Asia and South America, genotype 4 in southeast Asia, genotype 5 in South Africa and a sixth genotype was more recently found in Indonesia [58,61–67]. Within a given genotype, additional diversity exists. For example, intragenotype genetic distances may range from 13 to 19% and multiple subtypes within a genotype have been reported [68–70]. Moreover, mixed infections and recombinant viruses have been detected [71–73]. Within an individual, distinct variants of GBV-C have also been identified implying that viral adaptation may occur within individuals as well [74–78]. Interestingly, interferon sensitivity and cell tropism may differ among such variants [78–80]. Similarly, clinical isolates of GBV-C also vary in their ability to replicate in culture [80,81], suggesting that genotypic diversity may impact virologic phenotype. While the biological consequences of recombination and intrapatient diversity to GBV-C pathogenesis have not been examined, studies of HIV suggest that viral diversity may result in altered cell tropism, virulence and/or drug susceptibility [82].
GBV-C: impact of co-infection on HIV disease
In 1998, two reports raised interest on GBV-C in HIV disease. Toyoda et al. first reported that GBV-C co-infection had no adverse effect on the clinical course of HIV in a cohort of 41 HIV-positive Japanese patients with hemophilia [83]. GBV-C viremia was detected in 11 (27%) out of the 41 patients. Interestingly, patients co-infected with GBV-C showed lower mean HIV RNA levels. Moreover, there was a nonsignificant trend towards slower progression to AIDS and improved survival. In the same year, Heringlake et al. studied 197 HIV-positive German patients, of which 33 (17%) were GBV-C viremic [84]. They showed that higher baseline CD4 cell counts, slower progression to AIDS and improved survival were associated with GBV-C co-infection. These observations attracted much interest and were supported further by subsequent studies [85–87]. Similarly, in HIV patients receiving highly active antiretroviral therapy (HAART), Tillmann et al. reported a significant survival benefit of GBV-C infection on progression to AIDS [88]. An inverse relationship between GBV-C and HIV viral load was reported, suggesting an inhibition of HIV replication by GBV-C. In patients on HAART, Bjoerkmann et al. found that median GBV-C RNA levels increased, whereas GBV-C RNA levels decreased upon interruption of HAART and subsequent resumption of HIV replication, suggesting a reciprocal correlation between GBV-C and HIV viral dynamics [89]. Other studies demonstrated an improved initial response to HAART [90,91], a reduced risk of HIV viral rebound after initiating HAART [92] and a better quality of life in persons co-infected with GBV-C (Box 1) [93]. However, in the past several years, there has also been some controversy regarding the effects of GBV-C on the course of HIV infection, as some reports have failed to confirm a positive impact of GBV-C co-infection on HIV disease. Birk et al. studied 157 HIV-positive patients early after HIV seroconversion [94]. Among 36 (23%) patients co-infected with GBV-C, there was no impact of GBV-C co-infection on immunological and clinical outcomes of HIV-1 infection. Another study followed 230 HIV patients and detected GBV-C viremia in 62 patients (27%) [95]. GBV-C co-infection did not predict HIV outcome and no association of GBV-C viremia with the development of AIDS, HIV-related mortality or overall mortality was seen. Both studies included patients before effective antiretroviral treatment was available, possibly explaining conflicting results.
Box 1. Impact of GB virus C co-infection on HIV.
Lower HIV-1 RNA levels
Higher CD4 cell counts
Slower progression to AIDS
Improved response to HAART
Better quality of life
Reduction of mortality
HAART: Highly active antiretroviral therapy.
Several reports have explored the impact of GBV-C genotype on HIV disease progression. For example, Muerhoff et al. reported that CD4 cell counts were lower in HIV co-infected patients infected with GBV-C 2a than in patients with subtype 2b; however, other genotypes were not circulating at a sufficiently high prevalence for comparison among all GBV-C genotypes [96]. Subsequently, during HIV/HCV/GBV-C triple infection, GBV-C genotype 2 was associated with higher CD4 cell counts compared with GBV-C genotype 1 [97]. While similar findings have also been reported in a Brazilian cohort [67], a comparison of GBV-C genotype 2 versus non-2 infections in an Australian cohort observed no such difference in CD4 cell counts [98]. Thus, it is possible that GBV-C genotype could differentially impact HIV disease progression; however, further investigation in larger cohorts with multiple circulating GBV-C genotypes is warranted.
Differential effects of GBV-C co-infection in early versus advanced HIV infection may further explain the discrepancies reported regarding the association of GBV-C co-infection and HIV patient survival. In 2001, Xiang et al. reported that the beneficial effect of GBV-C co-infection was most pronounced among HIV patients with advanced immunodeficiency and CD4 T-cell counts <200/l [87]. This was further supported in the Multicentre AIDS Cohort Study in which no statistically significant effect of GBV-C co-infection on patient survival was observed early (12–18 months) after HIV seroconversion, whereas persistent GBV-C viremia over 5–6 years after seroconversion was associated with a significant survival benefit [33]. Likewise, in the Amsterdam Cohort Study, Van der Bij et al. observed that persistent GBV-C viremia was associated with prolonged survival [34]. In a meta-analysis evaluating the effect of GBV-C co-infection in late HIV disease, the authors found a significant reduction of mortality in HIV patients with GBV-C viremia [99]. Thus, differential effects of GBV-C co-infection in early versus late HIV disease may also account for the contradictory results regarding the association of GBV-C co-infection and patient survival.
Impact of GBV-C on HIV/HCV co-infection
Past or current GBV infection does not impact HCV replication, liver disease progression or response to interferon treatment in persons with HCV mono-infection [100–104]. However, a limited number of studies have reported a deleterious [105] or beneficial [106,107] impact of GBV-C on liver disease in the context of HCV mono-infection. While GBV-C has no reported impact on the outcome of HCV mono-infection, HIV clearly has a significant negative impact on HCV disease progression. For example, HCV RNA levels are significantly elevated during HIV/HCV co-infection compared with HCV mono-infection [108,109]. Moreover, HCV-related liver fibrosis, cirrhosis and end-stage liver disease are accelerated during HIV/HCV co-infection [109–118]. This is particularly relevant as HCV infection has emerged as an important cause of morbidity and mortality in HIV-infected individuals [114,119,120]. Similar to HCV, GBV-C is also sensitive to the antiviral effects of interferon [100,101,121,122]. Thus, it is important to consider how the sum effects of HIV/HCV/GBV-C triple infection may impact HIV disease progression, as well as liver-related disease. Particularly in persons receiving IFN treatment, clearance of GBV-C RNA could have a negative impact on HIV-related outcomes. In a recent study evaluating the efficacy of IFN-α-2a plus ribavirin (RBV) versus pegylated IFN-α-2a plus RBV for chronic HCV infection in individuals co-infected with HIV [97], GBV-C RNA was cleared in 50% of HIV/HCV/GBV-C triple infected patients treated with IFN (or pegylated IFN) plus RBV [97]. Interestingly, GBV-C clearance was not associated with a short-term loss of HIV control. Similar results have been reported elsewhere [123], although studies with longer follow-up periods will be necessary to determine whether IFN treatment-induced clearance of GBV-C has deleterious effects on long-term HIV disease progression.
GBV-C: possible molecular interactions with HIV
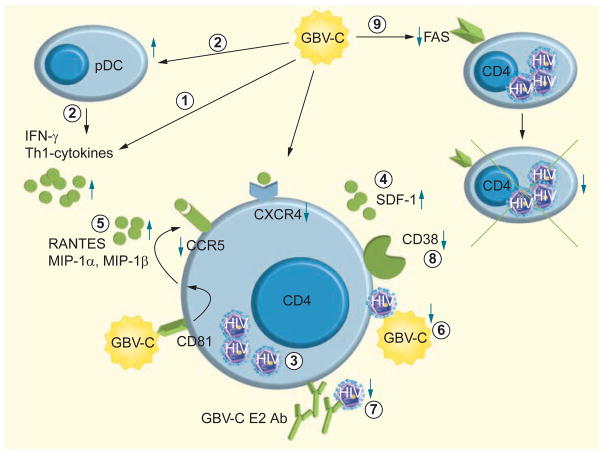
The exact mechanisms by which GBV-C inhibits HIV replication remain obscure, although recent research has identified several possible pathways (reviewed in [124]). The currently postulated mechanisms by which GBV-C may lead to a beneficial effect on the course of HIV infection are summarized in Figure 1.
Figure 1. Proposed molecular interactions of GB virus C with HIV.
(1) GBV-C alters the cytokine profile during HIV -infection, thereby stabilizing Th1 cytokine expression [127,128]. (2) A major source of Th1 cytokines are circulating CD80+ pDCs, which are increased in GBV-C co-infection [129]. (3) HIV replication is also inhibited by GBV-C proteins [87,132]. (4) GBV-C NS5A phosphoprotein induces SDF-1 release, thereby decreasing CXCR4, an important co-receptor of HIV [133–135]. (5) Furthermore, secretion of RANTES, MIP-1α and MIP-1β – natural ligands of the other HIV co-receptor CCR5 – is elevated during GBV-C co-infection leading to lower surface expression of CCR5 [131,137]. (6) Direct inhibition of HIV entry by GBV-C E2 protein has been proposed and interaction of GBV-C E2 with the HIV-1 fusion protein has been shown [141–144]. (7) Furthermore, GBV-C E2 Abs have been demonstrated to neutralize HIV-1 infection by inhibition of viral attachment [145]. (8) GBV-C alters T-cell activation leading to a lower percentage of T lymphocytes expressing CD38 [147]. (9) Finally, GBV-C co-infection leads to lower Fas expression on T and B lymphocytes, thereby reducing Fas-mediated apoptosis [151].
Ab: Antibody; CCR: Chemokine receptor; GBV-C: GB virus C; pDC: Plasmocytoid dendritic cell; RANTES: Regulated on activation, normal T-cell expressed and secreted; SDF-1: Stroma- derived factor-1.
Altering the cytokine profile
Numerous studies have underlined the importance of Th1 and Th2 helper T cytokine response in HIV infection, demonstrating that progression of HIV disease is associated with a shift from Th1 to Th2 cytokine profile [125], which can be reversed after initiating HAART [126]. Nunnari et al. showed that GBV-C co-infection alters the cytokine profile in HIV infection [127]. GBV-C viremia in HIV patients resulted in more stable Th1 cytokine serum levels (IL-2 and -12) compared with HIV patients without GBV-C infection in whom Th1 cytokine levels decreased and shifted towards Th2 cytokines (IL-4 and -10) over time. Further more, endogenous levels of IFN-γ were found to be higher in PBMCs from GBV-C/HIV co-infected subjects compared with HIV mono-infected patients [128]. GBV-C also induced expression of IFN-γ and downstream IFN response genes in PBMCs [129]. GBV-C infection results in increased cell number and activation levels of circulating CD80+ plasmocytoid dendritic cells (pDCs) in HIV patients, which are a major source of IFN-γ and other Th1 cytokines. It has been shown that pDCs are important in controlling HIV replication and high levels of HIV viral load are associated with pDC cell death via apoptosis and necrosis [130]. By preserving and boosting the innate antiviral response to infection with HIV, GBV-C may stabilize the antiviral response to HIV.
Modifying the expression of HIV co-receptors
HIV-1 entry into target cells depends, in addition to CD4, on its interaction with a secondary receptor, usually the chemokine receptors CCR5 or CXCR4. The β-chemokines MIP-1α and MIP-1β and RANTES (regulated on activation, normal T-cell expressed and secreted) are the natural ligands of CCR5, whereas SDF-1 and -2 are the natural ligands for CXCR4. In cultures of PBMCs, co-infection with HIV and GBV-C resulted in inhibition of HIV replication, as measured by the detection of p24 antigen in culture supernatants [80,87,131,132]. In further studies, expression of the NS5A phosphoprotein in a CD4+ Jurkat T-cell line led to a dose-dependent inhibition of HIV replication [133]. NS5A resulted in the release of SDF-1, thereby decreasing surface expression of CXCR4, partially explaining the observed inhibition of HIV replication. The NS5A protein from GBV-C genotypes 1, 2, 3 and 5 inhibited HIV replication [134]. This effect was related to NS5A amino acids 152–167 [135]. Interestingly, not only the NS5 protein from GBV-C but also from other members of the Flaviviridae family such as HCV, West Nile virus and yellow fever virus show inhibition of HIV replication [136].
Furthermore, GBV-C infection of PBMCs resulted in elevation of mRNA expression, not only for SDF-1, but also for RANTES, MIP-1α and MIP-1β, and higher secretion into culture supernatants, leading to lower surface expression of CCR5 [131]. Antibodies directed against these chemokines neutralized the inhibitory effect of GBV-C on HIV [131]. GBV-C E2 protein was shown to bind to CD81 (a member of the tetraspanin family) on the cell surface of CD4+ T lymphocytes in vitro [137]. This induced a dose-dependent release of RANTES and down-regulation of CCR5 surface expression, which was also found in GBV-C/HIV co-infected patients. However, direct interaction of GBV-C E2 with CD81 could not be confirmed by others [138]. Interestingly, in GBV-C/HIV co-infected patients in advanced stages of immunodeficiency, reduced expression of both HIV co-receptors, CCR5 and CXCR4, was detected [139]. Thus, modulation of chemokine receptor expression on CD4+ T lymphocytes likely represents one mechanism by which GBV-C co-infection influences HIV disease progression.
Directly inhibiting HIV-1 entry
Several groups have proposed that the GBV-C E2 protein modifies HIV disease progression [42]. GBV-C E2 protein inhibits HIV entry when added to CD4+ cells [140]. It has been suggested that GBV-C E2 protein directly targets HIV-1 particles and blocks entry of virions [141]. Synthetic peptides of the GBV-C E2 domain have been shown to interact with the HIV-1 fusion protein and to modify its conformation [142]. This indicates a possible alteration of the interaction of HIV-1 fusion protein with the cell membrane decreasing cellular membrane fusion in a dose-dependent manner [143,144]. Furthermore, Mohr et al. have found that naturally occurring GBV-C E2 antibodies from HIV-negative individuals and experimentally induced GBV-C E2 antibodies neutralize HIV-1 infection in vitro by inhibit of HIV attachment, but do not inhibiting HIV entry following attachment [145]. Therefore, further investigations of the exact mechanism of the GBV-C E2/HIV interaction may not only lead to better understanding of the beneficial effect of GBV-C on HIV disease, but also possibly open new therapeutic strategies for HIV-1 treatment.
Modulating T-cell activation
Chronic immune activation is a characteristic of progressive HIV disease. Thus, cellular immune responses are critical in the control of viral replication [146]. Recently, a lower percentage of T lymphocytes expressing CD38+CD4+, CD38+CD8+ and CCR5+CD8+ were observed in GBV-C viremic HIV patients [147]. This effect of GBV-C viremia was independent of HIV-1 viral load or CD4/CD8 cellular status. Thus, reduced activation of CD4+ and CD8+ T cells in GBV-C/HIV co-infected patients may be a potential mechanism of ameliorating HIV disease.
Influencing Fas-mediated apoptosis
It is postulated that enhancement of Fas (CD95/Apo-1) expression on virus-infected cells is important in controlling infection through elimination of infected cells by apoptosis. In HIV infected individuals, the percentage of Fas expressing T and B lymphocytes is higher compared with healthy controls [148]. Fas expression on CD4+ and CD8+ T-cells rises with progression of HIV disease and augments Fas-mediated cell death [149]. In HIV infected patients on HAART, spontaneous and Fas-mediated apoptosis of PBMCs is reduced [150]. Moenkemeyer et al. found that GBV-C co-infection in HIV patients not receiving HAART was associated with a lower percentage of Fas expressing cells compared with HIV mono-infected individuals [151]. Furthermore, this effect correlated directly with Fas-mediated apoptosis after priming cells with an anti-Fas monoclonal antibody in vitro. Lower expression of Fas on the cell surface and reduced Fas-mediated apoptosis may contribute to the beneficial effect of GBV-C co-infection on HIV disease progression.
Conclusion
GBV-C, a member of the Flaviviridae family, is distributed world-wide. GBV-C shares common routes of transmission with HCV and HIV, resulting in co- and triple-infection in humans. GBV-C does not cause any human disease on its own. Nonetheless, a beneficial effect of GBV-C co-infection on HIV disease progression has raised considerable interest in this virus. Several mechanisms of interaction between GBV-C and HIV have been proposed, for example, influencing antiviral cytokine production, HIV co-receptor expression, direct inhibition of HIV-1 entry, T-cell activation and Fas-mediated apoptosis. These mechanisms warrant further study to hopefully open new ways of treatment of HIV infection in the future.
Expert commentary & five-year view
In the past years, GBV-C has shifted from the research field of viral hepatitis to HIV, as GBV-C was found not to be a pathogenic agent of hepatitis but rather a beneficial modulator of HIV infection and disease progression. Recent studies focus on the identification of mechanisms of interaction between HIV, GBV-C and the immune system.
As there are several proposed ways of interaction, different clinical applications of GBV-C in treatment of HIV infection can be envisioned.
Characterization of the immunogenic domains of the GBV-C E2 protein responsible for inducing E2 antibodies and/or inhibiting HIV-1 entry may facilitate development of novel HIV-1 vaccines for the treatment and prevention of HIV disease.
Furthermore, GBV-C E2-derived peptides have been demonstrated to interact with HIV-1 cell fusion by directly inhibiting HIV-1. Thus, synthetic GBV-C peptides may open the way to the design of new therapeutic drugs for HIV.
Finally, deep understanding of the GBV-C-induced modulation of the immune system may provide further access to new therapeutic strategies of HIV infection.
Key issues.
GB virus C (GBV-C) is a member of the Flaviviridae family and closely related to HCV.
However, GBV-C replicates in lymphocytes rather than in hepatocytes.
No human disease has been associated with GBV-C infection.
Epidemiological studies have described an association between GBV-C co-infection and decreased morbidity and mortality in HIV-infected individuals.
Several mechanisms of interaction between GBV-C and HIV have been proposed, including altered cytokine profile, HIV co-receptor expression, T-cell activation, Fas-mediated apoptosis and direct inhibition of HIV replication.
Further understanding of the GBV-C/HIV interactions may lead to new therapeutic drugs and novel treatment strategies in HIV.
Footnotes
For reprint orders, please contact reprints@expert-reviews.com
Financial & competing interests disclosure
This work was supported by the HW and J Hector foundation and in part by NIH award R21 AI081564 to JT Blackard. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
- 1.Simons JN, Pilot-Matias TJ, Leary TP, et al. Identification of two flavivirus-like genomes in the GB hepatitis agent. Proc Natl Acad Sci USA. 1995;92(8):3401–3405. doi: 10.1073/pnas.92.8.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2•.Simons JN, Leary TP, Dawson GJ, et al. Isolation of novel virus-like sequences associated with human hepatitis. Nat Med. 1995;1(6):564–569. doi: 10.1038/nm0695-564. First identification of GB virus. [DOI] [PubMed] [Google Scholar]
- 3•.Linnen J, Wages J, Jr, Zhang-Keck ZY, et al. Molecular cloning and disease association of hepatitis G virus: a transfusion-transmissible agent. Science. 1996;271(5248):505–508. doi: 10.1126/science.271.5248.505. Second independent article identifying the GB virus. [DOI] [PubMed] [Google Scholar]
- 4.Alter HJ. The cloning and clinical implications of HGV and HGBV-C. N Engl J Med. 1996;334(23):1536–1537. doi: 10.1056/NEJM199606063342310. [DOI] [PubMed] [Google Scholar]
- 5.Leary TP, Muerhoff AS, Simons JN, et al. Sequence and genomic organization of GBV-C: a novel member of the flaviviridae associated with human non-A–E hepatitis. J Med Virol. 1996;48(1):60–67. doi: 10.1002/(SICI)1096-9071(199601)48:1<60::AID-JMV10>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 6•.Baggio-Zappia GL, Hernandes Granato CF. HIV-GB virus C co-infection: an overview. Clin Chem Lab Med. 2009;47(1):12–19. doi: 10.1515/CCLM.2009.001. Recent review on GB virus C (GBV-C) and its clinical role in HIV co-infection. [DOI] [PubMed] [Google Scholar]
- 7.Nerurkar VR, Chua PK, Hoffmann PR, Dashwood WM, Shikuma CM, Yanagihara R. High prevalence of GB virus C/hepatitis G virus infection among homosexual men infected with human immunodeficiency virus type 1: evidence for sexual transmission. J Med Virol. 1998;56(2):123–127. [PubMed] [Google Scholar]
- 8.Scallan MF, Clutterbuck D, Jarvis LM, Scott GR, Simmonds P. Sexual transmission of GB virus C/hepatitis G virus. J Med Virol. 1998;55(3):203–208. doi: 10.1002/(sici)1096-9071(199807)55:3<203::aid-jmv4>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 9.Bourlet T, Guglielminotti C, Evrard M, et al. Prevalence of GBV-C/hepatitis G virus RNA and E2 antibody among subjects infected with human immunodeficiency virus type 1 after parenteral or sexual exposure. J Med Virol. 1999;58(4):373–377. [PubMed] [Google Scholar]
- 10.Woelfle J, Berg T, Keller KM, Schreier E, Lentze MJ. Persistent hepatitis G virus infection after neonatal transfusion. J Pediatr Gastroenterol Nutr. 1998;26(4):402–407. doi: 10.1097/00005176-199804000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Jarvis LM, Davidson F, Hanley JP, Yap PL, Ludlam CA, Simmonds P. Infection with hepatitis G virus among recipients of plasma products. Lancet. 1996;348(9038):1352–1355. doi: 10.1016/s0140-6736(96)04041-x. [DOI] [PubMed] [Google Scholar]
- 12.Fischler B, Lara C, Chen M, Sönnerborg A, Nemeth A, Sällberg M. Genetic evidence for mother-to-infant transmission of hepatitis G virus. J Infect Dis. 1997;176(1):281–285. doi: 10.1086/517267. [DOI] [PubMed] [Google Scholar]
- 13.Zanetti AR, Tanzi E, Romanó L, et al. Multicenter trial on mother-to-infant transmission of GBV-C virus. The Lombardy Study Group on Vertical/ Perinatal Hepatitis Viruses Transmission. J Med Virol. 1998;54(2):107–112. doi: 10.1002/(sici)1096-9071(199802)54:2<107::aid-jmv7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 14.Tucker TJ, Smuts HE. Review of the epidemiology, molecular characterization and tropism of the hepatitis G virus/ GBV-C. Clin Lab. 2001;47(5–6):239–248. [PubMed] [Google Scholar]
- 15.Dawson GJ, Schlauder GG, Pilot-Matias TJ, et al. Prevalence studies of GB virus-C infection using reverse transcriptase-polymerase chain reaction. J Med Virol. 1996;50(1):97–103. doi: 10.1002/(SICI)1096-9071(199609)50:1<97::AID-JMV16>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 16.Hyland CA, Mison L, Solomon N, et al. Exposure to GB virus type C or hepatitis G virus in selected Australian adult and children populations. Transfusion. 1998;38(9):821–827. doi: 10.1046/j.1537-2995.1998.38998409001.x. [DOI] [PubMed] [Google Scholar]
- 17.Blair CS, Davidson F, Lycett C, et al. Prevalence, incidence, and clinical characteristics of hepatitis G virus/GB virus C infection in Scottish blood donors. J Infect Dis. 1998;178(6):1779–1782. doi: 10.1086/314508. [DOI] [PubMed] [Google Scholar]
- 18.El-Zayadi AR, Abe K, Selim O, Naito H, Hess G, Ahdy A. Prevalence of GBV-C/ hepatitis G virus viraemia among blood donors, health care personnel, chronic non-B non-C hepatitis, chronic hepatitis C and hemodialysis patients in Egypt. J Virol Methods. 1999;80(1):53–58. doi: 10.1016/s0166-0934(99)00036-1. [DOI] [PubMed] [Google Scholar]
- 19.Tucker TJ, Louw SJ, Robson SC, Isaacs S, Kirsch RE. High prevalence of GBV-C hepatitis G virus infection in a rural South African population. J Med Virol. 1997;53(3):225–228. [PubMed] [Google Scholar]
- 20.Ren FR, Wang Y, Li H, Chen HS, Zhao HY. Hepatitis G virus infection in screened Chinese blood donors. Vox Sang. 1998;74(1):51–52. [PubMed] [Google Scholar]
- 21.Tanaka Y, Mizokami M, Orito E, et al. GB virus C/hepatitis G virus infection among Colombian native Indians. Am J Trop Med Hyg. 1998;59(3):462–467. doi: 10.4269/ajtmh.1998.59.462. [DOI] [PubMed] [Google Scholar]
- 22.Bassit L, Kleter B, Ribeiro-dos-Santos G, et al. Hepatitis G virus: prevalence and sequence analysis in blood donors of São Paulo, Brazil. Vox Sang. 1998;74(2):83–87. doi: 10.1159/000030910. [DOI] [PubMed] [Google Scholar]
- 23.Masuko K, Mitsui T, Iwano K, et al. Infection with hepatitis GB virus C in patients on maintenance hemodialysis. N Engl J Med. 1996;334(23):1485–1490. doi: 10.1056/NEJM199606063342301. [DOI] [PubMed] [Google Scholar]
- 24.Thomas DL, Nakatsuji Y, Shih JW, et al. Persistence and clinical significance of hepatitis G virus infections in injecting drug users. J Infect Dis. 1997;176(3):586–592. doi: 10.1086/514078. [DOI] [PubMed] [Google Scholar]
- 25.Stark K, Bienzle U, Hess G, Engel AM, Hegenscheid B, Schluter V. Detection of the hepatitis G virus genome among injecting drug users, homosexual and bisexual men, and blood donors. J Infect Dis. 1996;174(6):1320–1323. doi: 10.1093/infdis/174.6.1320. [DOI] [PubMed] [Google Scholar]
- 26.Thomas DL, Vlahov D, Alter HJ, et al. Association of antibody to GB virus C (hepatitis G virus) with viral clearance and protection from reinfection. J Infect Dis. 1998;177(3):539–542. doi: 10.1086/514245. [DOI] [PubMed] [Google Scholar]
- 27.Tillmann HL, Heringlake S, Trautwein C, et al. Antibodies against the GB virus C envelope 2 protein before liver transplantation protect against GB virus C de novo infection. Hepatology. 1998;28(2):379–384. doi: 10.1002/hep.510280213. [DOI] [PubMed] [Google Scholar]
- 28.Kleinman S. Hepatitis G virus biology, epidemiology, and clinical manifestations: Implications for blood safety. Transfus Med Rev. 2001;15(3):201–212. doi: 10.1053/tmrv.2001.24589. [DOI] [PubMed] [Google Scholar]
- 29.Pilot-Matias TJ, Carrick RJ, Coleman PF, et al. Expression of the GB virus C E2 glycoprotein using the Semliki Forest virus vector system and its utility as a serologic marker. Virology. 1996;225(2):282–292. doi: 10.1006/viro.1996.0602. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka E, Tacke M, Kobayashi M, et al. Past and present hepatitis G virus infections in areas where hepatitis C is highly endemic and those where it is not endemic. J Clin Microbiol. 1998;36(1):110–114. doi: 10.1128/jcm.36.1.110-114.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tacke M, Schmolke S, Schlueter V, et al. Humoral immune response to the E2 protein of hepatitis G virus is associated with long-term recovery from infection and reveals a high frequency of hepatitis G virus exposure among healthy blood donors. Hepatology. 1997;26(6):1626–1633. doi: 10.1002/hep.510260635. [DOI] [PubMed] [Google Scholar]
- 32.Stapleton JT. GB virus type C/Hepatitis G virus. Semin Liver Dis. 2003;23(2):137–148. doi: 10.1055/s-2003-39943. [DOI] [PubMed] [Google Scholar]
- 33.Williams CF, Klinzman D, Yamashita TE, et al. Persistent GB virus C infection and survival in HIV-infected men. N Engl J Med. 2004;350(10):981–990. doi: 10.1056/NEJMoa030107. [DOI] [PubMed] [Google Scholar]
- 34.Van der Bij AK, Kloosterboer N, Prins M, et al. GB virus C co-infection and HIV-1 disease progression: the Amsterdam Cohort Study. J Infect Dis. 2005;191(5):678–685. doi: 10.1086/427559. [DOI] [PubMed] [Google Scholar]
- 35.Björkman P, Flamholc L, Widell A. GB virus C and survival in persons with HIV infection. N Engl J Med. 2004;350(25):2617–2618. doi: 10.1056/NEJM200406173502516. author reply 2617. [DOI] [PubMed] [Google Scholar]
- 36.Xiang J, Wünschmann S, Schmidt W, Shao J, Stapleton JT. Full-length GB virus C (hepatitis G virus) RNA transcripts are infectious in primary CD4-positive T cells. J Virol. 2000;74(19):9125–9133. doi: 10.1128/jvi.74.19.9125-9133.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tillmann HL, Manns MP. GB virus-C infection in patients infected with the human immunodeficiency virus. Antiviral Res. 2001;52(2):83–90. doi: 10.1016/s0166-3542(01)00172-3. [DOI] [PubMed] [Google Scholar]
- 38.Sauleda S, Reesink HJ, Esteban JI, Hess G, Esteban R, Guardia J. Profiles of GBV-C/ hepatitis G virus markers in patients coinfected with hepatitis C virus. J Med Virol. 1999;59(1):45–51. [PubMed] [Google Scholar]
- 39.Ruiz V, Espínola L, Mathet VL, Perandones CE, Oubiña JR. Design, development and evaluation of a competitive RT-PCR for quantitation of GBV-C RNA. J Virol Methods. 2006;136(1–2):58–64. doi: 10.1016/j.jviromet.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 40.Gómara MJ, Fernández L, Pérez T, Ercilla G, Haro I. Assessment of synthetic chimeric multiple antigenic peptides for diagnosis of GB virus C infection. Anal Biochem. 2010;396(1):51–58. doi: 10.1016/j.ab.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 41.Gómara MJ, Fernández L, Pérez T, et al. Diagnostic value of anti-GBV-C antibodies in HIV-infected patients. Chem Biol Drug Des. 2011;78(2):277–282. doi: 10.1111/j.1747-0285.2011.01143.x. [DOI] [PubMed] [Google Scholar]
- 42.Mohr EL, Stapleton JT. GB virus type C interactions with HIV: the role of envelope glycoproteins. J Viral Hepat. 2009;16(11):757–768. doi: 10.1111/j.1365-2893.2009.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Polgreen PM, Xiang J, Chang Q, Stapleton JT. GB virus type C/hepatitis G virus: a non-pathogenic flavivirus associated with prolonged survival in HIV-infected individuals. Microbes Infect. 2003;5(13):1255–1261. doi: 10.1016/j.micinf.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 44.Belyaev AS, Chong S, Novikov A, et al. Hepatitis G virus encodes protease activities which can effect processing of the virus putative nonstructural proteins. J Virol. 1998;72(1):868–872. doi: 10.1128/jvi.72.1.868-872.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saito S, Tanaka K, Kondo M, et al. Plus- and minus-stranded hepatitis G virus RNA in liver tissue and in peripheral blood mononuclear cells. Biochem Biophys Res Commun. 1997;237(2):288–291. doi: 10.1006/bbrc.1997.7103. [DOI] [PubMed] [Google Scholar]
- 46.Sergi C, Jundt K, Seipp S, et al. The distribution of HBV, HCV and HGV among livers with fulminant hepatic failure of different aetiology. J Hepatol. 1998;29(6):861–871. doi: 10.1016/s0168-8278(98)80112-8. [DOI] [PubMed] [Google Scholar]
- 47.Seipp S, Scheidel M, Hofmann WJ, et al. Hepatotropism of GB virus C (GBV-C): GBV-C replication in human hepatocytes and cells of human hepatoma cell lines. J Hepatol. 1999;30(4):570–579. doi: 10.1016/s0168-8278(99)80186-x. [DOI] [PubMed] [Google Scholar]
- 48.Cao MM, Ren H, Zhao P, Pan W, Chen QL, Qi ZT. Persistent replication of the GBV-C subgenomic replicons in Huh7 cells. J Virol Methods. 2009;157(2):168–174. doi: 10.1016/j.jviromet.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 49.Laskus T, Radkowski M, Wang LF, Vargas H, Rakela J. Lack of evidence for hepatitis G virus replication in the livers of patients coinfected with hepatitis C and G viruses. J Virol. 1997;71(10):7804–7806. doi: 10.1128/jvi.71.10.7804-7806.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mellor J, Haydon G, Blair C, Livingstone W, Simmonds P. Low level or absent in vivo replication of hepatitis C virus and hepatitis G virus/GB virus C in peripheral blood mononuclear cells. J Gen Virol. 1998;79(Pt 4):705–714. doi: 10.1099/0022-1317-79-4-705. [DOI] [PubMed] [Google Scholar]
- 51.Laskus T, Radkowski M, Wang LF, Vargas H, Rakela J. Detection of hepatitis G virus replication sites by using highly strand-specific Tth-based reverse transcriptase PCR. J Virol. 1998;72(4):3072–3075. doi: 10.1128/jvi.72.4.3072-3075.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Radkowski M, Kubicka J, Kisiel E, et al. Detection of active hepatitis C virus and hepatitis G virus/GB virus C replication in bone marrow in human subjects. Blood. 2000;95(12):3986–3989. [PubMed] [Google Scholar]
- 53.George SL, Varmaz D, Stapleton JT. GB virus C replicates in primary T and B lymphocytes. J Infect Dis. 2006;193(3):451–454. doi: 10.1086/499435. [DOI] [PubMed] [Google Scholar]
- 54.Handa A, Brown KE. GB virus C/hepatitis G virus replicates in human haematopoietic cells and vascular endothelial cells. J Gen Virol. 2000;81(Pt 10):2461–2469. doi: 10.1099/0022-1317-82-11-2837. [DOI] [PubMed] [Google Scholar]
- 55.Tucker TJ, Smuts HE, Eedes C, et al. Evidence that the GBV-C/hepatitis G virus is primarily a lymphotropic virus. J Med Virol. 2000;61(1):52–58. [PubMed] [Google Scholar]
- 56.Thiel H-J, Collett MS, Gould EA, et al. Virus Taxonomy: Eighth Report of the International Committee on Taxonomy Of Viruse. Elsevier Academic Press; London, UK: 2005. [Google Scholar]
- 57•.Stapleton JT, Foung S, Muerhoff AS, Bukh J, Simmonds P. The GB viruses: a review and proposed classification of GBV-A, GBV-C (HGV), and GBV-D in genus Pegivirus within the familyFlaviviridae. J Gen Virol. 2011;92(Pt 2):233–246. doi: 10.1099/vir.0.027490-0. Proposed new classification of the GB virus family. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Muerhoff AS, Dawson GJ, Desai SM. A previously unrecognized sixth genotype of GB virus C revealed by analysis of 5′ untranslated region sequences. J Med Virol. 2006;78(1):105–111. doi: 10.1002/jmv.20510. [DOI] [PubMed] [Google Scholar]
- 59.Smith DB, Basaras M, Frost S, et al. Phylogenetic analysis of GBV-C/hepatitis G virus. J Gen Virol. 2000;81(Pt 3):769–780. doi: 10.1099/0022-1317-81-3-769. [DOI] [PubMed] [Google Scholar]
- 60.Naito H, Abe K. Genotyping system of GBV-C/HGV type 1 to type 4 by the polymerase chain reaction using type-specific primers and geographical distribution of viral genotypes. J Virol Methods. 2001;91(1):3–9. doi: 10.1016/s0166-0934(00)00207-x. [DOI] [PubMed] [Google Scholar]
- 61.Muerhoff AS, Simons JN, Erker JC, Desai SM, Mushahwar IK. Identification of conserved nucleotide sequences within the GB virus C 5′ untranslated region: design of PCR primers for detection of viral RNA. J Virol Methods. 1996;62(1):55–62. doi: 10.1016/0166-0934(96)02088-5. [DOI] [PubMed] [Google Scholar]
- 62.Smith DB, Cuceanu N, Davidson F, et al. Discrimination of hepatitis G virus/GBV-C geographical variants by analysis of the 5′ non-coding region. J Gen Virol. 1997;78(Pt 7):1533–1542. doi: 10.1099/0022-1317-78-7-1533. [DOI] [PubMed] [Google Scholar]
- 63.Liu HF, Muyembe-Tamfum JJ, Dahan K, Desmyter J, Goubau P. High prevalence of GB virus C/hepatitis G virus in Kinshasa, Democratic Republic of Congo: a phylogenetic analysis. J Med Virol. 2000;60(2):159–165. [PubMed] [Google Scholar]
- 64.Tucker TJ, Smuts H, Eickhaus P, Robson SC, Kirsch RE. Molecular characterization of the 5′ non-coding region of South African GBV-C/HGV isolates: major deletion and evidence for a fourth genotype. J Med Virol. 1999;59(1):52–59. [PubMed] [Google Scholar]
- 65.Tucker TJ, Smuts HE. GBV-C/HGV genotypes: proposed nomenclature for genotypes 1–5. J Med Virol. 2000;62(1):82–83. doi: 10.1002/1096-9071(200009)62:1<82::aid-jmv12>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 66.Nishiya AS, Ribeiro-dos-Santos G, Bassit L, Focaccia R, Chamone DF, Sabino EC. Genotype distribution of the GB virus C in citizens of São Paulo City, Brazil. Rev Inst Med Trop Sao Paulo. 2003;45(4):213–216. doi: 10.1590/s0036-46652003000400007. [DOI] [PubMed] [Google Scholar]
- 67.Alcalde R, Nishiya A, Casseb J, Inocêncio L, Fonseca LA, Duarte AJ. Prevalence and distribution of the GBV-C/HGV among HIV-1-infected patients under anti-retroviral therapy. Virus Res. 2010;151(2):148–152. doi: 10.1016/j.virusres.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 68.Muerhoff AS, Leary TP, Sathar MA, Dawson GJ, Desai SM. African origin of GB virus C determined by phylogenetic analysis of a complete genotype 5 genome from South Africa. J Gen Virol. 2005;86(Pt 6):1729–1735. doi: 10.1099/vir.0.80854-0. [DOI] [PubMed] [Google Scholar]
- 69.Branco C, Esteves A, Piedade J, Parreira R. A new genotype 2 subcluster identified among GBV-C strains circulating in the Lisbon metropolitan area of Portugal. J Med Virol. 2010;82(3):452–459. doi: 10.1002/jmv.21703. [DOI] [PubMed] [Google Scholar]
- 70.Liu HF, Teng CW, Fukuda Y, et al. A novel subtype of GB virus C/hepatitis G virus genotype 1 detected uniquely in patients with hemophilia in Japan. J Med Virol. 2003;71(3):385–390. doi: 10.1002/jmv.10491. [DOI] [PubMed] [Google Scholar]
- 71.Neibecker M, Schwarze-Zander C, Rockstroh JK, Spengler U, Blackard JT. Evidence for extensive genotypic diversity and recombination of GB virus C (GBV-C) in Germany. J Med Virol. 2011;83(4):685–694. doi: 10.1002/jmv.22029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Worobey M, Holmes EC. Homologous recombination in GB virus C/hepatitis G virus. Mol Biol Evol. 2001;18(2):254–261. doi: 10.1093/oxfordjournals.molbev.a003799. [DOI] [PubMed] [Google Scholar]
- 73.Fan X, Xu Y, Detre K, Di Bisceglie AM. Direct evidence for GB virus C/hepatitis G virus (GBV-C/HGV) superinfection: elimination of resident viral strain by donor strain in a patient undergoing liver transplantation. J Med Virol. 2002;68(1):76–81. doi: 10.1002/jmv.10172. [DOI] [PubMed] [Google Scholar]
- 74.García F, Jr, García F, Bernal MC, Piédrola G, Maroto MC. Genomic variability of hepatitis G virus/GBV-C at the NS3 region: clinical implications. Microbios. 2000;102(401):17–25. [PubMed] [Google Scholar]
- 75.Zampino R, Pickering J, Iqbal M, Gaud U, Thomas HC, Karayiannis P. Hepatitis G virus/GBV-C persistence: absence of hypervariable E2 region and genetic analysis of viral quasispecies in serum and lymphocytes. J Viral Hepat. 1999;6(3):209–218. doi: 10.1046/j.1365-2893.1999.00162.x. [DOI] [PubMed] [Google Scholar]
- 76.Ruiz V, Giordano M, Rivero CW, et al. GB virus C quasispecies detected in plasma and lymphocyte subsets in a natural human infection. J Gen Virol. 2010;91(Pt 7):1687–1692. doi: 10.1099/vir.0.019877-0. [DOI] [PubMed] [Google Scholar]
- 77.Kato T, Mizokami M, Nakano T, et al. Heterogeneity in E2 region of GBV-C/ hepatitis G virus and hepatitis C virus. J Med Virol. 1998;55(2):109–117. [PubMed] [Google Scholar]
- 78.Shimizu YK, Hijikata M, Kiyohara T, Kitamura Y, Yoshikura H. Replication of GB virus C (hepatitis G virus) in interferon-resistant Daudi cells. J Virol. 1999;73(10):8411–8414. doi: 10.1128/jvi.73.10.8411-8414.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fogeda M, López-Alcorocho JM, Bartolomé J, Arocena C, Martín MA, Carreño V. Existence of distinct GB virus C/hepatitis G virus variants with different tropism. J Virol. 2000;74(17):7936–7942. doi: 10.1128/jvi.74.17.7936-7942.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xiang J, Martinez-Smith C, Gale M, Jr, et al. GB virus type C NS5A sequence polymorphisms: association with interferon susceptibility and inhibition of PKR-mediated eIF2alpha phosphorylation. J Interferon Cytokine Res. 2005;25(5):261–270. doi: 10.1089/jir.2005.25.261. [DOI] [PubMed] [Google Scholar]
- 81.George SL, Xiang J, Stapleton JT. Clinical isolates of GB virus type C vary in their ability to persist and replicate in peripheral blood mononuclear cell cultures. Virology. 2003;316(2):191–201. doi: 10.1016/s0042-6822(03)00585-3. [DOI] [PubMed] [Google Scholar]
- 82.Blackard JT, Cohen DE, Mayer KH. Human immunodeficiency virus superinfection and recombination: current state of knowledge and potential clinical consequences. Clin Infect Dis. 2002;34(8):1108–1114. doi: 10.1086/339547. [DOI] [PubMed] [Google Scholar]
- 83.Toyoda H, Fukuda Y, Hayakawa T, Takamatsu J, Saito H. Effect of GB virus C/hepatitis G virus co-infection on the course of HIV infection in hemophilia patients in Japan. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17(3):209–213. doi: 10.1097/00042560-199803010-00004. [DOI] [PubMed] [Google Scholar]
- 84.Heringlake S, Ockenga J, Tillmann HL, et al. GB virus C/hepatitis G virus infection: a favorable prognostic factor in human immunodeficiency virus-infected patients? J Infect Dis. 1998;177(6):1723–1726. doi: 10.1086/517431. [DOI] [PubMed] [Google Scholar]
- 85.Lefrère JJ, Roudot-Thoraval F, Morand-Joubert L, et al. Carriage of GB virus C/hepatitis G virus RNA is associated with a slower immunologic, virologic, and clinical progression of human immunodeficiency virus disease in coinfected persons. J Infect Dis. 1999;179(4):783–789. doi: 10.1086/314671. [DOI] [PubMed] [Google Scholar]
- 86.Yeo AE, Matsumoto A, Hisada M, Shih JW, Alter HJ, Goedert JJ. Effect of hepatitis G virus infection on progression of HIV infection in patients with hemophilia. Multicenter Hemophilia Cohort Study. Ann Intern Med. 2000;132(12):959–963. doi: 10.7326/0003-4819-132-12-200006200-00006. [DOI] [PubMed] [Google Scholar]
- 87•.Xiang J, Wünschmann S, Diekema DJ, et al. Effect of co-infection with GB virus C on survival among patients with HIV infection. N Engl J Med. 2001;345(10):707–714. doi: 10.1056/NEJMoa003364. Describes the beneficial clinical impact of GBV-C co-infection in a large HIV cohort. [DOI] [PubMed] [Google Scholar]
- 88•.Tillmann HL, Heiken H, Knapik-Botor A, et al. Infection with GB virus C and reduced mortality among HIV-infected patients. N Engl J Med. 2001;345(10):715–724. doi: 10.1056/NEJMoa010398. Independent finding of GBV-C co-infection in HIV patients on highly active antiretroviral therapy influencing disease progression. [DOI] [PubMed] [Google Scholar]
- 89.Björkman P, Flamholc L, Molnegren V, Marshall A, Güner N, Widell A. Enhanced and resumed GB virus C replication in HIV-1-infected individuals receiving HAART. AIDS. 2007;21(12):1641–1643. doi: 10.1097/QAD.0b013e32823bc9b7. [DOI] [PubMed] [Google Scholar]
- 90.Rodriguez B, Woolley I, Lederman MM, Zdunek D, Hess G, Valdez H. Effect of GB virus C co-infection on response to antiretroviral treatment in human immunodeficiency virus-infected patients. J Infect Dis. 2003;187(3):504–507. doi: 10.1086/368206. [DOI] [PubMed] [Google Scholar]
- 91.Mosam A, Sathar MA, Dawood H, Cassol E, Esterhuizen TM, Coovadia HM. Effect of GB virus C co-infection on response to generic HAART in African patients with HIV-1 clade C infection. AIDS. 2007;21(10):1377–1379. doi: 10.1097/QAD.0b013e3281532cb8. [DOI] [PubMed] [Google Scholar]
- 92.Antonucci G, Girardi E, Cozzi-Lepri A, et al. HepaICoNA Study GroupICoNA Study Group; Response to HAART and GB virus type C co-infection in a cohort of antiretroviral-naive HIV-infected individuals. Antivir Ther (Lond) 2005;10(1):109–117. [PubMed] [Google Scholar]
- 93.Tillmann HL, Manns MP, Claes C, Heiken H, Schmidt RE, Stoll M. GB virus C infection and quality of life in HIV-positive patients. AIDS Care. 2004;16(6):736–743. doi: 10.1080/09540120412331269576. [DOI] [PubMed] [Google Scholar]
- 94.Birk M, Lindbäck S, Lidman C. No influence of GB virus C replication on the prognosis in a cohort of HIV-1-infected patients. AIDS. 2002;16(18):2482–2485. doi: 10.1097/00002030-200212060-00017. [DOI] [PubMed] [Google Scholar]
- 95.Björkman P, Flamholc L, Nauclér A, Molnegren V, Wallmark E, Widell A. GB virus C during the natural course of HIV-1 infection: viremia at diagnosis does not predict mortality. AIDS. 2004;18(6):877–886. doi: 10.1097/00002030-200404090-00005. [DOI] [PubMed] [Google Scholar]
- 96.Muerhoff AS, Tillmann HL, Manns MP, Dawson GJ, Desai SM. GB virus C genotype determination in GB virus-C/ HIV co-infected individuals. J Med Virol. 2003;70(1):141–149. doi: 10.1002/jmv.10375. [DOI] [PubMed] [Google Scholar]
- 97.Schwarze-Zander C, Blackard JT, Zheng H, et al. AIDS Clinical Trial Group A5071 Study Team. GB virus C (GBV-C) infection in hepatitis C virus (HCV)/ HIV-coinfected patients receiving HCV treatment: importance of the GBV-C genotype. J Infect Dis. 2006;194(4):410–419. doi: 10.1086/505713. [DOI] [PubMed] [Google Scholar]
- 98.Berzsenyi MD, Bowden DS, Roberts SK, Revill PA. GB virus C genotype 2 predominance in a hepatitis C virus/HIV infected population associated with reduced liver disease. J Gastroenterol Hepatol. 2009;24(8):1407–1410. doi: 10.1111/j.1440-1746.2009.05920.x. [DOI] [PubMed] [Google Scholar]
- 99.Zhang W, Chaloner K, Tillmann HL, Williams CF, Stapleton JT. Effect of early and late GB virus C viraemia on survival of HIV-infected individuals: a meta-analysis. HIV Med. 2006;7(3):173–180. doi: 10.1111/j.1468-1293.2006.00366.x. [DOI] [PubMed] [Google Scholar]
- 100.Yu ML, Chuang WL, Dai CY, et al. GB virus C/hepatitis G virus infection in chronic hepatitis C patients with and without interferon-alpha therapy. Antiviral Res. 2001;52(3):241–249. doi: 10.1016/s0166-3542(01)00165-6. [DOI] [PubMed] [Google Scholar]
- 101.Oshita M, Hayashi N, Mita E, et al. GBV-C/HGV infection in chronic hepatitis C patients: its effect on clinical features and interferon therapy. J Med Virol. 1998;55(2):98–102. [PubMed] [Google Scholar]
- 102.Pereira LM, Spinelli V, Ximenes RA, et al. Chronic hepatitis C infection: influence of the viral load, genotypes, and GBV-C/ HGV co-infection on the severity of the disease in a Brazilian population. J Med Virol. 2002;67(1):27–32. doi: 10.1002/jmv.2188. [DOI] [PubMed] [Google Scholar]
- 103.Pawlotsky JM, Roudot-Thoraval F, Muerhoff AS, et al. GB virus C (GBV-C) infection in patients with chronic hepatitis C. Influence on liver disease and on hepatitis virus behaviour: effect of interferon alfa therapy. J Med Virol. 1998;54(1):26–37. doi: 10.1002/(sici)1096-9071(199801)54:1<26::aid-jmv5>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 104.Boodram B, Hershow RC, Klinzman D, Stapleton JT. GB virus C infection among young, HIV-negative injection drug users with and without hepatitis C virus infection. J Viral Hepat. 2011;18(4):e153–e159. doi: 10.1111/j.1365-2893.2010.01350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Manolakopoulos S, Morris A, Davies S, Brown D, Hajat S, Dusheiko G. Influence of GB virus C viraemia on the clinical, virological and histological features of early hepatitis C-related hepatic disease. J Hepatol. 1998;28(2):173–178. doi: 10.1016/0168-8278(88)80001-1. [DOI] [PubMed] [Google Scholar]
- 106.Hofer H, Aydin I, Neumueller-Guber S, et al. Prevalence and clinical significance of GB virus type C/hepatitis G virus co-infection in patients with chronic hepatitis C undergoing antiviral therapy. J Viral Hepat. 2011;18(7):513–517. doi: 10.1111/j.1365-2893.2010.01340.x. [DOI] [PubMed] [Google Scholar]
- 107.Strauss E, da Costa Gayotto LC, Fay F, Fay O, Fernandes HS, de Fischer Chamone DA. Liver histology in co-infection of hepatitis C virus (HCV) and hepatitis G virus (HGV) Rev Inst Med Trop Sao Paulo. 2002;44(2):67–70. doi: 10.1590/s0036-46652002000200003. [DOI] [PubMed] [Google Scholar]
- 108.Yokozaki S, Takamatsu J, Nakano I, et al. Immunologic dynamics in hemophiliac patients infected with hepatitis C virus and human immunodeficiency virus: influence of antiretroviral therapy. Blood. 2000;96(13):4293–4299. [PubMed] [Google Scholar]
- 109.Tedaldi EM, Baker RK, Moorman AC, et al. HIV Outpatient Study (HOPS) Investigators. Influence of co-infection with hepatitis C virus on morbidity and mortality due to human immunodeficiency virus infection in the era of highly active antiretroviral therapy. Clin Infect Dis. 2003;36(3):363–367. doi: 10.1086/345953. [DOI] [PubMed] [Google Scholar]
- 110.Bica I, McGovern B, Dhar R, et al. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis. 2001;32(3):492–497. doi: 10.1086/318501. [DOI] [PubMed] [Google Scholar]
- 111.Martinez-Sierra C, Arizcorreta A, Díaz F, et al. Progression of chronic hepatitis C to liver fibrosis and cirrhosis in patients coinfected with hepatitis C virus and human immunodeficiency virus. Clin Infect Dis. 2003;36(4):491–498. doi: 10.1086/367643. [DOI] [PubMed] [Google Scholar]
- 112.Mohsen AH, Easterbrook PJ, Taylor C, et al. Impact of human immunodeficiency virus (HIV) infection on the progression of liver fibrosis in hepatitis C virus infected patients. Gut. 2003;52(7):1035–1040. doi: 10.1136/gut.52.7.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Poynard T, Mathurin P, Lai CL, et al. PANFIBROSIS Group. A comparison of fibrosis progression in chronic liver diseases. J Hepatol. 2003;38(3):257–265. doi: 10.1016/s0168-8278(02)00413-0. [DOI] [PubMed] [Google Scholar]
- 114.Soto B, Sánchez-Quijano A, Rodrigo L, et al. Human immunodeficiency virus infection modifies the natural history of chronic parenterally-acquired hepatitis C with an unusually rapid progression to cirrhosis. J Hepatol. 1997;26(1):1–5. doi: 10.1016/s0168-8278(97)80001-3. [DOI] [PubMed] [Google Scholar]
- 115.Lesens O, Deschênes M, Steben M, Bélanger G, Tsoukas CM. Hepatitis C virus is related to progressive liver disease in human immunodeficiency virus-positive hemophiliacs and should be treated as an opportunistic infection. J Infect Dis. 1999;179(5):1254–1258. doi: 10.1086/314720. [DOI] [PubMed] [Google Scholar]
- 116.Ragni MV, Belle SH. Impact of human immunodeficiency virus infection on progression to end-stage liver disease in individuals with hemophilia and hepatitis C virus infection. J Infect Dis. 2001;183(7):1112–1115. doi: 10.1086/319273. [DOI] [PubMed] [Google Scholar]
- 117.Yee TT, Griffioen A, Sabin CA, Dusheiko G, Lee CA. The natural history of HCV in a cohort of haemophilic patients infected between 1961 and 1985. Gut. 2000;47(6):845–851. doi: 10.1136/gut.47.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Puoti M, Bonacini M, Spinetti A, et al. HIV-HCV Co-infection Study Group. Liver fibrosis progression is related to CD4 cell depletion in patients coinfected with hepatitis C virus and human immunodeficiency virus. J Infect Dis. 2001;183(1):134–137. doi: 10.1086/317644. [DOI] [PubMed] [Google Scholar]
- 119.Benhamou Y, Bochet M, Di Martino V, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group Hepatology. 1999;30(4):1054–1058. doi: 10.1002/hep.510300409. [DOI] [PubMed] [Google Scholar]
- 120.Pineda JA, Macías J. Progression of liver fibrosis in patients coinfected with hepatitis C virus and human immunodeficiency virus undergoing antiretroviral therapy. J Antimicrob Chemother. 2005;55(4):417–419. doi: 10.1093/jac/dkh555. [DOI] [PubMed] [Google Scholar]
- 121.Shindo M, Arai K, Okuno T. Long-term follow-up of hepatitis G virus/GB virus C replication in liver during and after interferon therapy in patients coinfected with hepatitis C and G viruses. J Gastroenterol. 1999;34(6):680–687. doi: 10.1007/s005350050319. [DOI] [PubMed] [Google Scholar]
- 122.Kao JH, Lai MY, Chen W, Chen PJ, Chen DS. Efficacy of ribavirin plus interferon alpha on viraemia of GB virus-C/hepatitis G virus: comparison with interferon alpha alone. J Gastroenterol Hepatol. 1998;13(12):1249–1253. [PubMed] [Google Scholar]
- 123.Piroth L, Carrat F, Larrat S, et al. Prevalence and impact of GBV-C, SEN-V and HBV occult infections in HIV-HCV co-infected patients on HCV therapy. J Hepatol. 2008;49(6):892–898. doi: 10.1016/j.jhep.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 124.Shankar EM, Balakrishnan P, Vignesh R, Velu V, Jayakumar P, Solomon S. Current Views on the Pathophysiology of GB Virus C Co-infection with HIV-1 Infection. Curr Infect Dis Rep. 2011;13(1):47–52. doi: 10.1007/s11908-010-0142-z. [DOI] [PubMed] [Google Scholar]
- 125.Siegal F. Interferon-producing plasmacytoid dendritic cells and the pathogenesis of AIDS. Res Initiat Treat Action. 2003;8(2):10–13. [PubMed] [Google Scholar]
- 126.Arrode G, Finke JS, Zebroski H, Siegal FP, Steinman RM. CD8+ T cells from most HIV-1-infected patients, even when challenged with mature dendritic cells, lack functional recall memory to HIV gag but not other viruses. Eur J Immunol. 2005;35(1):159–170. doi: 10.1002/eji.200425744. [DOI] [PubMed] [Google Scholar]
- 127.Nunnari G, Nigro L, Palermo F, et al. Slower progression of HIV-1 infection in persons with GB virus C co-infection correlates with an intact T-helper 1 cytokine profile. Ann Intern Med. 2003;139(1):26–30. doi: 10.7326/0003-4819-139-1-200307010-00009. [DOI] [PubMed] [Google Scholar]
- 128.Capobianchi MR, Lalle E, Martini F, et al. Influence of GBV-C infection on the endogenous activation of the IFN system in HIV-1 co-infected patients. Cell Mol Biol (Noisy-le-grand) 2006;52(1):3–8. [PubMed] [Google Scholar]
- 129.Lalle E, Sacchi A, Abbate I, et al. Activation of interferon response genes and of plasmacytoid dendritic cells in HIV-1 positive subjects with GB virus C co-infection. Int J Immunopathol Pharmacol. 2008;21(1):161–171. doi: 10.1177/039463200802100118. [DOI] [PubMed] [Google Scholar]
- 130.Meyers JH, Justement JS, Hallahan CW, et al. Impact of HIV on cell survival and antiviral activity of plasmacytoid dendritic cells. PLoS ONE. 2007;2(5):e458. doi: 10.1371/journal.pone.0000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xiang J, George SL, Wünschmann S, Chang Q, Klinzman D, Stapleton JT. Inhibition of HIV-1 replication by GB virus C infection through increases in RANTES, MIP-1alpha, MIP-1beta, and SDF-1. Lancet. 2004;363(9426):2040–2046. doi: 10.1016/S0140-6736(04)16453-2. [DOI] [PubMed] [Google Scholar]
- 132.Jung S, Knauer O, Donhauser N, et al. Inhibition of HIV strains by GB virus C in cell culture can be mediated by CD4 and CD8 T-lymphocyte derived soluble factors. AIDS. 2005;19(12):1267–1272. doi: 10.1097/01.aids.0000180097.50393.df. [DOI] [PubMed] [Google Scholar]
- 133.Xiang J, McLinden JH, Chang Q, Kaufman TM, Stapleton JT. An 85-aa segment of the GB virus type C NS5A phosphoprotein inhibits HIV-1 replication in CD4+ Jurkat T cells. Proc Natl Acad Sci USA. 2006;103(42):15570–15575. doi: 10.1073/pnas.0604728103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134•.Chang Q, McLinden JH, Stapleton JT, Sathar MA, Xiang J. Expression of GB virus C NS5A protein from genotypes 1, 2, 3 and 5 and a 30 aa NS5A fragment inhibit human immunodeficiency virus type 1 replication in a CD4+ T-lymphocyte cell line. J Gen Virol. 2007;88(Pt 12):3341–3346. doi: 10.1099/vir.0.83198-0. Describes an important modification of HIV co-receptor expression through the GBV-C NS5A protein. [DOI] [PubMed] [Google Scholar]
- 135.Xiang J, McLinden JH, Chang Q, Jordan EL, Stapleton JT. Characterization of a peptide domain within the GB virus C NS5A phosphoprotein that inhibits HIV replication. PLoS ONE. 2008;3(7):e2580. doi: 10.1371/journal.pone.0002580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xiang J, McLinden JH, Rydze RA, et al. Viruses within the Flaviviridae decrease CD4 expression and inhibit HIV replication in human CD4+ cells. J Immunol. 2009;183(12):7860–7869. doi: 10.4049/jimmunol.0902276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nattermann J, Nischalke HD, Kupfer B, et al. Regulation of CC chemokine receptor 5 in hepatitis G virus infection. AIDS. 2003;17(10):1457–1462. doi: 10.1097/00002030-200307040-00006. [DOI] [PubMed] [Google Scholar]
- 138.Kaufman TM, McLinden JH, Xiang J, Engel AM, Stapleton JT. The GBV-C envelope glycoprotein E2 does not interact specifically with CD81. AIDS. 2007;21(8):1045–1048. doi: 10.1097/QAD.0b013e3280f77412. [DOI] [PubMed] [Google Scholar]
- 139.Schwarze-Zander C, Neibecker M, Othman S, et al. GB virus C co-infection in advanced HIV type-1 disease is associated with low CCR5 and CXCR4 surface expression on CD4+ T-cells. Antivir Ther (Lond) 2010;15(5):745–752. doi: 10.3851/IMP1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jung S, Eichenmüller M, Donhauser N, et al. HIV entry inhibition by the envelope 2 glycoprotein of GB virus C. AIDS. 2007;21(5):645–647. doi: 10.1097/QAD.0b013e32803277c7. [DOI] [PubMed] [Google Scholar]
- 141•.Koedel Y, Eissmann K, Wend H, Fleckenstein B, Reil H. Peptides derived from a distinct region of GB virus C glycoprotein E2 mediate strain-specific HIV-1 entry inhibition. J Virol. 2011;85(14):7037–7047. doi: 10.1128/JVI.02366-10. Gives new insight into the interaction between GBV-C and HIV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Herrera E, Gomara MJ, Mazzini S, Ragg E, Haro I. Synthetic peptides of hepatitis G virus (GBV-C/HGV) in the selection of putative peptide inhibitors of the HIV-1 fusion peptide. J Phys Chem B. 2009;113(20):7383–7391. doi: 10.1021/jp900707t. [DOI] [PubMed] [Google Scholar]
- 143•.Herrera E, Tenckhoff S, Gómara MJ, et al. Effect of synthetic peptides belonging to E2 envelope protein of GB virus C on human immunodeficiency virus type 1 infection. J Med Chem. 2010;53(16):6054–6063. doi: 10.1021/jm100452c. Studies the interaction of the GBV-C E2 and HIV fusion protein. [DOI] [PubMed] [Google Scholar]
- 144.Haro I, Gómara MJ, Galatola R, et al. Study of the inhibition capacity of an 18-mer peptide domain of GBV-C virus on gp41-FP HIV-1 activity. Biochim Biophys Acta. 2011;1808(6):1567–1573. doi: 10.1016/j.bbamem.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 145.Mohr EL, Xiang J, McLinden JH, et al. GB virus type C envelope protein E2 elicits antibodies that react with a cellular antigen on HIV-1 particles and neutralize diverse HIV-1 isolates. J Immunol. 2010;185(7):4496–4505. doi: 10.4049/jimmunol.1001980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hazenberg MD, Stuart JW, Otto SA, et al. T-cell division in human immunodeficiency virus (HIV)-1 infection is mainly due to immune activation: a longitudinal analysis in patients before and during highly active antiretroviral therapy (HAART) Blood. 2000;95(1):249–255. [PubMed] [Google Scholar]
- 147.Maidana-Giret MT, Silva TM, Sauer MM, et al. GB virus type C infection modulates T-cell activation independently of HIV-1 viral load. AIDS. 2009;23(17):2277–2287. doi: 10.1097/QAD.0b013e32832d7a11. [DOI] [PubMed] [Google Scholar]
- 148.Kottilil S, Shin K, Jackson JO, et al. Innate immune dysfunction in HIV infection: effect of HIV envelope-NK cell interactions. J Immunol. 2006;176(2):1107–1114. doi: 10.4049/jimmunol.176.2.1107. [DOI] [PubMed] [Google Scholar]
- 149.Silvestris F, Cafforio P, Frassanito MA, et al. Overexpression of Fas antigen on T cells in advanced HIV-1 infection: differential ligation constantly induces apoptosis. AIDS. 1996;10(2):131–141. doi: 10.1097/00002030-199602000-00002. [DOI] [PubMed] [Google Scholar]
- 150.Gougeon ML, Lecoeur H, Sasaki Y. Apoptosis and the CD95 system in HIV disease: impact of highly active anti-retroviral therapy (HAART) Immunol Lett. 1999;66(1–3):97–103. doi: 10.1016/s0165-2478(98)00167-9. [DOI] [PubMed] [Google Scholar]
- 151.Moenkemeyer M, Schmidt RE, Wedemeyer H, Tillmann HL, Heiken H. GBV-C co-infection is negatively correlated to Fas expression and Fas-mediated apoptosis in HIV-1 infected patients. J Med Virol. 2008;80(11):1933–1940. doi: 10.1002/jmv.21305. [DOI] [PubMed] [Google Scholar]